Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis
Abstract
1. Introduction
2. Subacute Sclerosing Panencephalitis (SSPE)
2.1. Clinical Features and Epidemiology of SSPE
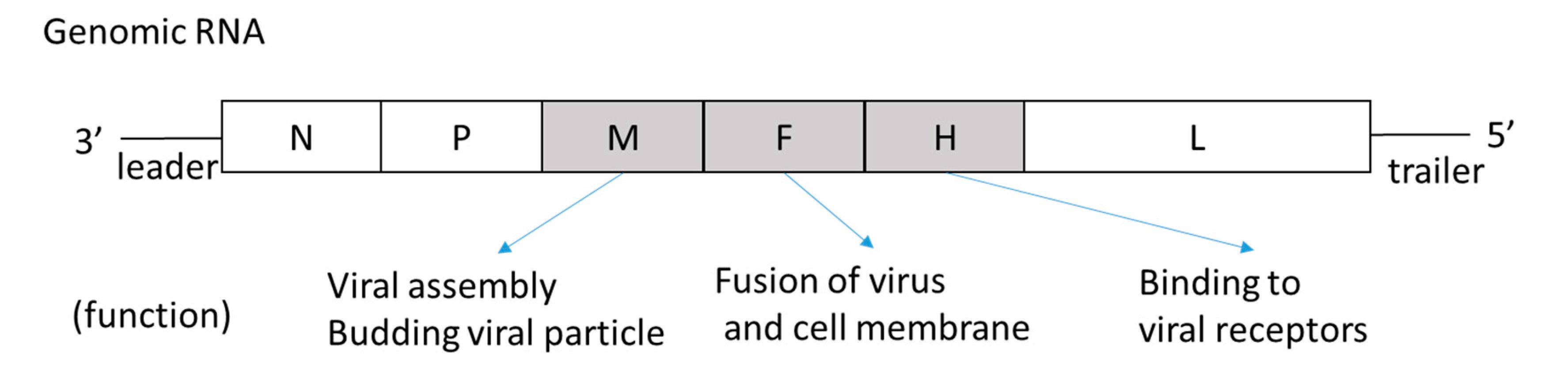
2.2. Etiology and Virological Characteristics of SSPE
3. Treatment
3.1. Inosine Pranobex
3.2. Interferons
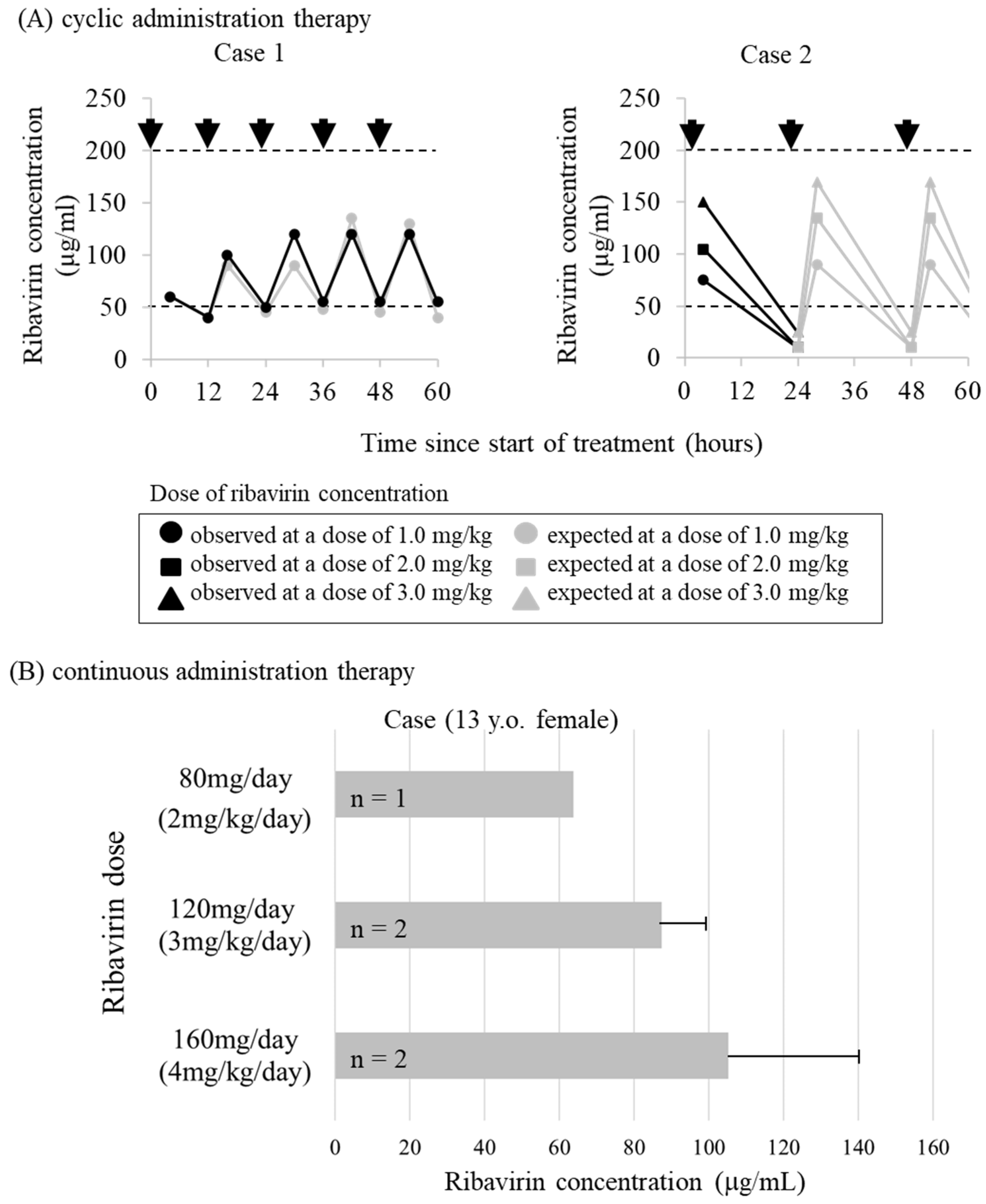
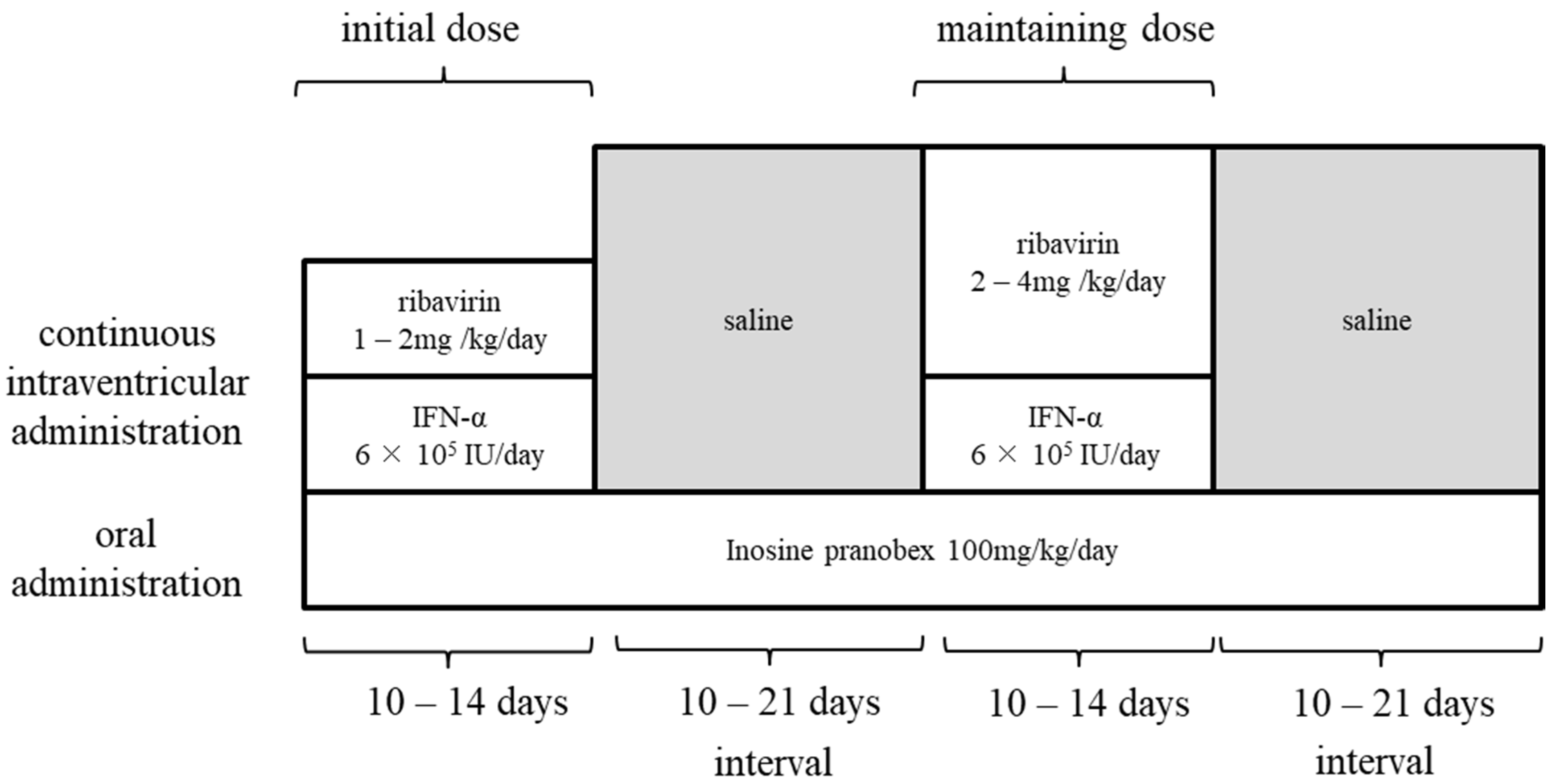
3.3. Ribavirin and Research on Treatment Methods
3.4. Other Existing Clinical Drugs
3.5. Preclinical Drugs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Immunization, Vaccines and Biologicals, Measles. Available online: https://www.who.int/immunization/diseases/measles/en/ (accessed on 1 November 2020).
- WHO New Measles Surveillance Data for 2019. Available online: https://www.who.int/news/item/15-05-2019-new-measles-surveillance-data-for-2019 (accessed on 1 November 2020).
- Cherry, J.; Demmler-Harrison, G.J.; Kaplan, S.L.; Steinbach, W.J.; Hotez, P.J. Feigin and Cherry’s Textbook of Pediatric Infectious Disease. In Measles Virus, 8th ed.; Elesevier: Philadelphia, PA, USA, 2019. [Google Scholar]
- Modlin, J.F.; Jabbour, J.T.; Witte, J.J.; Halsey, N.A. Epidemiologic studies of measles, measles vaccine, and subacute sclerosing panencephalitis. Pediatrics 1977, 59, 505–512. [Google Scholar]
- Jabbour, J.T.; Garcia, J.H.; Lemmi, H.; Ragland, J.; Duenas, D.A.; Sever, J.L. Subacute sclerosing panencephalitis. A multidisciplinary study of eight cases. JAMA 1969, 207, 2248–2254. [Google Scholar] [CrossRef]
- Dyken, P.R.; Swift, A.; DuRant, R.H. Long-term follow-up of patients with subacute sclerosing panencephalitis treated with inosiplex. Ann. Neurol. 1982, 11, 359–364. [Google Scholar] [CrossRef]
- Kandadai, R.M.; Yada, P.; Uppin, M.S.; Jabeen, S.A.; Cherian, A.; Kanikannan, M.A.; Borgohain, R.; Challa, S. Fulminant subacute sclerosing panencephalitis presenting with acute ataxia and hemiparesis in a 15-year-old boy. J. Clin. Neurol. 2014, 10, 354–357. [Google Scholar] [CrossRef]
- Prashanth, L.K.; Taly, A.B.; Ravi, V.; Sinha, S.; Rao, S. Long term survival in subacute sclerosing panencephalitis: An enigma. Brain Dev. 2006, 28, 447–452. [Google Scholar] [CrossRef]
- Risk, W.S.; Haddad, F.S. The variable natural history of subacute sclerosing panencephalitis: A study of 118 cases from the Middle East. Arch. Neurol. 1979, 36, 610–614. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, K.; Iinuma, K.; Ohtsuka, Y.; Ichiyama, T.; Kusuhara, K.; Nomura, K.; Mizuguchi, M.; Aiba, H.; Suzuki, Y.; et al. Survey of subacute sclerosing panencephalitis in Japan. J. Child Neurol. 2012, 27, 1529–1533. [Google Scholar] [CrossRef]
- Campbell, H.; Andrews, N.; Brown, K.E.; Miller, E. Review of the effect of measles vaccination on the epidemiology of SSPE. Int. J. Epidemiol. 2007, 36, 1334–1348. [Google Scholar] [CrossRef]
- Bellini, W.J.; Rota, J.S.; Lowe, L.E.; Katz, R.S.; Dyken, P.R.; Zaki, S.R.; Shieh, W.J.; Rota, P.A. Subacute sclerosing panencephalitis: More cases of this fatal disease are prevented by measles immunization than was previously recognized. J. Infect. Dis. 2005, 192, 1686–1693. [Google Scholar] [CrossRef]
- Schonberger, K.; Ludwig, M.S.; Wildner, M.; Weissbrich, B. Epidemiology of subacute sclerosing panencephalitis (SSPE) in Germany from 2003 to 2009: A risk estimation. PLoS ONE 2013, 8, e68909. [Google Scholar] [CrossRef]
- Wendorf, K.A.; Winter, K.; Zipprich, J.; Schechter, R.; Hacker, J.K.; Preas, C.; Cherry, J.D.; Glaser, C.; Harriman, K. Subacute Sclerosing Panencephalitis: The Devastating Measles Complication That Might Be More Common Than Previously Estimated. Clin. Infect. Dis. 2017, 65, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.C.; Hadler, S.C.; Dykewicz, C.A.; Reef, S.; Phillips, L. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 1998, 47, 1–57. [Google Scholar] [PubMed]
- Dawson, J.R. Cellular Inclusions in Cerebral Lesions of Lethargic Encephalitis. Am. J. Pathol. 1933, 9, 7–16. [Google Scholar] [PubMed]
- Horta-Barbosa, L.; Fuccillo, D.A.; Sever, J.L.; Zeman, W. Subacute sclerosing panencephalitis: Isolation of measles virus from a brain biopsy. Nature 1969, 221, 974. [Google Scholar] [CrossRef]
- Payne, F.E.; Baublis, J.V.; Itabashi, H.H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N. Engl. J. Med. 1969, 281, 585–589. [Google Scholar] [CrossRef]
- Delpeut, S.; Noyce, R.S.; Siu, R.W.; Richardson, C.D. Host factors and measles virus replication. Curr. Opin. Virol. 2012, 2, 773–783. [Google Scholar] [CrossRef]
- Young, V.A.; Rall, G.F. Making it to the synapse: Measles virus spread in and among neurons. Curr. Top. Microbiol. Immunol. 2009, 330, 3–30. [Google Scholar]
- Sato, Y.; Watanabe, S.; Fukuda, Y.; Hashiguchi, T.; Yanagi, Y.; Ohno, S. Cell-to-Cell Measles Virus Spread between Human Neurons Is Dependent on Hemagglutinin and Hyperfusogenic Fusion Protein. J. Virol. 2018, 92, 6. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Virus, 6th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bellini, W.J.; Englund, G.; Rozenblatt, S.; Arnheiter, H.; Richardson, C.D. Measles virus P gene codes for two proteins. J. Virol. 1985, 53, 908–919. [Google Scholar] [CrossRef]
- Shaffer, J.A.; Bellini, W.J.; Rota, P.A. The C protein of measles virus inhibits the type I interferon response. Virology 2003, 315, 389–397. [Google Scholar] [CrossRef]
- Schuhmann, K.M.; Pfaller, C.K.; Conzelmann, K.K. The measles virus V protein binds to p65 (RelA) to suppress NF-kappaB activity. J. Virol. 2011, 85, 3162–3171. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, B.; Komatsu, T.; Takeuchi, K.; Yokoo, J. Paramyxovirus accessory proteins as interferon antagonists. Microbiol. Immunol. 2001, 45, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E.; Lin, W.H.; Pan, C.H. Measles virus, immune control, and persistence. FEMS Microbiol. Rev. 2012, 36, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.A.; Parks, G.D. Paramyxoviridae: The Viruses and Their Replication, 6th ed.; Lippincott, Williams, and Wilkins: Philadelphia, PA, USA, 2013; Volume 1, p. 39. [Google Scholar]
- Plattet, P.; Alves, L.; Herren, M.; Aguilar, H.C. Measles Virus Fusion Protein: Structure, Function and Inhibition. Viruses 2016, 8, 112. [Google Scholar] [CrossRef]
- Rima, B.K.; Duprex, W.P. Molecular mechanisms of measles virus persistence. Virus Res. 2005, 111, 132–147. [Google Scholar] [CrossRef]
- Watanabe, S.; Shirogane, Y.; Suzuki, S.O.; Ikegame, S.; Koga, R.; Yanagi, Y. Mutant fusion proteins with enhanced fusion activity promote measles virus spread in human neuronal cells and brains of suckling hamsters. J. Virol. 2013, 87, 2648–2659. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohno, S.; Shirogane, Y.; Suzuki, S.O.; Koga, R.; Yanagi, Y. Measles virus mutants possessing the fusion protein with enhanced fusion activity spread effectively in neuronal cells, but not in other cells, without causing strong cytopathology. J. Virol. 2015, 89, 2710–2717. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, K.; Watanabe, M.; Ohara, S.; Sato, M.; Kawasaki, Y.; Hashimoto, Y.; Hosoya, M. Characteristics of viruses derived from nude mice with persistent measles virus infection. J. Virol. 2013, 87, 4170–4175. [Google Scholar] [CrossRef]
- Jin, L.; Beard, S.; Hunjan, R.; Brown, D.W.; Miller, E. Characterization of measles virus strains causing SSPE: A study of 11 cases. J. Neurovirol. 2002, 8, 335–344. [Google Scholar] [CrossRef]
- Rima, B.K.; Earle, J.A.; Yeo, R.P.; Herlihy, L.; Baczko, K.; ter Meulen, V.; Carabana, J.; Caballero, M.; Celma, M.L.; Fernandez-Munoz, R. Temporal and geographical distribution of measles virus genotypes. J. Gen. Virol. 1995, 76, 1173–1180. [Google Scholar] [CrossRef]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [CrossRef]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef]
- Mühlebach, M.D.; Mateo, M.; Sinn, P.L.; Prüfer, S.; Uhlig, K.M.; Leonard, V.H.J.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 4. [Google Scholar] [CrossRef] [PubMed]
- Naniche, D.; Varior-Krishnan, G.; Cervoni, F.; Wild, T.F.; Rossi, B.; Rabourdin-Combe, C.; Gerlier, D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 1993, 67, 6025–6032. [Google Scholar] [CrossRef] [PubMed]
- Dorig, R.E.; Marcil, A.; Chopra, A.; Richardson, C.D. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 1993, 75, 295–305. [Google Scholar] [CrossRef]
- Torisu, H.; Kusuhara, K.; Kira, R.; Bassuny, W.M.; Sakai, Y.; Sanefuji, M.; Takemoto, M.; Hara, T. Functional MxA promoter polymorphism associated with subacute sclerosing panencephalitis. Neurology 2004, 62, 457–460. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Takemoto, M.; Kira, R.; Kusuhara, K.; Torisu, H.; Sakai, Y.; Sanefuji, M.; Yukaya, N.; Hara, T. Association of toll-like receptor 3 gene polymorphism with subacute sclerosing panencephalitis. J. Neurovirol. 2008, 14, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Karakas-Celik, S.; Piskin, I.E.; Keni, M.F.; Calik, M.; Iscan, A.; Dursun, A. May TLR4 Asp299Gly and IL17 His161Arg polymorphism be associated with progression of primary measles infection to subacute sclerosing panencephalitis? Gene 2014, 547, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, V.; Demirbilek, V.; Gurses, C.; Yentur, S.P.; Uysal, S.; Yapici, Z.; Yilmaz, G.; Muncey, A.; Cokar, O.; Onal, E.; et al. Interleukin (IL)-12, IL-2, interferon-gamma gene polymorphisms in subacute sclerosing panencephalitis patients. J. Neurovirol. 2007, 13, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kira, R.; Nakao, F.; Ihara, K.; Bassuny, W.M.; Kusuhara, K.; Nihei, K.; Takeshita, K.; Hara, T. Contribution of the interleukin 4 gene to susceptibility to subacute sclerosing panencephalitis. Arch. Neurol. 2002, 59, 822–827. [Google Scholar] [CrossRef]
- Piskin, I.E.; Karakas-Celik, S.; Calik, M.; Abuhandan, M.; Kolsal, E.; Genc, G.C.; Iscan, A. Association of interleukin 18, interleukin 2, and tumor necrosis factor polymorphisms with subacute sclerosing panencephalitis. DNA Cell Biol. 2013, 32, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Yentur, S.P.; Aydin, H.N.; Gurses, C.; Demirbilek, V.; Kuru, U.; Uysal, S.; Yapici, Z.; Baris, S.; Yilmaz, G.; Cokar, O.; et al. Granzyme B gene polymorphism associated with subacute sclerosing panencephalitis. Neuropediatrics 2014, 45, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Piskin, I.E.; Calik, M.; Abuhandan, M.; Kolsal, E.; Celik, S.K.; Iscan, A. PD-1 gene polymorphism in children with subacute sclerosing panencephalitis. Neuropediatrics 2013, 44, 187–190. [Google Scholar] [PubMed]
- Sliva, J.; Pantzartzi, C.N.; Votava, M. Inosine Pranobex: A Key Player in the Game Against a Wide Range of Viral Infections and Non-Infectious Diseases. Adv. Ther. 2019, 36, 1878–1905. [Google Scholar] [CrossRef] [PubMed]
- Campoli-Richards, D.M.; Sorkin, E.M.; Heel, R.C. Inosine pranobex. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 1986, 32, 383–424. [Google Scholar] [CrossRef]
- Huttenlocher, P.R.; Mattson, R.H. Isoprinosine in subacute sclerosing panencephalitis. Neurology 1979, 29, 763–771. [Google Scholar] [CrossRef]
- Haddad, F.S.; Risk, W.S. Isoprinosine treatment in 18 patients with subacute sclerosing panencephalitis: A controlled study. Ann. Neurol. 1980, 7, 185–188. [Google Scholar] [CrossRef]
- Jones, C.E.; Dyken, P.R.; Huttenlocher, P.R.; Jabbour, J.T.; Maxwell, K.W. Inosiplex therapy in subacute sclerosing panencephalitis. A multicentre, non-randomised study in 98 patients. Lancet 1982, 1, 1034–1037. [Google Scholar] [CrossRef]
- Yalaz, K.; Anlar, B.; Oktem, F.; Aysun, S.; Ustacelebi, S.; Gurcay, O.; Gucuyener, K.; Renda, Y. Intraventricular interferon and oral inosiplex in the treatment of subacute sclerosing panencephalitis. Neurology 1992, 42, 488–491. [Google Scholar] [CrossRef]
- Gascon, G.; Yamani, S.; Crowell, J.; Stigsby, B.; Nester, M.; Kanaan, I.; Jallu, A. Combined oral isoprinosine-intraventricular alpha-interferon therapy for subacute sclerosing panencephalitis. Brain Dev. 1993, 15, 346–355. [Google Scholar] [CrossRef]
- Anlar, B.; Yalaz, K.; Oktem, F.; Kose, G. Long-term follow-up of patients with subacute sclerosing panencephalitis treated with intraventricular alpha-interferon. Neurology 1997, 48, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Gascon, G.G.; International Consortium on Subacute Sclerosing, P. Randomized treatment study of inosiplex versus combined inosiplex and intraventricular interferon-alpha in subacute sclerosing panencephalitis (SSPE): International multicenter study. J. Child Neurol. 2003, 18, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K. Subacute sclerosing panencephalitis. J. Neurol. 2008, 255, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Cianchetti, C.; Fratta, A.L.; Muntoni, F.; Marrosu, G.; Marrosu, M.G. Toxic effect of intraventricular interferon-alpha in subacute sclerosing panencephalitis. Ital. J. Neurol. Sci. 1994, 15, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Shigeta, S.; Nakamura, K.; De Clercq, E. Inhibitory effect of selected antiviral compounds on measles (SSPE) virus replication in vitro. Antivir. Res. 1989, 12, 87–97. [Google Scholar] [CrossRef]
- Taber, L.H.; Knight, V.; Gilbert, B.E.; McClung, H.W.; Wilson, S.Z.; Norton, H.J.; Thurson, J.M.; Gordon, W.H.; Atmar, R.L.; Schlaudt, W.R. Ribavirin aerosol treatment of bronchiolitis associated with respiratory syncytial virus infection in infants. Pediatrics 1983, 72, 613–618. [Google Scholar]
- McIntosh, K.; Kurachek, S.C.; Cairns, L.M.; Burns, J.C.; Goodspeed, B. Treatment of respiratory viral infection in an immunodeficient infant with ribavirin aerosol. Am. J. Dis. Child 1984, 138, 305–308. [Google Scholar] [CrossRef]
- McClung, H.W.; Knight, V.; Gilbert, B.E.; Wilson, S.Z.; Quarles, J.M.; Divine, G.W. Ribavirin aerosol treatment of influenza B virus infection. JAMA 1983, 249, 2671–2674. [Google Scholar] [CrossRef]
- Wilson, S.Z.; Gilbert, B.E.; Quarles, J.M.; Knight, V.; McClung, H.W.; Moore, R.V.; Couch, R.B. Treatment of influenza A (H1N1) virus infection with ribavirin aerosol. Antimicrob Agents Chemother 1984, 26, 200–203. [Google Scholar] [CrossRef]
- Gilbert, B.E.; Knight, V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents Chemother 1986, 30, 201–205. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.B.; King, I.J.; Webb, P.A.; Scribner, C.L.; Craven, R.B.; Johnson, K.M.; Elliott, L.H.; Belmont-Williams, R. Lassa fever. Effective therapy with ribavirin. N. Engl. J. Med. 1986, 314, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.L.; Schluger, N.W.; Roberts, R.B. Severe measles pneumonitis in adults: Evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin. Infect. Dis. 1994, 19, 454–462. [Google Scholar] [CrossRef]
- Gururangan, S.; Stevens, R.F.; Morris, D.J. Ribavirin response in measles pneumonia. J. Infect. 1990, 20, 219–221. [Google Scholar] [CrossRef]
- Ogle, J.W.; Toltzis, P.; Parker, W.D.; Alvarez, N.; McIntosh, K.; Levin, M.J.; Lauer, B.A. Oral ribavirin therapy for subacute sclerosing panencephalitis. J. Infect. Dis. 1989, 159, 748–750. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Hosoya, M.; Ishii, T.; Shigeta, S.; Suzuki, H. Effect of ribavirin on subacute sclerosing panencephalitis virus infections in hamsters. Antimicrob Agents Chemother 1994, 38, 653–655. [Google Scholar] [CrossRef][Green Version]
- Ishii, T.; Hosoya, M.; Mori, S.; Shigeta, S.; Suzuki, H. Effective ribavirin concentration in hamster brains for antiviral chemotherapy for subacute sclerosing panencephalitis. Antimicrob Agents Chemother 1996, 40, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hosoya, M.; Kimura, K.; Ohno, K.; Mori, S.; Takahashi, K.; Shigeta, S. The cooperative effect of interferon-alpha and ribavirin on subacute sclerosing panencephalitis (SSPE) virus infections, in vitro and in vivo. Antivir. Res. 1998, 37, 29–35. [Google Scholar] [CrossRef]
- Connor, E.; Morrison, S.; Lane, J.; Oleske, J.; Sonke, R.L.; Connor, J. Safety, tolerance, and pharmacokinetics of systemic ribavirin in children with human immunodeficiency virus infection. Antimicrob Agents Chemother 1993, 37, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Shigeta, S.; Mori, S.; Tomoda, A.; Shiraishi, S.; Miike, T.; Suzuki, H. High-dose intravenous ribavirin therapy for subacute sclerosing panencephalitis. Antimicrob Agents Chemother 2001, 45, 943–945. [Google Scholar] [CrossRef]
- Tomoda, A.; Shiraishi, S.; Hosoya, M.; Hamada, A.; Miike, T. Combined treatment with interferon-alpha and ribavirin for subacute sclerosing panencephalitis. Pediatr. Neurol. 2001, 24, 54–59. [Google Scholar] [CrossRef]
- Solomon, T.; Hart, C.A.; Vinjamuri, S.; Beeching, N.J.; Malucci, C.; Humphrey, P. Treatment of subacute sclerosing panencephalitis with interferon-alpha, ribavirin, and inosiplex. J. Child Neurol. 2002, 17, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, A.; Nomura, K.; Shiraishi, S.; Hamada, A.; Ohmura, T.; Hosoya, M.; Miike, T.; Sawaishi, Y.; Kimura, H.; Takashima, H.; et al. Trial of intraventricular ribavirin therapy for subacute sclerosing panencephalitis in Japan. Brain Dev. 2003, 25, 514–517. [Google Scholar] [CrossRef]
- Hosoya, M.; Mori, S.; Tomoda, A.; Mori, K.; Sawaishi, Y.; Kimura, H.; Shigeta, S.; Suzuki, H. Pharmacokinetics and effects of ribavirin following intraventricular administration for treatment of subacute sclerosing panencephalitis. Antimicrob Agents Chemother 2004, 48, 4631–4635. [Google Scholar] [CrossRef]
- Cutler, R.W.; Page, L.; Galicich, J.; Watters, G.V. Formation and absorption of cerebrospinal fluid in man. Brain 1968, 91, 707–720. [Google Scholar] [CrossRef]
- Lishner, M.; Perrin, R.G.; Feld, R.; Messner, H.A.; Tuffnell, P.G.; Elhakim, T.; Matlow, A.; Curtis, J.E. Complications associated with Ommaya reservoirs in patients with cancer. The Princess Margaret Hospital experience and a review of the literature. Arch. Intern. Med. 1990, 150, 173–176. [Google Scholar] [CrossRef]
- Miyazaki, K.; Hashimoto, K.; Suyama, K.; Sato, M.; Abe, Y.; Watanabe, M.; Kanno, S.; Maeda, H.; Kawasaki, Y.; Hosoya, M. Maintaining Concentration of Ribavirin in Cerebrospinal Fluid by a New Dosage Method; 3 Cases of Subacute Sclerosing Panencephalitis Treated Using a Subcutaneous Continuous Infusion Pump. Pediatr. Infect. Dis. J. 2019, 38, 496–499. [Google Scholar] [CrossRef]
- Berger, B.; Vienenkoetter, B.; Korporal, M.; Rocco, A.; Meinck, H.M.; Steiner, T. Accidental intoxication with 60 mg intrathecal baclofen: Survived. Neurocrit. Care 2012, 16, 428–432. [Google Scholar] [CrossRef]
- Gutierrez, J.; Issacson, R.S.; Koppel, B.S. Subacute sclerosing panencephalitis: An update. Dev. Med. Child Neurol. 2010, 52, 901–907. [Google Scholar] [CrossRef]
- Ravikumar, S.; Crawford, J.R. Role of carbamazepine in the symptomatic treatment of subacute sclerosing panencephalitis: A case report and review of the literature. Case Rep. Neurol. Med. 2013, 2013, 327647. [Google Scholar] [CrossRef]
- Lo, M.K.; Jordan, R.; Arvey, A.; Sudhamsu, J.; Shrivastava-Ranjan, P.; Hotard, A.L.; Flint, M.; McMullan, L.K.; Siegel, D.; Clarke, M.O.; et al. GS-5734 and its parent nucleoside analog inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017, 7, 43395. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, D.; van Nieuwkoop, S.; Smits, S.L.; Neyts, J.; Fouchier, R.A.; van den Hoogen, B.G. Antiviral Activity of Favipiravir (T-705) against a Broad Range of Paramyxoviruses In Vitro and against Human Metapneumovirus in Hamsters. Antimicrob Agents Chemother 2016, 60, 4620–4629. [Google Scholar] [CrossRef] [PubMed]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Maeda, H.; Miyazaki, K.; Watanabe, M.; Norito, S.; Maeda, R.; Kume, Y.; Ono, T.; Chishiki, M.; Suyama, K.; et al. Antiviral effect of favipiravir (T-705) against measles and subacute sclerosing panencephalitis viruses. Jpn. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Tani, H.; Fukuma, A.; Fukushi, S.; Taniguchi, S.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Sato, Y.; Suzuki, T.; Nagata, N.; Hasegawa, H.; et al. Efficacy of T-705 (Favipiravir) in the Treatment of Infections with Lethal Severe Fever with Thrombocytopenia Syndrome Virus. mSphere 2016, 1, e00061. [Google Scholar] [CrossRef]
- White, L.K.; Yoon, J.J.; Lee, J.K.; Sun, A.; Du, Y.; Fu, H.; Snyder, J.P.; Plemper, R.K. Nonnucleoside inhibitor of measles virus RNA-dependent RNA polymerase complex activity. Antimicrob. Agents Chemother. 2007, 51, 2293–2303. [Google Scholar] [CrossRef]
- Yoon, J.J.; Krumm, S.A.; Ndungu, J.M.; Hoffman, V.; Bankamp, B.; Rota, P.A.; Sun, A.; Snyder, J.P.; Plemper, R.K. Target analysis of the experimental measles therapeutic AS-136A. Antimicrob. Agents Chemother. 2009, 53, 3860–3870. [Google Scholar] [CrossRef]
- Ndungu, J.M.; Krumm, S.A.; Yan, D.; Arrendale, R.F.; Reddy, G.P.; Evers, T.; Howard, R.; Natchus, M.G.; Saindane, M.T.; Liotta, D.C.; et al. Non-nucleoside inhibitors of the measles virus RNA-dependent RNA polymerase: Synthesis, structure-activity relationships, and pharmacokinetics. J. Med. Chem. 2012, 55, 4220–4230. [Google Scholar] [CrossRef]
- Tadokoro, T.; Jahan, M.L.; Ito, Y.; Tahara, M.; Chen, S.; Imai, A.; Sugimura, N.; Yoshida, K.; Saito, M.; Ose, T.; et al. Biophysical characterization and single-chain Fv construction of a neutralizing antibody to measles virus. FEBS J. 2020, 287, 145–159. [Google Scholar] [CrossRef]
- Ader, N.; Brindley, M.; Avila, M.; Orvell, C.; Horvat, B.; Hiltensperger, G.; Schneider-Schaulies, J.; Vandevelde, M.; Zurbriggen, A.; Plemper, R.K.; et al. Mechanism for active membrane fusion triggering by morbillivirus attachment protein. J. Virol. 2013, 87, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Alves, L.; Khosravi, M.; Ader-Ebert, N.; Origgi, F.; Schneider-Schaulies, J.; Zurbriggen, A.; Plemper, R.K.; Plattet, P. Molecular determinants defining the triggering range of prefusion F complexes of canine distemper virus. J. Virol. 2014, 88, 2951–2966. [Google Scholar] [CrossRef] [PubMed]
- Plemper, R.K.; Erlandson, K.J.; Lakdawala, A.S.; Sun, A.; Prussia, A.; Boonsombat, J.; Aki-Sener, E.; Yalcin, I.; Yildiz, I.; Temiz-Arpaci, O.; et al. A target site for template-based design of measles virus entry inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 5628–5633. [Google Scholar] [CrossRef] [PubMed]
- Welsch, J.C.; Talekar, A.; Mathieu, C.; Pessi, A.; Moscona, A.; Horvat, B.; Porotto, M. Fatal measles virus infection prevented by brain-penetrant fusion inhibitors. J. Virol. 2013, 87, 13785–13794. [Google Scholar] [CrossRef]
- Watanabe, M.; Hashimoto, K.; Abe, Y.; Kodama, E.N.; Nabika, R.; Oishi, S.; Ohara, S.; Sato, M.; Kawasaki, Y.; Fujii, N.; et al. A Novel Peptide Derived from the Fusion Protein Heptad Repeat Inhibits Replication of Subacute Sclerosing Panencephalitis Virus In Vitro and In Vivo. PLoS ONE 2016, 11, e0162823. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, K.; Hosoya, M. Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis. Molecules 2021, 26, 427. https://doi.org/10.3390/molecules26020427
Hashimoto K, Hosoya M. Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis. Molecules. 2021; 26(2):427. https://doi.org/10.3390/molecules26020427
Chicago/Turabian StyleHashimoto, Koichi, and Mitsuaki Hosoya. 2021. "Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis" Molecules 26, no. 2: 427. https://doi.org/10.3390/molecules26020427
APA StyleHashimoto, K., & Hosoya, M. (2021). Advances in Antiviral Therapy for Subacute Sclerosing Panencephalitis. Molecules, 26(2), 427. https://doi.org/10.3390/molecules26020427





