Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence
Abstract
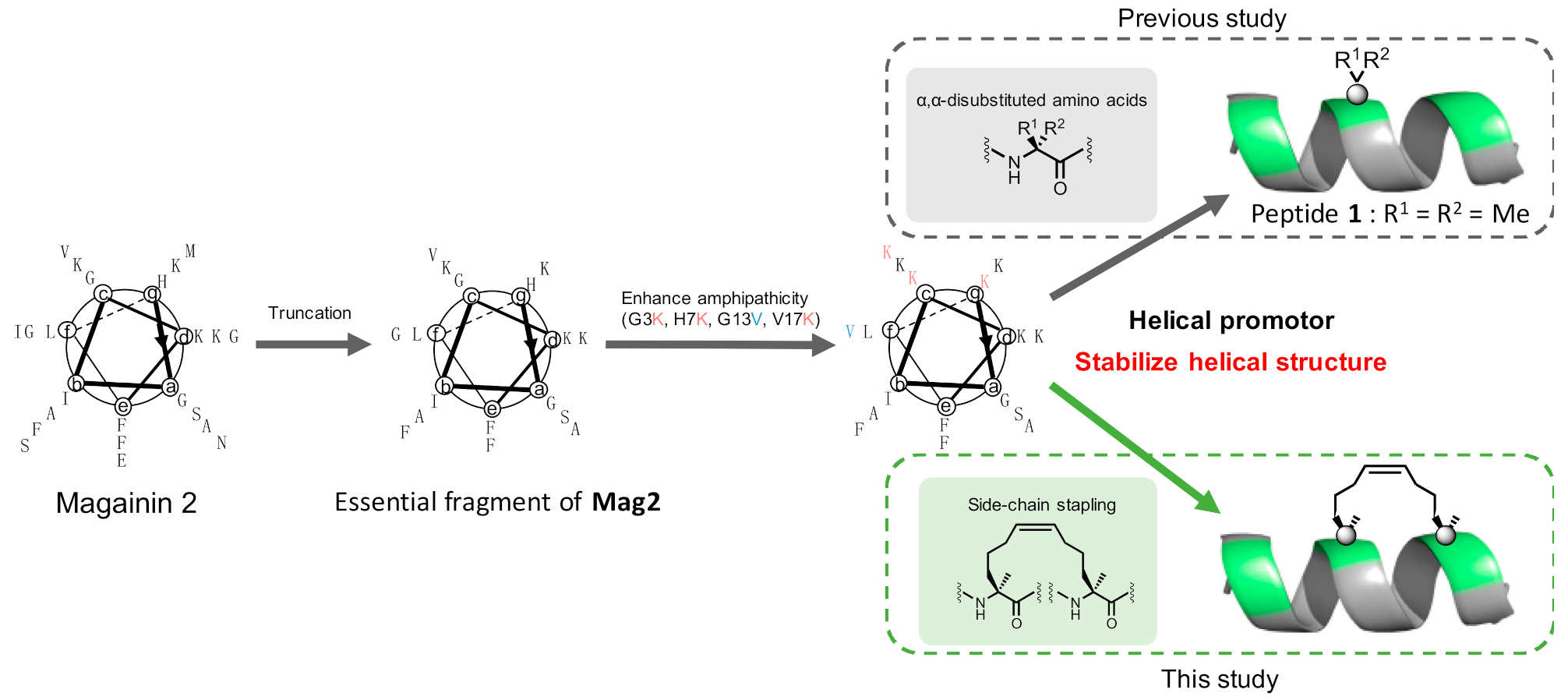
1. Introduction
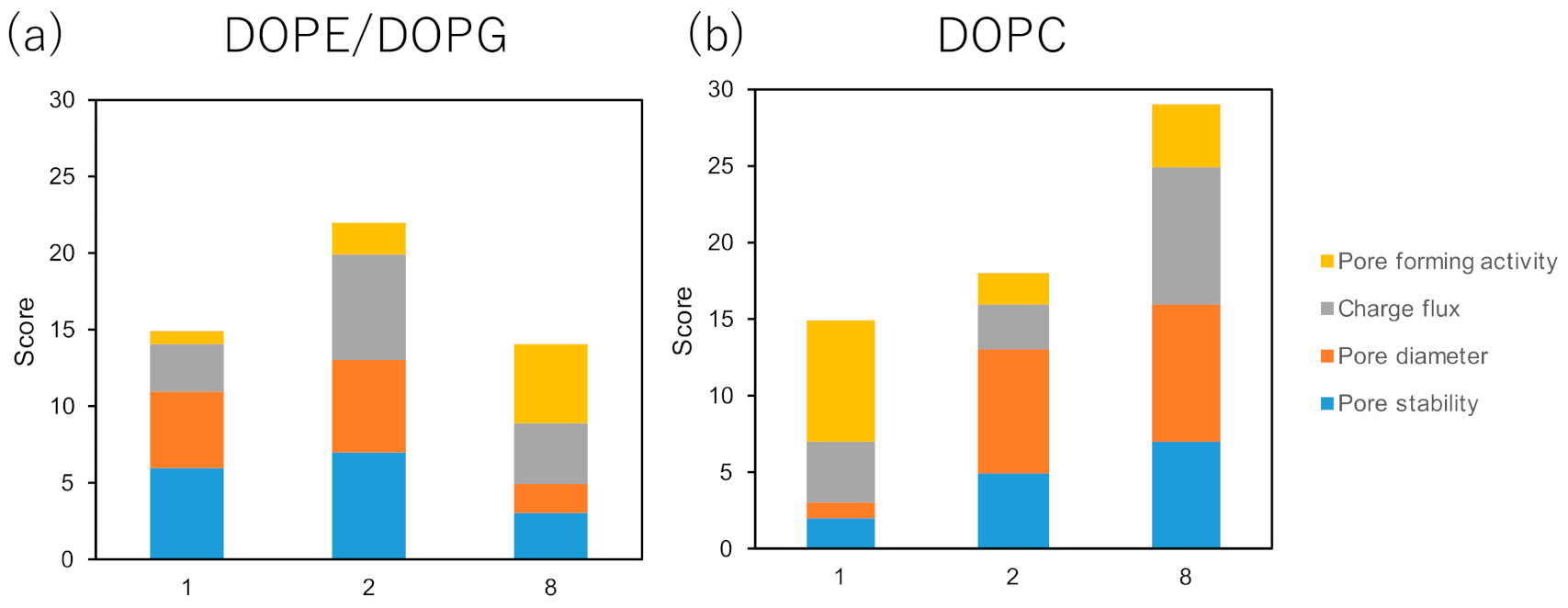
2. Results
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Peptide Synthesis
4.3. CD Spectrometry
4.4. Antimicrobial Activity
4.5. Hemolysis Activity
4.6. Channel Current Measurement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, M.K.; Kang, N.; Ko, S.J.; Park, J.; Park, E.; Shin, D.W.; Kim, S.H.; Lee, S.A.; Lee, J.I.; Lee, S.H.; et al. Antibacterial and Antibiofilm Activity and Mode of Action of Magainin 2 against Drug-Resistant Acinetobacter baumannii. Int. J. Mol. Sci. 2018, 19, 3041. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Teng, P.; Bai, G.; Lin, X.; Zuo, X.; Cao, C.; Cai, J. Helical Antimicrobial Sulfono-γ-AApeptides. J. Med. Chem. 2015, 58, 4802–4811. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Cohen, T.; Grad, H.Y.; Hanage, P.W.; O’Brien, F.T.; Lipsitch, M. Origin and Proliferation of multi-drug resistance in Bacterial pathogen. Microbiol. Biol. Rev. 2015, 79, 101–116. [Google Scholar] [CrossRef]
- Goto, C.; Hirano, M.; Hayashi, K.; Kikuchi, Y.; Hara-Kudo, Y.; Misawa, T.; Demizu, Y. Development of amphipathic antimicrobial peptide foldamers based on Magainin 2 sequence. ChemMedChem 2019, 14, 1911–1916. [Google Scholar] [CrossRef]
- Walensky, L.D.; Kung, A.L.; Escher, I.; Malla, T.J.; Burbuto, S.; Wright, R.D.; Wagner, G.; Verdine, G.L.; Korsmeyer, S. Activation of apoptosis in Vivo by a hydrocarbon stapled BH3 Helix. Science 2004, 305, 1466–1470. [Google Scholar] [CrossRef]
- Porter, E.A.; Wang, X.; Lee, H.S.; Weisblum, B.; Gellman, S.H. Non-haemolytic-amino-acid oligomers. Nature 2000, 404, 565. [Google Scholar] [CrossRef]
- Ali, A.M.; Atmaj, J.; Oosterwijk, N.V.; Groves, M.R.; Domling, A. Stapling peptides inhibitors: A new windows for target drug discovery. Comput. Struct. Biotechnol. J. 2019, 7, 263–281. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Pu, Q.; He, T.; Zhang, Q.; Wu, W.; Xia, X.; Zhang, J. Novel stapling by lysin tethering provides stable and low hemolytic cationic antimicrobial peptides. J. Med. Chem. 2020, 63, 4081–4089. [Google Scholar] [CrossRef]
- Yokoo, H.; Misawa, T.; Demizu, Y. De Novo design of cel-penetrating foldamers. Chem. Rec. 2020, 20, 912–921. [Google Scholar] [CrossRef]
- Mourtada, R.; Herce, H.D.; Yin, D.J.; Moroco, J.A.; Walse, T.E.; Engen, J.R.; Walensky, L.D. Design of stapled antimicrobial peptides that are stable, nontoxic, and kill antibiotic-resistant bacteria in mice. Nat. Biotechnol. 2019, 37, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumari, V.; Nagaraj, R. N-Terminal fatty acylation of peptides spanning the cationic C-terminal segment of bovine -defensin-2 results in salt-resistant antibacterial activity. Biophys. Chem. 2015, 199, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zweytick, D.; Pabst, G.; Abuja, M.P.; Jilek, A.; Blondelle, E.S.; Andrä, J.; Jerala, R.; Monreal, D.; Tejada, M.G.; Lohner, K. Influence of N-acylation of a peptide derived from human lactoferricin on membrane selectivity. Biochim. Biophys. Acta. 2006, 1758, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, P.; Cheetham, G.A.; Walston, J.; Abadir, P.; Cui, H. Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug. Chem. 2015, 26, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Kato, T.; Oba, M.; Misawa, T.; Hattori, T.; Ohoka, N.; Tanaka, M.; Naito, M.; Kurihara, M.; Demizu, Y. Development of a cell-penetrating peptide that exhibits responsive changes in its secondary structure in the cellular environment. Sci. Rep. 2016, 6, 33003. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, Y.; Sakashita, S.; Shimizu, K.; Usui, K.; Kawano, R. Channel current analysis estimates the pore-formation and the penetration of transmembrane peptides. Analyst 2018, 143, 3540–3543. [Google Scholar] [CrossRef]
- Sekiya, Y.; Shimizu, K.; Kitahashi, Y.; Ohyama, A.; Kawamura, I.; Kawano, R. Electrophysiological analysis of membrane disruption by bombinin and its isomer using the lipid bilayer system. ACS Appl. Bio Mater. 2019, 2, 1542–1548. [Google Scholar] [CrossRef]
- Saigo, N.; Izumi, K.; Kawano, R. Electrophysiological analysis of antimicrobial peptides in diverse species. ACS Omega 2019, 4, 13124–13130. [Google Scholar] [CrossRef]
- Misawa, T.; Goto, C.; Shibata, N.; Hirano, M.; Kikuchi, Y.; Naito, M.; Demizu, Y. Rational design of novel amphipathic antimicrobial peptides focused on distribution of cationic acid residues. MedChemComm. 2019, 10, 896–900. [Google Scholar] [CrossRef]
- Strandberg, E.; Zerweck, J.; Horn, D.; Pritz, G.; Berditsch, M.; Bürck, J.; Wedhwani, P.; Ulrich, S.A. Influence of hydrophobic residues on the activity of the antimicrobial peptide magainin 2 and its synergy with PGLa. J. Pept. Sci. 2015, 21, 436–445. [Google Scholar] [CrossRef]
- Akishiba, M.; Takeuchi, T.; Kawaguchi, Y.; Sakamoto, K.; Yu, H.H.; Nakase, I.; Takatani-Nakase, T.; Madani, F.; Gräslund, A.; Futaki, S. Cytosolic antibody delivery by lipid-sensitive endosomolytic peptide. Nat. Chem. 2017, 9, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Tsuji, Y.; Sato, K.; Osaki, T.; Kamiya, K.; Hirano, M.; Ide, T.; Miki, N.; Takeuchi, S. Automated parallel recordings of topologically identified single ion channels. Sci. Rep. 2013, 3, 1995. [Google Scholar] [CrossRef] [PubMed]


| Peptide | Sequence | MIC (µM) | Hemolysis (µM) | |||
|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | |||||
| S.a. | E.c. (DH5α) | P.a. | MDRP | |||
| Mag2 | H-GIGKFLHSAKKFGKAFVGEIMNS-NH2 | 100 | 3.125 | 12.5 | 12.5 | >100 |
| 1 | H-GIKKFLKSXKKFVKXFK-NH2 | 12.5 | 3.125 | 3.125 | 3.125 | 50 |
| 2 | H-S5IKKS5LKSAKKFVKAFK-NH2 | 3.125 | 1.56 | 1.56 | 1.56 | 50 |
| 3 | H-GIKKS5LKSS5KKFVKAFK-NH2 | 3.125 | 1.56 | 3.125 | 0.78 | 6.25 |
| 4 | H-GIKKFLKS5AKKS5VKAFK-NH2 | 6.25 | 1.56 | 1.56 | 0.78 | 3.125 |
| 5 | H-GIKKFLKSS5KKFS5KAFK-NH2 | 12.5 | 1.56 | 6.25 | 3.125 | 1.56 |
| 6 | H-GIKKFLKSAKKS5VKAS5K-NH2 | 3.125 | 1.56 | 1.56 | 0.78 | 3.125 |
| 7 | H-GIKKR8LKSAKKS5VKAFK-NH2 | 25 | 3.125 | 3.125 | 1.56 | 6.25 |
| 8 | H-GIKKFLKR8AKKFVKS5FK-NH2 | >50 | 6.25 | >50 | 3.125 | 0.19 |
| 9 | H-GIKKFLKSR8KKFVKAS5K-NH2 | 6.25 | 3.125 | 6.25 | 3.125 | 3.125 |
| Peptide | Sequence | MIC (µM) | Hemolysis (µM) | |||
|---|---|---|---|---|---|---|
| Gram Positive | Gram Negative | |||||
| S.a. | E.c. (DH5α) | P.a. | MDRP | |||
| 2 | H-S5IKKS5LKSAKKFVKAFK-NH2 | 3.125 | 1.56 | 1.56 | 1.56 | 50 |
| C6-2 | CH3(CH2)4CO-S5IKKS5LKSAKKFVKAFK-NH2 | 1.56 | 1.56 | 3.125 | 3.125 | 12.5 |
| C12-2 | CH3(CH2)10CO-S5IKKS5LKSAKKFVKAFK-NH2 | 6.25 | 6.25 | 12.5 | 25 | 0.78 |
| C18-2 | CH3(CH2)16CO-S5IKKS5LKSAKKFVKAFK-NH2 | >50 | >50 | >50 | >50 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, M.; Saito, C.; Yokoo, H.; Goto, C.; Kawano, R.; Misawa, T.; Demizu, Y. Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence. Molecules 2021, 26, 444. https://doi.org/10.3390/molecules26020444
Hirano M, Saito C, Yokoo H, Goto C, Kawano R, Misawa T, Demizu Y. Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence. Molecules. 2021; 26(2):444. https://doi.org/10.3390/molecules26020444
Chicago/Turabian StyleHirano, Motoharu, Chihiro Saito, Hidetomo Yokoo, Chihiro Goto, Ryuji Kawano, Takashi Misawa, and Yosuke Demizu. 2021. "Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence" Molecules 26, no. 2: 444. https://doi.org/10.3390/molecules26020444
APA StyleHirano, M., Saito, C., Yokoo, H., Goto, C., Kawano, R., Misawa, T., & Demizu, Y. (2021). Development of Antimicrobial Stapled Peptides Based on Magainin 2 Sequence. Molecules, 26(2), 444. https://doi.org/10.3390/molecules26020444






