Abstract
A series of 12 silica gel-bound enaminones and their Cu(II) complexes were prepared and tested for their suitability as heterogeneous catalysts in azomethine imine-alkyne cycloadditions (CuAIAC). Immobilized Cu(II)–enaminone complexes showed promising catalytic activity in the CuAIAC reaction, but these new catalysts suffered from poor reusability. This was not due to the decoordination of copper ions, as the use of enaminone ligands with additional complexation sites resulted in negligible improvement. On the other hand, reusability was improved by the use of 4-aminobenzoic acid linker, attached to 3-aminopropyl silica gel via an amide bond to the enaminone over the more hydrolytically stable N-arylenamine C-N bond. The study showed that silica gel-bound Cu(II)–enaminone complexes are readily available and suitable heterogeneous catalysts for the synthesis of 6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazoles.
1. Introduction
1,3-Dipolar cycloadditions of azomethine imines are important reactions to obtain pyrazoles with variable degree of saturation [1,2]. Since the end of the 20th century, this field has gained much attention; most azomethine imines have been recognized as stable compounds that are easy to prepare, store, and handle [1,2]. In this context, 1-alkylidene-3-oxopyrazolidin-1-ium-2-ides (3-oxopyrazolidin-1-azomethine imines), accessible by condensation of 1,2-unsubstituted pyrazolidin-3-ones with aldehydes or ketones, have been extensively used for regio- and stereoselective synthesis of pyrazolo[1,2-a]pyrazoles (bicyclic pyrazolidinones). Bicyclic pyrazolidinones exhibit antibiotic [3,4,5] and anti-Alzheimer activity [6], as well as inhibition of lymphocyte-specific protein tyrosine kinase [7,8] and Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) [9]. The most prominent examples of bioactive bicyclic pyrazolidinones are Eli Lilly’s γ-lactam antibiotics, which exhibit antibiotic activity similar to that of penicillins and cephalosporins (Figure 1) [3,4,5]. These antibiotics are based on 6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole scaffold, which is accessible by [3 + 2] cycloaddition of 3-oxopyrazolidin-1-ium-2-ides to acetylenes [1,2]. In this context, copper-catalyzed azomethine imine-alkyne cycloadditions (CuAIAC) [1,2,10,11,12,13,14,15,16] provide easy access to 6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazoles in a regio- and stereoselective manner under mild conditions that are compliant with requirements of “click” chemistry (Figure 1) [17,18,19,20,21,22,23]. In contrast to the CuAAC reaction, which is catalyzed only by Cu(I), the azomethine imine analogue (CuAIAC) is also catalyzed by Cu(II) [10,11,12,24,25,26,27]. This is a major advantage in terms of catalyst scope and simplicity of workup as the use of reducing agent, such as sodium ascorbate, can be avoided when Cu(II) catalyst is used (Figure 1).
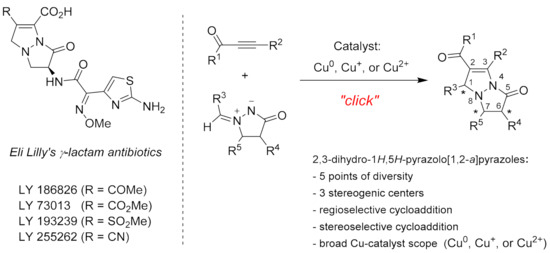
Figure 1.
Examples of bioactive bicyclic pyrazolidinones (left) and CuAIAC reaction (right).
Alkyl 2-substituted-3-(dimethylamino)propenoates and related enaminones are readily available and stable enamino-masked β-keto aldehydes, which are useful 1,3-dielectrophilic reagents in synthetic organic chemistry. Acid-catalyzed reactions with N-, C-, and O-nucleophiles take place under mild conditions by substitution of the dimethylamino group to give β-functionalized propenoates. With ambident nucleophiles, enaminones undergo cyclization into different heterocyclic systems [28,29,30,31,32,33]. Enaminones are also used as alkenes in cycloaddition reactions [34,35,36,37,38] and as bidentate N, O ligands [39,40,41,42,43,44,45,46,47,48,49] and tetradentate acacen-type ligands [27,50,51,52,53,54] to coordinate metal ions.
In recent years, an important part of our ongoing research on the chemistry of 3-pyrazolidinones [55] has been focused on CuAIAC reactions catalyzed by Cu(0) [56,57], Cu(I) [58,59,60,61], and Cu(II) [27]. In extension, we were interested in the use of immobilized Cu(II) complexes with enaminone-type ligands attached to the solid support in CuAIAC reactions. In contrast to the rather extensive use of immobilized copper complexes in azide-alkyne cycloadditions (CuAAC) [62], their applications in CuAIAC reactions are almost unknown [26]. 3-Aminopropyl silica gel-immobilized Cu(II)-enaminone complexes would be easy to prepare via a transamination reaction [28,29,30,31,32,33,63,64], could serve as heterogeneous Cu(II) catalysts for the synthesis of pyrazolo[1,2-a]pyrazoles, and would complement well the known examples of heterogeneous Cu(0)- [41], Cu(I)- [65,66,67,68,69], and Cu(II)-catalysts [26] in the CuAIAC reaction. Herein, we report the results of this study confirming the suitability of these new enaminone-based heterogeneous copper catalysts in regioselective [3 + 2] cycloadditions of 1-benzylidene-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ides to methyl propiolate leading to methyl 1-aryl-7,7-dimethyl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates.
2. Results
2.1. Synthesis and Catalytic Activity of Silica Gel-Bound Cu–Enaminone Complexes 5a–g
First, the starting enaminones 2a–g were prepared from active methylene compounds 1a–g by treatment with N,N-dimethylformamide dimethylacetal (DMFDMA) or tert-butoxy-bis(dimethylamino)methane (TBDMAM) at 20–110 °C following literature procedure [27]. Next, the enaminones 2a–g were reacted with equimolar amount of 3-aminopropyl silica gel (3) in methanol for 48 h to give the immobilized enaminones 4a–g. Subsequent treatment of 4a–g with one equivalent of Cu(OAc)2·H2O in methanol at room temperature for 48 h then furnished the desired complexes 5a–g. The complex 3-Cu was prepared by treatment of 3 with Cu(OAc)2·H2O in methanol (Scheme 1). Absorption bands at around 1600 cm−1 (C = O/C = N) in the IR spectra of compounds 5a–h, the results of combustion analyses for compounds 5a–g, and the results of characterization of the catalyst 5f by SEM and EDX spectroscopy were in line with attachment of copper–enaminone complexes to 3 (For characterization details see the Supporting Information).
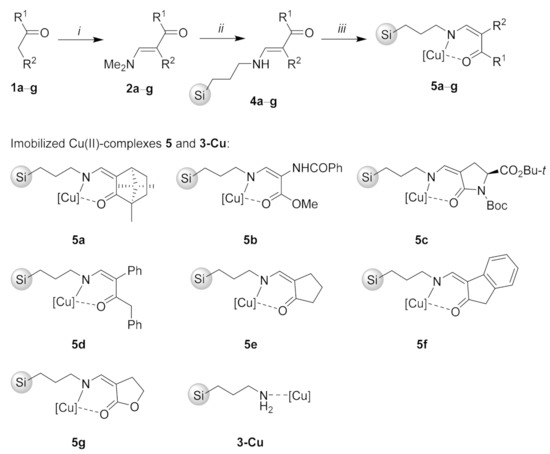
Scheme 1.
Reaction conditions: (i) DMFDMA or TBDMAM, CH2Cl2 or toluene, 20–110 °C; (ii) 3-aminopropyl silica gel (3), MeOH, 20 °C, 48 h; (iii) Cu(OAc)2·H2O, MeOH, 20 °C, 48 h.
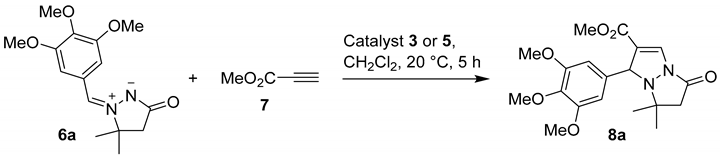
Compounds 5a–g and 3-Cu were then evaluated for their catalytic activity in [3 + 2] cycloaddition of (Z)-3,3-dimethyl-5-oxo-2-(3,4,5-trimethoxybenzylidene)pyrazolidin-2-ium-1-ide (6a) to methyl propiolate (7). The reaction was performed in CH2Cl2 at room temperature for 5 h with 30 mg (~20 mol%) catalyst loading (Table 1). Quantitative conversion was obtained only with 2-indanone-derived catalyst 5f (Table 1, entry 6), while the conversion above 50% was also obtained from related enamino ketone-derived catalysts 5d and 5e (Table 1, entries 4 and 5). Catalysts 5a–c and 5g were less active and the respective conversions ranged from 33% to 47% (Table 1, entries 1–3 and 7). Moderate activity of 3-Cu (Table 1, entry 8) was in line with complexation of Cu(OAc)2 to 3-aminopropyl silica gel (3), which itself was found inactive (Table 1, entry 9).

Table 1.
Evaluation of catalytic activity of 5a–g, 3-Cu, and 3 in model cycloaddition reaction 1.
The most active catalyst 5f was tested further. The model reaction was carried out varying reaction time (1–3 h) and catalyst loading (10–30 mg). The results are presented in Table 2. In the presence of 30 mg of the catalyst, the conversion was around 50% after one hour, around 90% after two hours, and 100% after three hours (Table 2, entries 1–3). Complete conversion was also achieved with 25 mg and 20 mg of the catalyst (Table 2, entries 4 and 5), while further lowering of the catalyst loading to 15 mg (89%) and to 10 mg (61%) gave incomplete conversions (Table 2, entries 6 and 7).

Table 2.
Evaluation of catalyst 5f in model cycloaddition reaction 1.
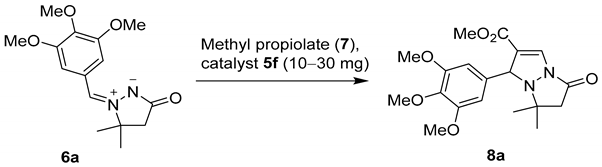
Next, the substrate scope was investigated using 20 mg (~13 mol%) of catalyst 5f in reactions with azomethine imines 6a–f (Scheme 2). After 3 h, only dipoles 6a and 6d were transformed quantitatively into the corresponding cycloadducts 8a and 8d, while conversions of other dipoles ranged from 23% to 95%. The highest conversions (95–100%) were obtained with 3,4,5-trimethoxyphenyl- (6a), phenyl- (6d), and 4-nitrophenyl-substituted dipole (6f), whereas poor conversions (23–29%) were observed with 4-methoxy- (6b), 4-methyl- (6c), and 4-chloro-substituted dipole (6e). Since closely related Cu0- and Cu+-catalyzed cycloadditions did not show any significant substrate dependence [56,57], incomplete conversions may seem surprising, yet they are explainable by much shorter reaction time (i.e., 12–48 h [56,57] vs. 3 h in the present case). Quantitative conversion of dipole 6e into cycloadduct 8e after 48 h was in line with this rationale (Scheme 2).
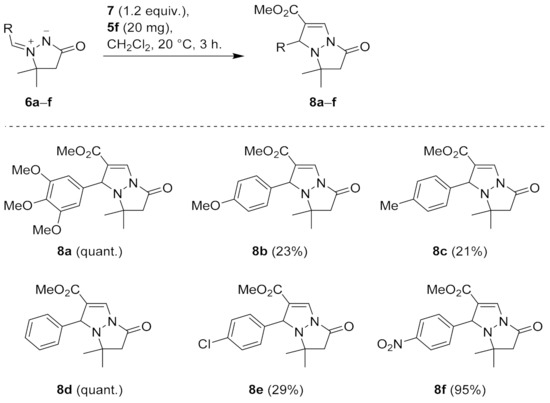
Scheme 2.
The conversions in CuAIAC reactions of dipoles 6a–f with methyl propiolate (7) catalyzed by 20 mg (~13 mol%) of 5f. The conversions were determined by 1H NMR of the crude reaction mixtures.
To further explore the reaction scope, azomethine imine 6a was reacted also with nonpolar phenylacetylene in the presence of catalyst 5f under the above standard reaction conditions. This reaction gave no conversion, even after prolonged treatment for 150 h. This result indicated a limitation of the reaction scope to polar electron-poor alkynes.
Reusability of the catalyst 5f in the standard model reaction (6a + 7 → 8a, 3 h, 30 mg of 5f) was tested next. Much to our disappointment, the quantitative conversion in the first run dropped significantly in the second (29%) and the third run (5%) and the catalyst was inactive upon the third run (Figure 2). If poor reusability of catalyst 5f is explainable by decomplexation of copper ions from the heterogeneous ligand 4f, then reusability should be improved by stronger coordination of copper(II) to the ligand. Therefore, we decided to address the reusability issue by attaching stronger coordinating acacen ligands 9a,b [27,50,51,52,53,54,70] and pyridine-enaminone ligands 9c [71] to 3-aminopropyl silica gel (3).
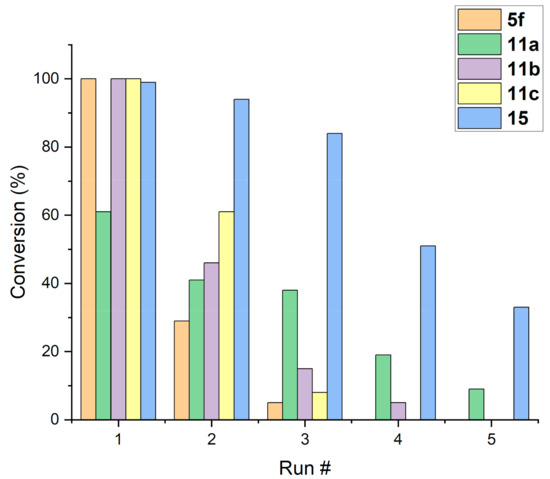
Figure 2.
Reusability of catalysts 5f ( ), 11a (
), 11a ( ), 11b (
), 11b ( ), 11c (
), 11c ( ), and 15 (
), and 15 ( ) in the model reaction 6a + 7 → 8a. The conversions were determined by 1H NMR.
) in the model reaction 6a + 7 → 8a. The conversions were determined by 1H NMR.
 ), 11a (
), 11a ( ), 11b (
), 11b ( ), 11c (
), 11c ( ), and 15 (
), and 15 ( ) in the model reaction 6a + 7 → 8a. The conversions were determined by 1H NMR.
) in the model reaction 6a + 7 → 8a. The conversions were determined by 1H NMR.
2.2. Synthesis and Catalytic Activity of Silica Gel-Bound Cu–Enaminone Complexes 11a–c and 15
Bis-enaminone compounds 9a and 9b [70] (Scheme 3) contain two terminal N,N-(dimethyl)enaminone groups that enable transaminative attachment to 3-aminopropyl silica gel (3). Thus, treatment of 9a and 9b with 3 in methanol at room temperature afforded the immobilized acacen ligands 10a and 10b, which were subsequently reacted with Cu(OAc)2·H2O in methanol to furnish the desired immobilized Cu–acacen complexes 11a and 11b (Scheme 3). To obtain pyridine-type catalyst 11c, bis-enaminone ligand 9c [71], was reacted with 3-aminopropyl silica gel (3) to give silica gel-bound ligand 10c, followed by treatment with Cu(OAc)2·H2O in methanol to furnish the copper complex 11c (Scheme 3). Absorption bands at around 1600 cm−1 (C = O/C = N) in the IR spectra of compounds 11a–c and the combustion analyses for compounds 11a–c were in line with attachment of copper–enaminone complexes to 3 (For characterization details see the Supporting Information).
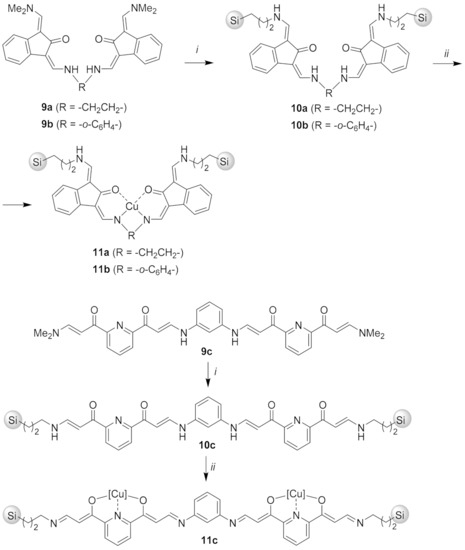
Scheme 3.
Reaction conditions: (i) 3-aminopropyl silica gel (3), MeOH, 20 °C, 48 h; (ii) Cu(OAc)2·H2O, MeOH, 20 °C, 48 h.
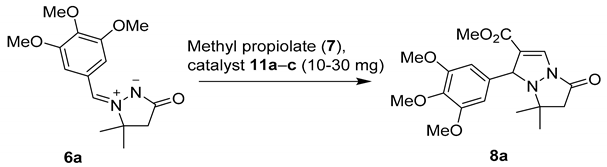
With the desired new catalysts 11a–c in our hands, we first examined their catalytic activity in model cycloaddition (6a + 7 → 8a, Table 3). After 3 h in the presence of 30 mg (~20 mol%) of the catalyst 11, 1,2-ethylenediamine-based catalyst 11a showed only moderate performance (61% conversion, Table 3, entry 1), while activities of 1,2-phenylenediamine-based catalyst 11b and pyridine-based catalyst 11c (Table 3, entries 2 and 3) were similar to that of catalyst 5f (cf. Table 2, entry 3). Further evaluation of catalysts 11b and 11c in terms of catalyst loading (Table 3, entries 4–7) and reaction time (Table 3, entries 8–11) confirmed the performance of 11b and 11c, which was similar to that of catalyst 5f (cf. Table 2, entries 1–7).

Table 3.
Catalytic activity of heterogeneous Cu(II) catalysts 11a–c in model reaction 1.
The substrate scope of catalysts 11a–c was then checked by measuring conversions in the reactions of azomethine imines 6a–f with methyl propiolate (7) in dichloromethane using ~13 mol% (20 mg) catalyst loading (Scheme 4). Quantitative conversions after 3 h were achieved only with dipole 6a in the presence of catalysts 11b and 11c, and with electron-poor dipole 6f, relatively good conversions above 80% were obtained with all three catalysts. The conversions after 3 h were low to moderate (15–69%) with dipoles 6b–e. For the most part, these results were in line with those obtained with catalyst 5f. Notably, also the less reactive dipole 6e underwent full conversion within 48 h with catalyst 11c (Scheme 4, cf. Scheme 3).
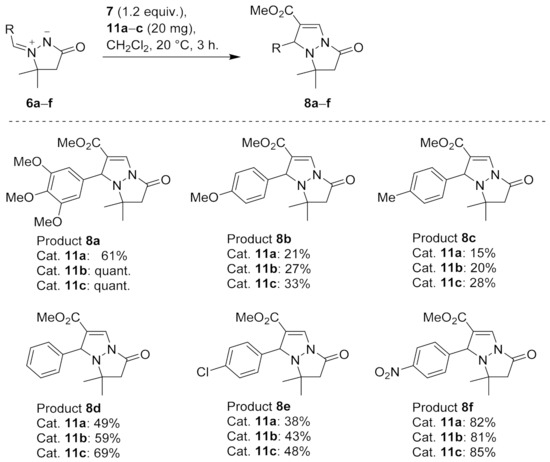
Scheme 4.
The conversions in CuAIAC reactions of dipoles 6a–f with methyl propiolate (7) catalyzed by ~13 mol% of 11a–c. The conversions were determined by 1H NMR of the crude reaction mixtures.
To our disappointment, reusability tests for catalysts 11a–c in the standard model reaction (6a + 7 → 8a, 3 h, 30 mg of 11) revealed only minor improvement of reusability of catalysts 11a–c in comparison to catalyst 5f. Initially highly active catalysts 11b and 11c became inactive upon the third run (see Figure 2 at the end of Section 2.1). On the basis of these data, it became clear that decoordination of Cu(II) from the ligand was not the main reason for low reusability of 5f and 11a–c. We then considered that loss of catalytic activity could also be explainable by detachment of Cu(II)-enaminone complex from 3-aminopropyl silica gel (3), for example, through hydrolytic cleavage of the enamine C-N bond, as proposed in Scheme 5. Hydrolysis of enaminone complex 5 gives the complex 5′, which can release Cu(II)-1,3-dicarbonyl complex 5″ in solution through decoordination from aminopropyl silica gel 3.
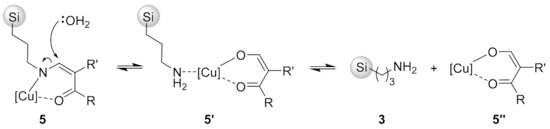
Scheme 5.
A plausible mechanism for detachment of Cu(II)-enaminone complex from 3.
According to the proposed mechanism, the use of hydrolytically more stable enamine C-N bond should reduce detachment of Cu(II)-enaminone complex from the solid support and, thus, improve reusability of the catalyst. To confirm this hypothesis, we prepared silica gel-bound enaminone 14 using 4-aminobenzoic acid (12) as a bifunctional linker, which was bound to 3-aminopropyl silica gel (3) via a robust amide bond and to the enaminone 2f through a stronger N-arylenamine C-N bond (Scheme 6) [28,29,30,31,32,33,63,64]. Acid-catalyzed transamination of 2f with 4-aminobenzoic acid (12) gave the carboxy-functionalized enaminone 13, which was amidated with 3 using 1,1′-carbonyldiimidazole (CDI) as activating reagent. Subsequent treatment of the silica gel-bound enaminone 14 with copper(II) acetate in methanol then furnished the desired catalyst 15 (Scheme 6). Absorption bands at around 1600 cm−1 (C = O/C = N) in the IR spectra of compound 15 and the combustion analyses for 15 were in line with attachment of copper–enaminone complex to 3 (For characterization details see the Supporting Information).
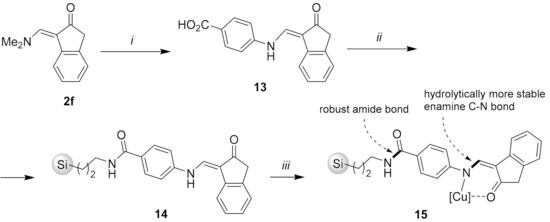
Scheme 6.
Reaction conditions: (i) 4-aminobenzoic acid (12), 37% aq. HCl (1 equiv.), MeOH, 20 °C; (ii) CDI, MeCN, 20 °C, 1 h, then 3-aminopropyl silica gel (3), MeCN, 20 °C, 120 h; (iii) Cu(OAc)2·H2O, MeOH, 20 °C, 48 h.
Activity and reusability of catalysts 5f, 11a–c, and 15 were tested in the standard model reaction (6a + 7 → 8a, CH2Cl2, 20 °C, 3 h, 30 mg catalyst loading). The results are summarized in Figure 2. In the first run, quantitative conversion was obtained with catalysts 5f, 11b, 11c, and 15, while catalyst 11a gave only 61% conversion. The catalytic activity of 5f and 11b,c dropped significantly and they became practically inactive after the second run. Surprisingly, the initially least active catalyst 11a lost catalytic activity more slowly than analogues 5f and 11b,c and remained only weakly active in the fifth run. On the other hand, catalyst 15 gave a near quantitative conversion in the second run (94%), followed by a gradual decrease of catalytic activity leading to 31% conversion in the fifth run. Thus, the reusability of N-arylenaminone catalyst 15 was significantly better than that of N-alkylenaminone analogues 5 and 11 (Figure 2). This result was consistent with the hypothesis that the decrease of catalytic activity was largely due to the detachment of the copper-enaminone complex from the solid support by hydrolysis of the C-N bond of the enamine (cf. Scheme 5).
3. Conclusions
Transamination of enaminones 2a–g and bis-enaminones 9a–c with 3-aminopropyl silica gel (3) in methanol gives the corresponding silica gel-bound enaminones 4a–g and 10a–c. Subsequent treatment of the immobilized enaminones 4a–g and 10a–c with copper(II) acetate in methanol gives the corresponding silica gel-bound Cu(II) complexes 5a–g and 11a–c. Both reactions are general and take place with different types of enaminones 2 and 9 under mild conditions. The obtained copper(II) complexes 5a–g and 11a–c exhibit catalytic activity in azomethine imine-alkyne cycloadditions (CuAIAC). The 2-indanone-derived catalyst 5f and the bis-enaminone-derived catalysts 11b and 11c showed the most promising activity, unfortunately, with poor reusability. The main cause of the poor reusability appears to be hydrolytic cleavage of the Cu(II)-enaminone complex from the 3-aminopropyl silica gel (3), rather than decomplexation of the copper(II) ions from the ligand. This hypothesis was confirmed by the synthesis of a modified catalyst 15 with hydrolytically more stable enamine C-N bond of the enamine attached to 3-aminopropyl silica gel (3) via a robust amide bond. Catalyst 15 exhibited better reusability while still retaining the same catalytic activity as analogues 5 and 11. In conclusion, silica gel-bound Cu(II)-enaminone complexes 5, 11, and 15 are easily available heterogeneous catalysts for the regioselective synthesis of pyrazolo[1,2-a]pyrazoles via [3 + 2] cycloaddition of 3-pyrazolidinone-derived azomethine imines to terminal ynones.
4. Materials and Methods
4.1. General Information
All solvents and reagents were used as received. Melting points were determined on SRS OptiMelt MPA100—Automated Melting Point System (Stanford Research Systems, Sunnyvale, CA, USA). The 1H NMR, 13C NMR, and 2D NMR spectra were recorded in CDCl3 and DMSO-d6 as solvents using Me4Si as the internal standard on a Bruker Avance III UltraShield 500 plus instrument (Bruker, Billerica, MA, USA) at 500 MHz for 1H and at 126 MHz for 13C nucleus, respectively. IR spectra were recorded on a Bruker FTIR Alpha Platinum spectrophotometer (Bruker, Billerica, MA, USA). Microanalyses were performed by combustion analysis on a Perkin-Elmer CHN Analyzer 2400 II (PerkinElmer, Waltham, MA, USA). Mass spectra were recorded on an Agilent 6224 Accurate Mass TOF LC/MS (Agilent Technologies, Santa Clara, CA, USA). Parallel stirring was carried out on a Tehtnica Vibromix 313 EVT orbital shaker (400 rpm in all cases) (Domel, Železniki, Slovenia). Flash column chromatography was performed on silica gel (Silica gel 60, particle size: 0.035–0.070 mm, Sigma-Aldrich, St. Louis, MO, USA).
Active methylene compounds 1a–g, 3-aminopropyl silica gel (3) (for preparative chromatography, 40–63 µm, 0.9 mmol/g amino groups, pore size ~9 nm), 4-aminobenzoic acid (12), Cu(OAc)2·H2O, N,N-dimethylformamide dimethylacetal (DMFDMA, for synthesis, ≥96%), tert-butoxy-bis(dimethylamino)methane (TBDMAM, technical grade), and 1,1′-carbonyldiimidazole (CDI) are commercially available (Sigma-Aldrich). Enaminones 2a [72], 2b [73], 2c [74], 2d [75], 2e [76], 2f [77], and 2g [78], bis-enaminones 9a, 9b [70], and 9c [71], and azomethine imines 6a,f [79], 6b,e [80], 6c [81], and 6d [82] were prepared following the literature procedures.
4.2. Synthesis of 3-Aminopropyl Silica Gel-Bound Copper(II)-Catalyst 3-Cu
A mixture of 3-aminopropyl silica gel (3) (5.015 g, 4.5 mmol of amino group), Cu(OAc)2·H2O (903 mg, 4.5 mmol), and methanol (25 mL) was stirred at 20 °C for 48 h. The insoluble material was collected by filtration, washed carefully with methanol until the filtrate was colorless (around 10 × 5 mL), and air-dried to give the copper(II) catalyst 3-Cu. Blue powder (5.163 g).
4.3. General Procedure for the Synthesis of 3-Aminopropyl Silica Gel-Bound Copper(II) Catalysts 5a–g
Enaminone 2 (1.381 mmol) was added to a suspension of 3-aminopropyl silica gel (3) (1.534 g, 1.381 mmol of amino group) in methanol (4 mL) and the mixture was stirred at 20 °C for 48 h. The insoluble material was collected by filtration, washed with methanol until the filtrate was colorless (around 10 × 5 mL), and air-dried to give 4. The immobilized enaminone 4 was resuspended in methanol (8 mL), Cu(OAc)2·H2O (275 mg, 1.381 mmol) was added, and the mixture was stirred at room temperature for 48 h. The insoluble material was collected by filtration, washed carefully with methanol until the filtrate was colorless (around 10 × 5 mL), and air-dried to give the copper(II) catalyst 5. The following compounds were prepared in this manner:
4.3.1. Compound 5a
Prepared from 2a (934 mg, 4.5 mmol) and 3 (5.015 g, 4.5 mmol of amino group) in MeOH (15 mL); then Cu(OAc)2·H2O (903 mg, 4.5 mmol), MeOH (25 mL). Blue powder (5.392 g), νmax 1558 (C = O/C = N), 1418 cm−1.
4.3.2. Compound 5b
Prepared from 2b (1.119 g, 4.5 mmol) and 3 (5.015 g, 4.5 mmol of amino group) in MeOH (15 mL); then Cu(OAc)2·H2O (903 mg, 4.5 mmol), MeOH (25 mL). Blue powder (5.611 g), νmax 1558 (C = O/C = N), 1419 cm−1.
4.3.3. Compound 5c
Prepared from 2c (1.119 g, 4.5 mmol) and 3 (5.015 g, 4.5 mmol of amino group) in MeOH (15 mL); then Cu(OAc)2·H2O (903 mg, 4.5 mmol), MeOH (25 mL). Blue powder (5.415 g), νmax 1565 (C = O/C = N), 1418 cm−1.
4.3.4. Compound 5d
Prepared from 2d (366 mg, 1.4 mmol) and 3 (1.534 g, 1.4 mmol of amino group) in MeOH (4 mL); then Cu(OAc)2·H2O (275 mg, 1.4 mmol), MeOH (8 mL). Blue powder (1.643 g), νmax 1567 (C = O/C = N), 1416 cm−1.
4.3.5. Compound 5e
Prepared from 2e (366 mg, 1.4 mmol) and 3 (1.534 g, 1.4 mmol of amino group) in MeOH (4 mL); then Cu(OAc)2·H2O (275 mg, 1.4 mmol), MeOH (8 mL). Light brown powder (1.598 g), νmax 1565 (C = O/C = N), 1416 cm−1.
4.3.6. Compound 5f
Prepared from 2f (844 mg, 4.5 mmol) and 3 (5.015 g, 4.5 mmol of amino group) in MeOH (15 mL); then Cu(OAc)2·H2O (903 mg, 4.5 mmol), MeOH (25 mL). Dark brown powder (5.514 g), νmax 1606 (C = O/C = N), 1436 cm−1.
4.3.7. Compound 5g
Prepared from 2g (195 mg, 1.4 mmol) and 3 (1.534 g, 1.4 mmol of amino group) in MeOH (4 mL); then Cu(OAc)2·H2O (275 mg, 1.4 mmol), MeOH (8 mL). Blue powder (1.607 g), νmax 1565 (C = O/C = N), 1416 cm−1.
4.4. General Procedure for the Synthesis of Silica Gel-Bound Copper(II) Catalysts 11a–c
Bis-enaminone 9 (0.5 mmol) was added to a suspension of 3-aminopropyl silica gel (3) (1.111 g, 1 mmol of amino group) in methanol (4 mL) and the mixture was stirred at 20 °C for 48 h. The insoluble material was collected by filtration, washed with methanol until the filtrate was colorless (around 10 × 5 mL), and air-dried to give the silica gel-bound bis-enaminone 10. The immobilized enaminone 10 was resuspended in methanol (8 mL), Cu(OAc)2·H2O (200 mg, 1 mmol) was added, and the mixture was stirred at room temperature for 48 h. The insoluble material was collected by filtration, washed carefully with methanol until the filtrate was colorless (around 10 × 5 mL), and air-dried to give the copper(II) catalyst 11. The following compounds were prepared in this manner:
4.4.1. Compound 11a
Prepared from 9a (100 mg, 0.2 mmol) and 3 (490 g, 0.4 mmol of amino group); then Cu(OAc)2·H2O (80 mg, 0.4 mmol). Brown powder (527 mg), νmax 1569 (C = O/C = N), 1411 cm−1.
4.4.2. Compound 11b
Prepared from 9b (200 mg, 0.4 mmol) and 3 (884 g, 0.8 mmol of amino group); then Cu(OAc)2·H2O (160 mg, 0.8 mmol). Green-blue powder (906 mg), νmax 1564 (C = O/C = N), 1417 cm−1.
4.4.3. Compound 11c
Prepared from 9c (322 mg, 0.56 mmol) and 3 (1.265 g, 1.12 mmol of amino group); then Cu(OAc)2·H2O (160 mg, 0.8 mmol). Brown powder (1.294 mg), νmax 1552 (C = O/C = N), 1414 cm−1.
4.5. Synthesis of 3-Aminopropyl Silica Gel-Bound Copper(II) Complex 15
4.5.1. (E)-4-{[(2-Oxo-2,3-dihydro-1H-inden-1-ylidene)methyl]amino}benzoic acid (13)
Enaminone 2f (374 mg, 2 mmol) was added to a mixture of 4-aminobenzoic acid (12) (274 mg, 2 mmol), methanol (10 mL), and 37% aq. HCl (0.15 mL, 1.8 mmol) and the mixture was stirred at room temperature for 12 h. The precipitate was collected by filtration and washed with methanol (2 × 5 mL) and diethyl ether (2 × 5mL) to give 13. Beige solid (385 mg, 69%); E/Z = 85:15; mp 263–264 °C (with slow decomposition above 200 °C); νmax/cm−1 (ATR) 3014, 2813, 1669 (C = O), 1594, 1566, 1424, 1261, 1180, 1199, 1092, 947, 848, 753, 713, 634; δH (500 MHz; DMSO-d6; Me4Si): major isomer 3.49 (2H, s), 7.09 (1H, td, J = 7.5, 1.1 Hz), 7.23–7.27 (2H, m), 7.52 (2H, d, J = 8.8 Hz), 7.67 (1H, d, J = 7.6 Hz), 7.92 (2H, d, J = 8.8 Hz), 8.39 (1H, d, J = 12.2 Hz), 11.03 (1H, d, J = 12.2 Hz), 12.74 (1H, s), minor isomer 3.46 (3H, s), 7.19 (1H, td, J = 7.5, 1.1 Hz), 7.23–7.27 (1H, m), 7.33 (2H, br d, J = 7.4 Hz), 7.48 (2H, br d, J = 8.8 Hz), 7.71 (1H, d, J = 13.4 Hz), 8.04 (1H, d, J = 7.4 Hz), 9.47 (1H, d, J = 13.3 Hz), 12.74 (1H, s); δC (126 MHz; DMSO-d6; Me4Si): major isomer 41.9, 111.5, 115.6, 117.6, 124.7, 124.8, 124.9, 126.9, 131.1, 134.3, 134.4, 140.0, 143.8, 166.8, 204.4, minor isomer 41.4, 112.8, 116.2, 121.9, 124.6, 124.7, 125.7, 126.8, 131.1, 131.8, 135.5, 138.2, 145.5, 166.9, 202.4; HRMS (ESI): MH+, found 280.0968 (MH+). [C17H14NO3]+ requires 280.0968; (found: C, 71.98; H, 4.24; N, 4.73. C17H13NO3·¼H2O requires C, 71.95; H, 4.79; N, 4.94%).
4.5.2. Synthesis of Silica Gel-Bound Enaminone 14
1,1′-Carbonyldiimidazole (85 mg, 0.52 mmol) was added to a stirred suspension of carboxylic acid 13 (140 mg, 0.5 mmol) in acetonitrile (5 mL) and the mixture was stirred at room temperature for 1 h. Then, 3-aminopropyl silica gel (3) (500 mg, 0.45 mmol of NH2 group) was added and the suspension was stirred at room temperature for 120 h. Ethanol (2 mL) was added and the insoluble material was collected by filtration using a short column with fritted bottom (d = 1.5 cm, l = 10 cm) and the functionalized silica gel 14 was washed with EtOH-MeCN (1:1, 3 × 5 mL), EtOH (2 × 5 mL), DMF (2 × 5 mL), EtOH (3 mL), and Et2O (2 × 5mL) and air-dried. Brown powder (536 mg, 31%, loading ~0.3 mmol/g); FT-IR (ATR): νmax 1603 (C = O/C = N) cm−1.
4.5.3. Synthesis of Silica Gel-Bound Copper(II) Catalyst 15
3-Enaminopropyl silica gel 14 (300 mg, ~0.1 mmol of the enaminone) was added to a solution of Cu(OAc)2·H2O (50 mg, 0.25 mmol) in methanol (10 mL) and the mixture was stirred at 20 °C for 48 h. The insoluble material was collected by filtration, washed carefully with methanol until the filtrate was colorless (around 5 × 5 mL), and air-dried to give the copper(II) catalyst 15. Brown powder (280 mg, 81%, loading ~0.3 mmol/g); FT-IR (ATR): νmax 1603 (C = O/C = N) cm−1.
4.6. Synthesis of Methyl 1-Aryl-7,7-dimethyl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates 8a–f by [3 + 2] Cycloadditions of Azomethine Imines 6a–f to Methyl Propiolate (7) in the Presence of Catalysts 3-Cu, 5, 11, and 15
4.6.1. Determination of Conversion. General Procedure A
Catalyst 3-Cu, 5, 11, or 15 (10–30 mg) was added to a mixture of azomethine imine 6a–f (25–37 mg [83], 0.125 mmol), methyl propiolate (7) (12.5 μL, 0.15 mmol), and CH2Cl2 (4 mL) and the mixture was stirred at room temperature for 1–5 h. The reaction mixture was filtered to remove the catalyst and the filtrate was evaporated in vacuo to give 8a–f and 1H NMR spectrum of the residue was measured in CDCl3 to determine the conversion. 1H NMR data of compounds 8a,f [79], 8d [56,84], and 8e [56] were in agreement with the literature data.
4.6.2. Determination of Reusability of Catalysts. General Procedure B
Catalyst 5, 11, or 15 (30 mg) was added to a mixture of azomethine imine 6a (37 mg, 0.125 mmol), methyl propiolate (7) (12.5 μL, 0.15 mmol), and CH2Cl2 (4 mL) and the mixture was stirred at room temperature for 3 h. Stirring was stopped, the catalyst was allowed to settle down for 2 min, and the supernatant was carefully decanted and filtered. Dichloromethane (4 mL) was added to the catalyst, the mixture was stirred for 2 min, the catalyst was allowed to settle down for 2 min, and the supernatant was carefully decanted and filtered. The catalyst was washed once more with dichloromethane (4 mL) as described above. The combined filtrate was evaporated in vacuo and 1H NMR spectrum of the residue was measured to determine conversion, while the washed catalyst was used in the next run.
4.6.3. Synthesis of 1-aryl-7,7-dimethyl-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylates 8b and 8c. General Procedure C
Catalyst 5f (120 mg) was added to a mixture of azomethine imine 6b or 6c (0.5 mmol), methyl propiolate (7) (50 μL, 0.6 mmol), and CH2Cl2 (5 mL) and the mixture was stirred at room temperature for 24 h. The catalyst was removed by filtration and washed with dichloromethane (3 mL). The combined filtrate was evaporated in vacuo and the residue was purified by flash column chromatography (Et2O). Fractions containing the product 8 were combined and evaporated in vacuo to give 8b and 8c.
7,7-Dimethyl-1-(4-methoxyphenyl)-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylate (8b). Prepared from azomethine imine 6b (116 mg, 0.5 mmol), methyl propiolate (7) (50 μL, 0.6 mmol), and CH2Cl2 (4 mL). Yellow oil (74 mg, 47%); νmax/cm−1 (ATR) 2955, 1696 (C = O), 1599, 1511, 1444, 1408, 1371, 1323, 1200, 1173, 1099, 1031, 959, 824, 727; δH (500 MHz; CDCl3; Me4Si): 1.14 (3H, s), 1.22 (3H, s), 2.38 (1H, d, J = 15.7 Hz), 2.86 (1H, d, J = 15.7 Hz), 3.62 (3H, s), 3.79 (3H, s), 5.43 (1H, d, J = 1.3 Hz), 6.87 (2H, d, J = 8.7 Hz), 7.35 (2H, d, J = 8.7 Hz), 7.48 (1H, d, J = 1.3 Hz); δC (126 MHz; DMSO-d6; Me4Si): 19.1, 25.1, 49.6, 51.6, 55.3, 64.1, 64.4, 113.9, 117.1, 129.0, 129.3, 134.2, 159.3, 164.3, 166.5; HRMS (ESI): MH+, found 317.1493 (MH+). [C17H21N2O4]+ requires 317.1496.
7,7-Dimethyl-1-(4-methylphenyl)-5-oxo-6,7-dihydro-1H,5H-pyrazolo[1,2-a]pyrazole-2-carboxylate (8c). Prepared from azomethine imine 6c (98 mg, 0.45 mmol), methyl propiolate (7) (50 μL, 0.6 mmol), and CH2Cl2 (4 mL). Yellow solid (83 mg, 61%); mp 152–155 °C; νmax/cm−1 (ATR) 3082, 2946, 1731 (C = O), 1687 (C = O), 1601, 1514, 1323, 1275, 1225, 1192, 1120, 1099, 1039, 1007, 947, 818, 733; δH (500 MHz; CDCl3; Me4Si): 1.12 (3H, s), 1.19 (3H, s), 2.33 (3H, s), 2.38 (1H, d, J = 14.7 Hz), 2.82 (1H, d, J = 14.7 Hz), 3.58 (3H, s), 5.40 (1H, d, J = 1.2 Hz), 7.12 (2H, d, J = 7.8 Hz), 7.29 (2H, d, J = 8.1 Hz), 7.46 (1H, d, J = 1.5 Hz); δC (126 MHz; DMSO-d6; Me4Si): 19.1, 21.3, 25.1, 49.5, 51.6, 64.4, 64.6, 117.0, 127.8, 129.2, 129.5, 137.6, 139.1, 164.3, 166.7; HRMS (ESI): MH+, found 301.1546 (MH+). [C17H21N2O3]+ requires 301.1547.
Following the above Procedure C, also known cycloadducts 8a,d–f were obtained in the following isolated yields: compound 8a (92%), 8d (89%), 8e (84%), and 8f (88%). Spectral data for compounds 8a [56,79], 8d [56,84], 8e [56], and 8f [56,79] were in agreement with the literature data.
Supplementary Materials
The following are available online, copies of 1H and 13C NMR spectra of new compounds 8b, 8c, and 13, copies of IR spectra of catalysts 5a–g, 11a–c, and 15.
Author Contributions
Individual contributions of the authors are the following: conceptualization, U.Š., D.S., and J.S.; methodology, U.Š., D.S., U.G., F.P., B.Š., and J.S.; validation, U.Š., D.S, U.G., F.P., B.Š., and J.S.; formal analysis, U.Š., D.S., and J.S.; investigation, U.Š., D.S., and J.S.; resources, U.G., F.P., B.Š., and J.S.; data curation, U.Š., D.S., U.G., F.P., B.Š., and J.S.; writing—original draft preparation, U.G., F.P., B.Š., and J.S.; writing—review and editing, U.Š., D.S, U.G., F.P., B.Š., and J.S.; supervision, U.G., F.P., B.Š., and J.S.; project administration, U.G., F.P., B.Š., and J.S.; funding acquisition, U.G., F.P., B.Š., and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Slovenian Research Agency (ARRS), research core funding No. P1-0179.
Data Availability Statement
The data presented in this study are available in the Supporting Information.
Acknowledgments
We thank EN-FIST Centre of Excellence, Trg Osvobodilne fronte 13, 1000 Ljubljana, Slovenia, for using BX FTIR spectrophotometer. We thank Marjan Marinšek, University of Ljubljana, for characterization of the catalyst 5f by SEM and EDX spectroscopy.
Conflicts of Interest
The authors declare no conflict of interest.
ample Availability
Samples of the compounds 5a–g, 8a–f, 11a–c, and 13–15 are available from the authors.
References and Notes
- Grošelj, U.; Svete, J. [3+2] Cycloadditions of Azomethine Imines. Org. React. 2020, 103, 529–930. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M.; Yus, M. 1,3-Dipolar cycloadditions of azomethine imines. Org. Biomol. Chem. 2015, 13, 8596–8636. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, L.N.; Sigmund, S.K.; Fisher, J.W. Bicyclic pyrazolidinones, a new class of antibacterial agent based on the β-lactam model. Tetrahedron Lett. 1987, 28, 285–288. [Google Scholar] [CrossRef]
- Ternansky, R.J.; Draheim, S.E. The chemistry of substituted pyrazolidinones; applications to the synthesis of bicyclic derivatives. Tetrahedron 1992, 48, 777–796. [Google Scholar] [CrossRef]
- Ternansky, R.J.; Draheim, S.E.; Pike, A.J.; Counter, F.T.; Eudaly, J.A.; Kasher, J.S. Structure-activity relationship within a series of pyrazolidinone antibacterial agents. 2. Effect of side-chain modification on in vitro activity and pharmacokinetic parameters. J. Med. Chem. 1993, 36, 3224–3229. [Google Scholar] [CrossRef]
- Kosower, E.M.; Hershkowitz, E. 1,5-Diazabicyclo [3,3,0----] octanediones and pharmaceutical compositions containing them. IL 94658 A, 1994. Chem. Abstr. 1995, 122, 214077. [Google Scholar]
- Sabat, M.; Van Rens, J.C.; Brugel, T.A.; Maier, J.; Laufersweiler, M.J.; Golebiowski, A.; De, B.; Easwaran, V.; Hsieh, L.C.; Rosegen, J.; et al. The development of novel 1,2-dihydro-pyrimido[4,5-c]pyridazine-based inhibitors of lymphocyte specific kinase (Lck). Bioorg. Med. Chem. Lett. 2006, 16, 4257–4261. [Google Scholar] [CrossRef]
- VanRens, J.C.; Sabat, M.; Brugel, T.A.; Maier, J.; Laufersweiler, M.J.; Golebiowski, A.; Berberich, S.; De, B. Synthesis of novel pyrimido[4,5-c]pyridazines and 1,2-dihydro-3a,7,9,9b-tetraaza-cyclopenta[a]naphthalen-3-ones as potent inhibitors of lymphocyte specific kinase (LCK). Heterocycles 2006, 68, 2037–2044. [Google Scholar] [CrossRef]
- Strašek, N.; Lavrenčič, L.; Oštrek, A.; Slapšak, D.; Grošelj, U.; Klemenčič, M.; Brodnik Žugelj, H.; Wagger, J.; Novinec, M.; Svete, J. Tetrahydro-1H,5H-pyrazolo[1,2-a]pyrazole-1-carboxylates as inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase. Bioorg. Chem. 2019, 89, 102982. [Google Scholar] [CrossRef]
- Grošelj, U.; Požgan, F.; Štefane, B.; Svete, J. Copper-Catalyzed Azomethine Imine–Alkyne Cycloadditions (CuAIAC). Synthesis 2018, 50, 4501–4524. [Google Scholar] [CrossRef]
- Požgan, F.; Al Mamari, H.; Grošelj, U.; Svete, J.; Štefane, B. Synthesis of Non-Racemic Pyrazolines and Pyrazolidines by [3+2] Cycloadditions ofAzomethine Imines. Molecules 2018, 23, 3. [Google Scholar] [CrossRef]
- Decuypère, E.; Plougastel, L.; Audisio, D.; Taran, F. Sydnone–alkyne cycloaddition: Applications in synthesis and bioconjugation. Chem. Commun. 2017, 53, 11515–11527. [Google Scholar] [CrossRef]
- Nelina-Nemtseva, J.I.; Gulevskaya, A.V.; Suslonov, V.V.; Misharev, A.D. 1,3-Dipolar cycloaddition of azomethine imines to ethynyl hetarenes: A synthetic route to 2,3-dihydropyrazolo[1,2-a]pyrazol-1(5H)-one based heterobiaryls. Tetrahedron 2018, 74, 1101–1109. [Google Scholar] [CrossRef]
- Decuypère, E.; Bernard, S.; Feng, M.; Porte, K.; Riomet, M.; Thuéry, P.; Audisio, D.; Taran, F. Copper-Catalyzed Aza-Iminosydnone-Alkyne Cycloaddition Reaction Discovered by Screening. ACS Catal. 2018, 8, 11882–11888. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, F.; Wang, F.; Wu, J.; Chen, W. Copper-Catalyzed Sequential Azomethine Imine-Alkyne Cycloaddition and Umpolung Thiolation Reactions. Adv. Synth. Catal. 2017, 359, 2768–2772. [Google Scholar] [CrossRef]
- Koler, A.; Paljevac, M.; Cmager, N.; Iskra, J.; Kolar, M.; Krajnc, P. Poly(4-vinylpyridine) polyHIPEs as catalysts for cycloaddition click reaction. Polymer 2017, 126, 402–407. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3-----]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Fokin, V.V.; Matyjaszewski, K. CuAAC: The Quintessential Click Reaction. In Organic Chemistry–Breakthroughs and Perspectives, 1st ed.; Ding, K., Dai, L.-X., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 247–277. [Google Scholar]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide–alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalyzed azide–alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef] [PubMed]
- Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Copper(II) ionic liquid catalyzed cyclization–aromatization of hydrazones with dimethyl acetylenedicarboxylate: A green synthesis of fully substituted pyrazoles. New J. Chem. 2013, 37, 2037–2042. [Google Scholar] [CrossRef]
- Hori, M.; Sakakura, A.; Ishihara, K. Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Imines with Propioloylpyrazoles Induced by Chiral π−Cation Catalysts. J. Am. Chem. Soc. 2014, 136, 13198–13201. [Google Scholar] [CrossRef]
- Comas-Barceló, J.; Blanco-Ania, D.; van den Broek, S.A.M.W.; Nieuwland, P.J.; Harrity, J.P.A.; Rutjes, F.P.J.T. Cu-catalyzed pyrazole synthesis in continuous Flow. Catal. Sci. Technol. 2016, 6, 4718–4723. [Google Scholar] [CrossRef]
- Tomažin, U.; Grošelj, U.; Počkaj, M.; Požgan, F.; Štefane, B.; Svete, J. Combinatorial Synthesis of Acacen-Type Ligands and Their Coordination Compounds. ACS Comb. Sci. 2017, 19, 386–396. [Google Scholar] [CrossRef]
- Huang, J.; Yu, F. Recent Advances in Organic Synthesis Based on N,N-Dimethyl Enaminones. Synthesis 2021, 53. in press. [Google Scholar] [CrossRef]
- Fu, L.; Wan, J.-P. C3-Functionalized Chromones Synthesis by Tandem C-H Elaboration and Chromone Annulation of Enaminones. Asian J. Org. Chem. 2021, in press. [Google Scholar] [CrossRef]
- Stanovnik, B. Enaminone, enaminoesters, and related compounds in the metal-free synthesis of pyridines and fused pyridines. Eur. J. Org. Chem. 2019, 5120–5132. [Google Scholar] [CrossRef]
- Gaber, H.M.; Bagley, M.C.; Muhammad, Z.A.; Gomha, S.M. Recent developments in chemical reactivity of N,N-dimethylenamino ketones as synthons for various heterocycles. RSC Adv. 2017, 7, 14562–14610. [Google Scholar] [CrossRef]
- Chattopadhyay, A.K.; Hanessian, S. Cyclic enaminones. Part I: Stereocontrolled synthesis using diastereoselective and catalytic asymmetric methods. Chem. Commun. 2015, 51, 16437–16449. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.K.; Hanessian, S. Cyclic enaminones. Part II: Applications as versatile intermediates in alkaloid synthesis. Chem. Commun. 2015, 51, 16450–16467. [Google Scholar] [CrossRef]
- Efimov, I.V.; Matveeva, M.D.; Luque, R.; Bakulev, V.A.; Voskressensky, L.G. [3+2] Anionic Cycloaddition of Isocyanides to Acyclic Enamines and Enaminones: A New, Simple, and Convenient Method for the Synthesis of 2,4-Disubstituted Pyrroles. Eur. J. Org. Chem. 2020, 1108–1113. [Google Scholar] [CrossRef]
- Feng, J.; He, T.; Xie, Y.; Yu, Y.; Baell, J.B.; Huang, F. I2-Promoted [4 + 2] cycloaddition of in situ generated azoalkenes with enaminones: Facile andefficient synthesis of 1,4-dihydropyridazines andpyridazines. Org. Biomol. Chem. 2020, 18, 9483–9493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yan, B.-W.; Fan, S.-X.; Tian, J.-S.; Loh, T.-P. Regioselective Formal [4 + 2] Cycloadditions of Enaminones with Diazocarbonyls through RhIII-Catalyzed C–H Bond Functionalization. Org. Lett. 2018, 20, 3975–3979. [Google Scholar] [CrossRef] [PubMed]
- Prek, B.; Bezenšek, J.; Stanovnik, B. Synthesis of pyridines with an amino acid residue by [2+2] cycloadditions of electron-poor acetylenes on enaminone systems derived from N-Boc protected amino acids. Tetrahedron 2017, 73, 5260–5267. [Google Scholar] [CrossRef]
- Wan, J.-P.; Cao, S.; Liu, Y. Base-Promoted Synthesis of N-Substituted 1,2,3-Triazoles via Enaminone−Azide Cycloaddition Involving Regitz Diazo Transfer. Org. Lett. 2016, 18, 6034–6037. [Google Scholar] [CrossRef]
- Fu, L.; Cao, X.; Wan, J.-P.; Liu, Y. Synthesis of Enaminone-Pd(II) Complexes and Their Application in Catalysing Aqueous Suzuki-Miyaura Cross Coupling Reaction. Chin. J. Chem. 2020, 38, 254–258. [Google Scholar] [CrossRef]
- Huma, R.; Mahmud, T.; Mitu, L.; Ashraf, M.; Iqgal, A.; Iftikhar, K.; Hayat, A. Synthesis, Characterization, Molecular Docking and Enzyme InhibitionStudies of Some Novel Enaminone Derivatives and Their Complexes with Cu(II), Cd(II) and Co(II) Ions. Rev. Chim. 2019, 70, 3564–3569. [Google Scholar] [CrossRef]
- Sasinska, A.; Leduc, J.; Frank, M.; Czympiel, L.; Fischer, T.; Christiansen, S.H.; Mathur, S. Competitive interplay of deposition and etching processes in atomic layer growth of cobalt and nickel metal films. J. Mater. Res. 2018, 33, 4241–4250. [Google Scholar] [CrossRef]
- Chopin, N.; Novitchi, G.; Médebielle, M.; Pilet, G. A versatile ethanolamine-derived trifluoromethyl enaminone ligand for the elaboration of nickel(II) and copper(II)–dysprosium(III) multinuclear complexes with magnetic properties. J. Fluorine Chem. 2015, 179, 169–174. [Google Scholar] [CrossRef]
- Jeragh, B.; Elassar, A.-Z. Enaminone Complexes: Synthesis, Characterization amd Bioactivity. Chem. Sci. Trans. 2015, 4, 113–120. [Google Scholar] [CrossRef][Green Version]
- Chopin, N.; Médebielle, M.; Pilet, G. 8-Aminoquinoline and 2-aminopyridine trifluoromethyl-derived enaminone ligands and their Co(II), Ni(II) and Zn(II) metal complexes: Structural arrangements due to F···H interactions. J. Fluor. Chem. 2013, 155, 89–96. [Google Scholar] [CrossRef]
- Beckmann, U.; Hägele, G.; Frank, W. Square-Planar 2-Toluenido(triphenylphosphane)nickel(II) Complexes Containing Bidentate N,O Ligands: An Example of Planar Chirality. Eur. J. Inorg. Chem. 2010, 1670–1678. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Hu, Y.-Y. Syntheses and structures of copper complexes of tetradentate enaminones derived from condensation of benzoylacetone and ferrocenoylacetone with 1,2-bis(2-aminophenoxy)ethane. J. Coord. Chem. 2009, 62, 1302–1312. [Google Scholar] [CrossRef]
- Pilet, G.; Tommasino, J.-B.; Fenain, F.; Matrak, R.; Médebielle, M. Trifluoromethylated enaminones and their explorative coordination chemistry with Cu(II): Synthesis, redox properties and structural characterization of the complexes. Dalton Trans. 2008, 5621–5626. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Yang, H.-M.; Song, H.-B.; Liu, Y.-H. Syntheses and crystal structures of a ferrocene-containing enaminone and its copper complex. Polyhedron 2004, 23, 1541–1546. [Google Scholar] [CrossRef]
- Aumann, R.; Göttker-Schnetmann, I.; Fröhlich, R.; Saarenketo, P.; Holst, C. Enaminone Substituents Attached to Cyclopentadienes: 3E/3Z Stereochemistry of 1-Metalla-1,3,5-hexatriene Intermediates (M = Cr,W) as a Functional Criterion for the Formation of Cyclopentadienes and Six-Membered Heterocycles, Respectively. Chem. Eur. J. 2001, 7, 711–720. [Google Scholar] [CrossRef]
- Weber, B.; Jäger, E.-G. Structure and Magnetic Properties of Iron(II/III) Complexes with N2O22– Coordinating Schiff Base Like Ligands. Eur. J. Inorg. Chem. 2009, 465–477. [Google Scholar] [CrossRef]
- Schuhmann, K.; Jäger, E.-G. Equilibrium Studies of the Axial Addition of Chiral Bases to Chiral Metal Schiff Base Complexes. Eur. J. Inorg. Chem. 1998, 2051–2054. [Google Scholar] [CrossRef]
- Jäger, E.-G.; Keutel, H.; Rudolph, M.; Krebs, B.; Wiesemann, F. Activation of Molecular Oxygen by Biomimetic Schiff Base Complexes of Manganese, Iron, and Cobalt. Chem. Ber. 1996, 129, 503–514. [Google Scholar] [CrossRef]
- Liao, J.-H.; Cheng, K.-Y.; Fang, J.-M.; Cheng, M.-C.; Wang, Y. Oxidation of Alkenes and Sulfides with Transition Metal Catalysts. J. Chin. Chem. Soc. 1995, 42, 847–860. [Google Scholar] [CrossRef]
- Weissenfels, M.; Thust, U.; Mühlstädt, M. Über Umsetzungen von Hydroxymethylenecyclanonen. Die Synthese von 2,3-Benzo-5,6-cyclano-1,4-diazepinen. J. Prakt. Chem. 1963, 20, 117–124. [Google Scholar] [CrossRef]
- Grošelj, U.; Svete, J. Recent advances in the synthesis of polysubstituted 3-pyrazolidinones. Arkivoc 2015, 2015, 175–205. [Google Scholar] [CrossRef]
- Pušavec Kirar, E.; Grošelj, U.; Mirri, G.; Požgan, F.; Strle, G.; Štefane, B.; Jovanovski, V.; Svete, J. “Click” Chemistry: Application of Copper Metal in Cu-Catalyzed Azomethine Imine−Alkyne Cycloadditions. J. Org. Chem. 2016, 81, 5988–5997. [Google Scholar] [CrossRef]
- Mirnik, J.; Pušavec Kirar, E.; Ričko, S.; Grošelj, U.; Golobič, A.; Požgan, F.; Štefane, B.; Svete, J. Cu0-catalysed 1,3-dipolar cycloadditions of α-amino acid derived N,N-cyclic azomethine imines to ynones. Tetrahedron 2017, 73, 3329–3337. [Google Scholar] [CrossRef]
- Pezdirc, L.; Stanovnik, B.; Svete, J. Copper(I) Iodide-Catalyzed Cycloadditions of (1Z,4R*,5R*)-4-Benzamido-5-phenylpyrazolidin-3-on-1-azomethine Imines to Ethyl Propiolate. Aust. J. Chem. 2009, 62, 1661–1666. [Google Scholar] [CrossRef]
- Pušavec, E.; Mirnik, J.; Šenica, L.; Grošelj, U.; Stanovnik, B.; Svete, J.Z. Cu(I)-catalyzed [3+2] Cycloadditions of tert-Butyl (S)-(3-Oxopent-4-yn-2-yl)carbamate to 1-Benzylidenepyrazole-3-one-derived Azomethine Imines Naturforsch. Z. Naturforsch. B. 2014, 69, 615–626. [Google Scholar] [CrossRef]
- Pušavec Kirar, E.; Drev, M.; Mirnik, J.; Grošelj, U.; Golobič, A.; Dahmann, G.; Požgan, F.; Štefane, B.; Svete, J. Synthesis of 3D-Rich Heterocycles: Hexahydropyrazolo[1,5-a]pyridin-2(1H)-ones and Octahydro-2H-2a,2a1-diazacyclopenta[cd]inden-2-ones. J. Org. Chem. 2016, 81, 8920–8933. [Google Scholar] [CrossRef]
- Pušavec Kirar, E.; Grošelj, U.; Golobič, A.; Požgan, F.; Pusch, S.; Weber, C.; Andernach, L.; Štefane, B.; Opatz, T.; Svete, J. Absolute Configuration Determination of 2,3-Dihydro-1H,5H;-pyrazolo[1,2-a]pyrazoles Using Chiroptical Methods at Different Wavelengths. J. Org. Chem. 2016, 81, 11802–11812. [Google Scholar] [CrossRef]
- Shiri, P.; Aboonajmi, J. A systematic review on silica-, carbon-, and magnetic materials-supported copper species as efficient heterogeneous nanocatalysts in “click” reactions. Beilstein J. Org. Chem. 2020, 16, 551–586. [Google Scholar] [CrossRef] [PubMed]
- Stanovnik, B.; Svete, J. Synthesis of Heterocycles from Alkyl 3-(Dimethylamino)propenoates and Related Enaminones. Chem. Rev. 2004, 104, 2433–2480. [Google Scholar] [CrossRef] [PubMed]
- Stanovnik, B.; Svete, J. Alkyl 2-Substituted 3-(Dimethylamino)propenoates and Related Compounds-Versatile Reagents in Heterocyclic Chemistry. Synlett 2000, 1077–1091. [Google Scholar] [CrossRef]
- Keller, M.; Sido, A.S.S.; Pale, P.; Sommer, J. Copper(I) Zeolites as Heterogeneous and Ligand-Free Catalysts: [3+2] Cycloaddition of Azomethine Imines. Chem. Eur. J. 2009, 15, 2810–2817. [Google Scholar] [CrossRef]
- Chassaing, S.; Alix, A.; Bonongari, T.; Sido, K.S.S.; Keller, M.; Kuhn, P.; Louis, B.; Sommer, J.; Pale, P. Copper(I)-Zeolites as New Heterogeneous and Green Catalysts for Organic Synthesis. Synthesis 2010, 1557–1567. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K.; Nakagawa, Y.; Oishi, T.; Yamaguchi, K. Scope and reaction mechanism of an aerobic oxidative alkyne homocoupling catalyzed by a di-copper-substituted silicotungstate. Catal. Today 2010, 157, 359–363. [Google Scholar] [CrossRef]
- Oishi, T.; Yoshimura, K.; Yamaguchi, K.; Mizuno, N. An Efficient Copper-mediated 1,3-Dipolar Cycloaddition of Pyrazolidinone-based Dipoles to Terminal Alkynes to Produce N,N-Bicyclic Pyrazolidinone Derivatives. Chem. Lett. 2010, 39, 1086–1087. [Google Scholar] [CrossRef]
- Yoshimura, K.; Oshi, T.; Yamaguchi, K.; Mizuno, N. An Efficient, Ligand-Free, Heterogeneous Supported Copper Hydroxide Catalyst for the Synthesis of N,N-Bicyclic Pyrazolidinone Derivatives. Chem. Eur. J. 2011, 17, 3827–3831. [Google Scholar] [CrossRef]
- Tomažin, U.; Alič, B.; Kristl, A.; Ručigaj, A.; Grošelj, U.; Požgan, F.; Krajnc, M.; Štefane, B.; Šebenik, U.; Svete, J. Synthesis of polyenaminones by acid-catalyzed amino–enaminone ‘click’ polymerisation. Eur. Polym. J. 2018, 108, 603–616. [Google Scholar] [CrossRef]
- Zupanc, A.; Kotnik, T.; Štanfel, U.; Brodnik Žugelj, H.; Kristl, A.; Ručigaj, A.; Matoh, L.; Pahovnik, D.; Grošelj, U.; Opatz, T.; et al. Chemical recycling of polyenaminones by transamination reaction via amino–enaminone polymerisation/depolymerisation. Eur. Polym. J. 2019, 121, 109282. [Google Scholar] [CrossRef]
- Grošelj, U.; Rečnik, S.; Svete, J.; Meden, A.; Stanovnik, B. Stereoselective synthesis of (1R,3R,4R)-3-(1,2,4-triazolo[4,3-x]azin-3-yl)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ones. Tetrahedron Asymmetry 2002, 13, 821–833. [Google Scholar] [CrossRef]
- Yamato, E.; Okumura, K. 2-Acylamino-3-(indol-3-yl)acrylic acid esters. JP 50058063 A, 1975. Chem. Abstr. 1975, 83, 193075y. [Google Scholar]
- Malavašič, Č.; Brulc, B.; Čebašek, P.; Dahmann, G.; Heine, N.; Bevk, D.; Grošelj, U.; Meden, A.; Stanovnik, B.; Svete, J. Combinatorial Solution-Phase Synthesis of (2S,4S)-4-Acylamino-5-oxopyrrolidine-2-carboxamides. J. Comb. Chem. 2007, 9, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalik, M.M.; Eltoukhy, A.M.; Agamy, S.M.; Elnagdi, M.H. Enaminones as Building Blocks in Heterocyclic Synthesis: New Syntheses of Nicotinic Acid and Thienopyridine Derivatives. J. Heterocycl. Chem. 2004, 41, 431–434. [Google Scholar] [CrossRef]
- Schuda, P.F.; Ebner, C.B.; Morgan, T.M. The Synthesis of Mannich Bases from Ketones and Esters via Enaminones. Tetrahedron Lett. 1986, 23, 2567–2570. [Google Scholar] [CrossRef]
- Altomare, C.; Cellamare, S.; Summo, L.; Catto, M.; Carotti, A.; Thull, U.; Carrupt, P.-A.; Testa, B.; Stoeckli-Evans, H. Inhibition of Monoamine Oxidase-B by Condensed Pyridazines and Pyrimidines: Effects of Lipophilicity and Structure-Activity Relationships. J. Med. Chem. 1998, 41, 3812–3820. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Ives, J.L. Reaction of Singlet Oxygen with Enamino Lactones. Conversion of Lactones to α-Keto Lactones. J. Org. Chem. 1978, 43, 3238–3240. [Google Scholar] [CrossRef]
- Turk, C.; Svete, J.; Stanovnik, B.; Golič, L.; Golič-Grdadolnik, S.; Golobič, A.; Selič, L. Regioselective 1,3-Dipolar Cycloadditions of (1Z)-1-(Arylmethylidene)-5,5-dimethyl-3-oxopyrazolidin-1-ium-2-ide Azomethine Imines to Acetylenic Dipolarophiles. Helv. Chim. Acta 2001, 84, 146–156. [Google Scholar] [CrossRef]
- Sibi, M.P.; Rane, D.; Stanley, L.M.; Soeta, T. Copper(II)-Catalyzed Exo and Enantioselective Cycloadditions of Azomethine Imines. Org. Lett. 2008, 10, 2971–2974. [Google Scholar] [CrossRef]
- Petek, N.; Grošelj, U.; Svete, J.; Požgan, F.; Kočar, D.; Štefane, B. Eosin Y-Catalyzed Visible-Light-Mediated Aerobic Transformation of Pyrazolidine-3-One Derivatives. Catalysts 2020, 10, 981. [Google Scholar] [CrossRef]
- Shintani, R.; Fu, G.C. A New Copper-Catalyzed [3 + 2] Cycloaddition: Enantioselective Coupling of Terminal Alkynes with Azomethine Imines to Generate Five-Membered Nitrogen Heterocycles. J. Am. Chem. Soc. 2003, 125, 10778–10779. [Google Scholar] [CrossRef] [PubMed]
- 0.125 Mmol of dipoles 6a–g correspond to 37 mg of dipole 6a, 29 mg of 6b, 27 mg of 6c, 25 mg of 6d, 30 mg of 6e, 28 mg of 6f, and 31 mg of 6g.
- Arai, T.; Ogino, Y. Chiral Bis(Imidazolidine)Pyridine-Cu Complex-Catalyzed Enantioselective [3+2]-Cycloaddition of Azomethine Imines with Propiolates. Molecules 2012, 17, 6170–6178. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).