Nanoindentation of Graphene/Phospholipid Nanocomposite: A Molecular Dynamics Study
Abstract
1. Introduction
2. Results
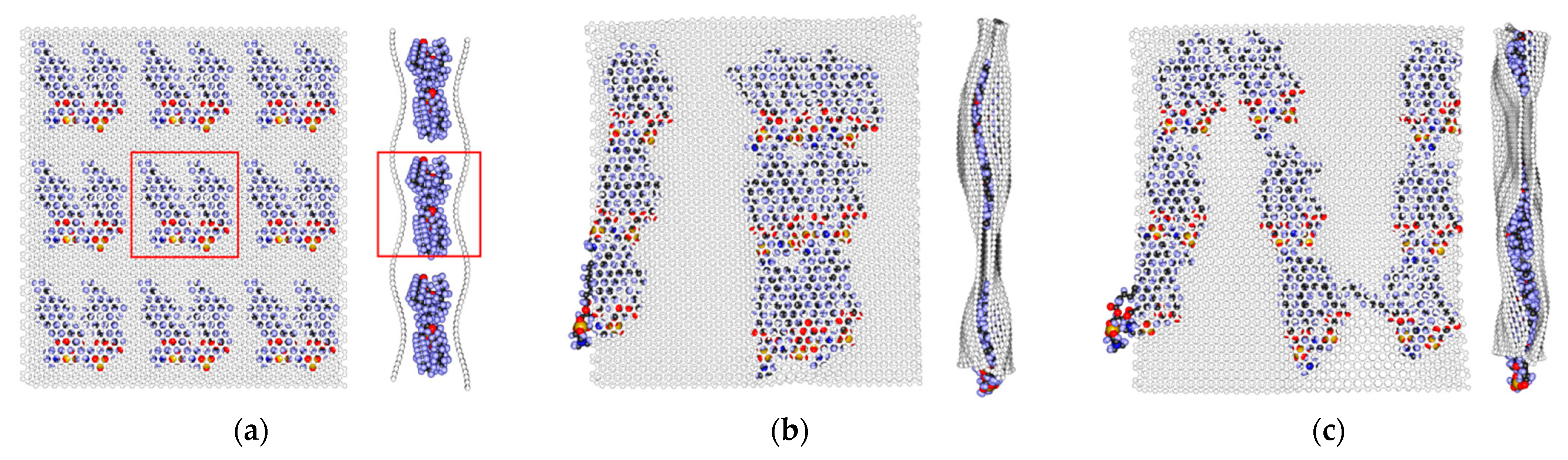
2.1. Atomistic Model
2.2. Nanoindentation
2.3. Analysis of Graphene Sheets Strength during Nanoindentation
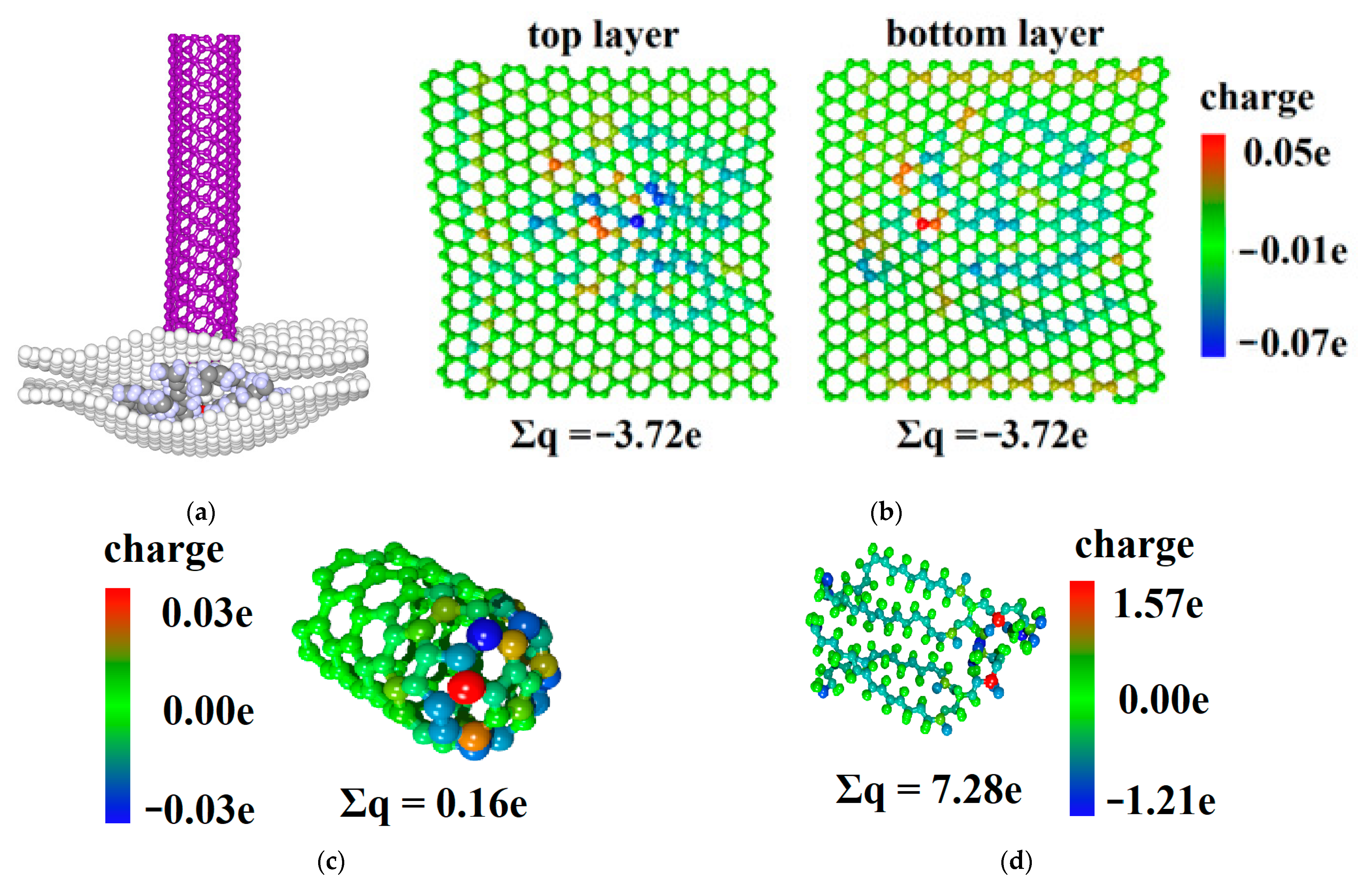
2.4. Analysis of Electron Transfer in the CNT/Graphene/DPPC System during Nanoindentation
3. Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.M.; Goncalves, I.C.; Magalhaes, F.D. The rise of graphene. Colloid. Surface. B 2013, 11, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, A.; Cantón-Vitoria, R.; Psarrou, M.N.; Economopoulos, S.P.; Tagmatarchis, N. Functionalized graphene and targeted applications—Highlighting the road from chemistry to applications. Prog. Mater. Sci. 2020, 114, 100683. [Google Scholar]
- Nandanapalli, K.R.; Mudusu, D.; Lee, S. Functionalization of graphene layers and advancements in device applications. Carbon 2019, 152, 954–985. [Google Scholar] [CrossRef]
- Rivela, T.; Yesylevskyy, S.O.; Ramseyer, C. Structures of single, double and triple layers of lipids adsorbed on graphene: Insights from all-atom molecular dynamics simulations. Carbon 2017, 118, 358–369. [Google Scholar] [CrossRef]
- Lima, L.M.C.; Fu, W.; Jiang, L.; Kros, A.; Schneider, G.F. Graphene-stabilized lipid monolayer heterostructures: A novel biomembrane superstructure. Nanoscale 2016, 8, 18646–18653. [Google Scholar]
- Li, W.; Moon, S.; Wojcik, M.; Xu, K. Direct Optical Visualization of Graphene and Its Nanoscale Defects on Transparent Substrates. Nano Lett. 2016, 16, 5022–5026. [Google Scholar] [CrossRef]
- Okamoto, Y.; Tsuzuki, K.; Iwasa, S.; Ishikawa, R.; Sandhu, A.; Tero, R. Fabrication of Supported Lipid Bilayer on Graphene Oxide. J. Phys. Conf. Ser. 2012, 352, 012017. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Sekula-Neuner, S.; Lenhert, S.; Fuchs, H.; Gizeli, E. Convergence of Dip-Pen Nanolithography and Acoustic Biosensors towards a Rapid-Analysis Multi-Sample Microsystem. Analyst 2012, 137, 3076–3082. [Google Scholar] [CrossRef]
- Bog, U.; Laue, T.; Grossmann, T.; Beck, T.; Wienhold, T.; Richter, B.; Hirtz, M.; Fuchs, H.; Kalt, H.; Mappes, T. On-Chip Microlasers for Biomolecular Detection via Highly Localized Deposition of a Multifunctional Phospholipid Ink. Lab Chip 2013, 13, 2701–2707. [Google Scholar] [CrossRef]
- Bog, U.; Brinkmann, F.; Wondimu, S.F.; Wienhold, T.; Kraemmer, S.; Koos, C.; Kalt, H.; Hirtz, M.; Fuchs, H.; Koeber, S.; et al. Densely Packed Microgoblet Laser Pairs for Cross-Referenced Biomolecular Detection. Adv. Sci. 2015, 2, 1500066. [Google Scholar] [CrossRef] [PubMed]
- Lahcen, A.A.; Rauf, S.; Beduk, T.; Durmus, C.; Aljedaibi, A.; Timur, S.; Alshareef, H.N.; Amine, A.; Wolfbeis, O.S.; Salama, K.N.; et al. Electrochemical sensors and biosensors using laser-derived graphene: A comprehensive review. Biosens. Bioelectron. 2020, 168, 112565. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.K.; Dinu, C.Z.; Guldi, D.M.; Sergeyev, V.G.; Creager, S.E.; Cooke, J.P.; Guiseppi-Elie, A. Nanobiosensing with graphene and carbon quantum dots: Recent advances. Mater. Today 2020, 39, 23–46. [Google Scholar] [CrossRef]
- Chauhan, G.; Shaik, A.A.; Kulkarni, N.S.; Gupta, V. The preparation of lipid-based drug delivery system using melt extrusion. Drug Discov. Today 2020, 25, 1930–1946. [Google Scholar]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Phospholipids: Unique carriers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2017, 39, 166–179. [Google Scholar] [CrossRef]
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloid. Surface. B 2020, 185, 110596. [Google Scholar] [CrossRef]
- Willems, N.; Urtizberea, A.; Verre, A.F.; Iliut, M.; Lelimousin, M.; Hirtz, M.; Vijayaraghavan, A.; Sansom, M.S.P. Biomimetic Phospholipid Membrane Organization on Graphene and Graphene Oxide Surfaces: A Molecular Dynamics Simulation Study. ACS Nano 2017, 11, 1613–1625. [Google Scholar]
- Monasterio, B.G.; Alonso, B.; Sot, J.; García-Arribas, A.B.; Gil-Cartón, D.; Valle, M.; Zurutuza, A.; Goñi, F.M. Coating graphene oxide with lipid bilayers greatly decreases its hemolytic properties. Langmuir 2017, 33, 8181–8191. [Google Scholar] [CrossRef]
- Durso, M.; Borrachero-Conejo, A.I.; Bettini, C.; Treossi, E.; Scidà, A.; Saracino, E.; Gazzano, M.; Christian, M.; Morandi, V.; Tuci, G.; et al. Biomimetic graphene for enhanced interaction with the external membrane of astrocytes. J. Mater. Chem. B 2018, 6, 5335–5342. [Google Scholar] [CrossRef]
- Hai, L.; He, D.; He, X.; Wang, K.; Yang, X.; Liu, J.; Cheng, H.; Huang, X.; Shangguan, J. Facile fabrication of a resveratrol loaded phospholipid@reduced graphene oxide nanoassembly for targeted and near-infrared laser-triggered chemo/photothermal synergistic therapy of cancer in vivo. J. Mater. Chem. B 2017, 5, 5783–5792. [Google Scholar] [CrossRef]
- Kuo, C.J.; Chiang, H.C.; Tseng, C.A.; Chang, C.; Ulaganathan, R.K.; Ling, T.-T.; Chang, Y.-J.; Chen, C.-C.; Chen, Y.-R.; Chen, Y.-T. Lipid-modified graphene-transistor biosensor for monitoring amyloid- aggregation. ACS Appl. Mater. Interfaces 2018, 10, 12311–12316. [Google Scholar] [CrossRef]
- Huang, Y.; Yanga, Z.; Lu, Z. Nanoindentation of bio-inspired graphene/nickel nanocomposites: A molecular dynamics simulation. Comput. Mater. Sci. 2021, 186, 109969. [Google Scholar] [CrossRef]
- Shuang, F.; Aifantis, K.E. Dislocation-graphene interactions in Cu/graphene composites and the effect of boundary conditions: A molecular dynamics study. Carbon 2021, 172, 50–70. [Google Scholar] [CrossRef]
- Peng, W.; Sun, K.; Zhang, M.; Shi, J.; Chen, J. Effects of graphene coating on the plastic deformation of single crystal copper nano-cuboid under different nanoindentation modes. Mater. Chem. Phys. 2019, 225, 1–7. [Google Scholar] [CrossRef]
- Song, Z.; Wang, Y.; Xu, Z. Mechanical responses of the bio-nano interface: A molecular dynamics study of graphene-coated lipid membrane. Theor. Appl. Mech. Lett. 2015, 5, 231–235. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, S.; Liu, S. Atomistic simulation on nanomechanical response of indented graphene/nickel system. Comput. Mater. Sci. 2017, 130, 16–20. [Google Scholar]
- Slepchenkov, M.M.; Glukhova, O.E. Improving the Sensory Properties of Layered Phospholipid-Graphene Films Due to the Curvature of Graphene Layers. Polymers 2020, 12, 1710. [Google Scholar] [CrossRef]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I. A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Chemistry Software, HyperChem, Molecular Modeling. Available online: http://www.hyper.com/ (accessed on 1 December 2020).
- Slepchenkov, M.M.; Glukhova, O.E. Influence of the curvature of deformed graphene nanoribbons on their electronic and adsorptive properties: Theoretical investigation based on the analysis of the local stress field for an atomic grid. Nanoscale 2012, 11, 3335–3344. [Google Scholar]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. J. Chem. Phys. 1995, 23, 1833–1840. [Google Scholar]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulations of complex materials properties. Phys. Rev. B 1998, 58, 7260–7268. [Google Scholar] [CrossRef]
- DFTB+ Density Functional Based Tight Binding. Available online: https://dftbplus.org (accessed on 1 December 2020).
- Xia, Y.; Shi, C.-Y.; Xiong, W.; Hou, X.-L.; Fang, J.G.; Wang, W. Shear Stress-sensitive Carriers for Localized Drug Delivery. Curr. Pharm. Des. 2016, 22, 5855–5867. [Google Scholar] [PubMed]
- Godoy-Gallardo, M.; Ek, P.K.; Jansman, M.M.T.; Wohl, B.M.; Hosta-Rigau, L. Interaction between drug delivery vehicles and cells under the effect of shear stress. Biomicrofluidics 2015, 9, 052605. [Google Scholar] [CrossRef] [PubMed]

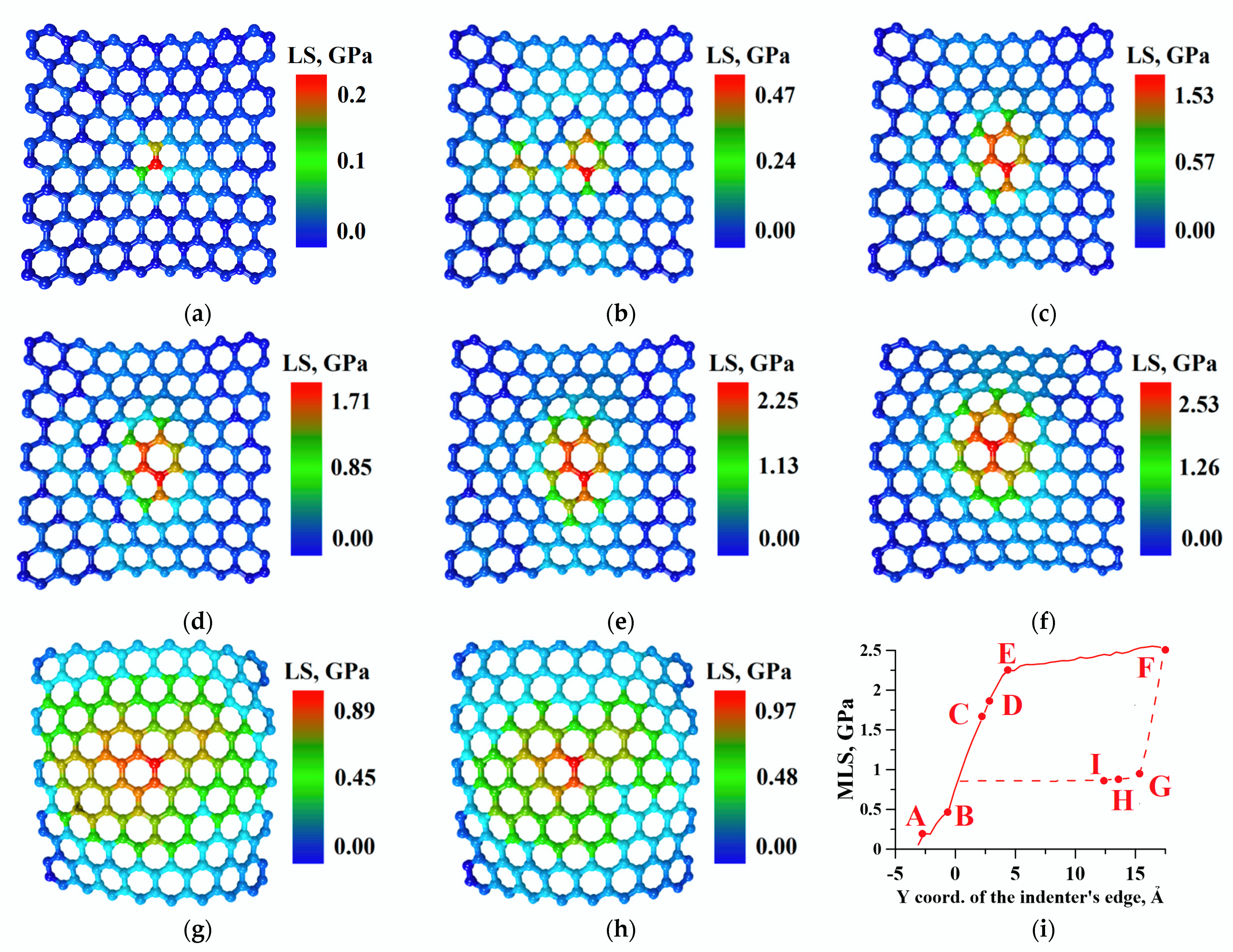
| Key Point | Y Coordinate of the Indenter’s Edge, Å | Energy of Adhesion between Bilayer Graphene and Phospholipid, kcal/mol | MLS, GPa |
|---|---|---|---|
| A | −2.6 (FS) | −4125.1 | 0.2 |
| B | −0.6 (FS) | −4124.03 | 0.47 |
| C | 1.9 (FS) | −4125.85 | 1.53 |
| D | 2.4 (FS) | −4127.13 | 1.71 |
| E | 4.4 (FS) | −4114.53 | 2.25 |
| F | 17.4(FS) | −4116.33 | 2.53 |
| G | 15.4 (RS) | −4166.4 | 0.89 |
| H | 13.4 (RS) | −4172.05 | 0.97 |
| I | 12.4 (RS) | −4172.45 | 0.97 |
| CNT | Upper Layer of Graphene Sheet | DPPC | Lower Layer of Graphene Sheet | |
|---|---|---|---|---|
| Initial moment of time | 0.00 e | −3.45 e | 6.09 e | −2.63 e |
| Point F | 0.16 e | −3.72 e | 7.28 e | −3.72 e |
| Point I | −0.01 e | −3.28 e | 6.87 e | −3.58 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shunaev, V.V.; Glukhova, O.E. Nanoindentation of Graphene/Phospholipid Nanocomposite: A Molecular Dynamics Study. Molecules 2021, 26, 346. https://doi.org/10.3390/molecules26020346
Shunaev VV, Glukhova OE. Nanoindentation of Graphene/Phospholipid Nanocomposite: A Molecular Dynamics Study. Molecules. 2021; 26(2):346. https://doi.org/10.3390/molecules26020346
Chicago/Turabian StyleShunaev, Vladislav V., and Olga E. Glukhova. 2021. "Nanoindentation of Graphene/Phospholipid Nanocomposite: A Molecular Dynamics Study" Molecules 26, no. 2: 346. https://doi.org/10.3390/molecules26020346
APA StyleShunaev, V. V., & Glukhova, O. E. (2021). Nanoindentation of Graphene/Phospholipid Nanocomposite: A Molecular Dynamics Study. Molecules, 26(2), 346. https://doi.org/10.3390/molecules26020346







