The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection
Abstract
1. Introduction
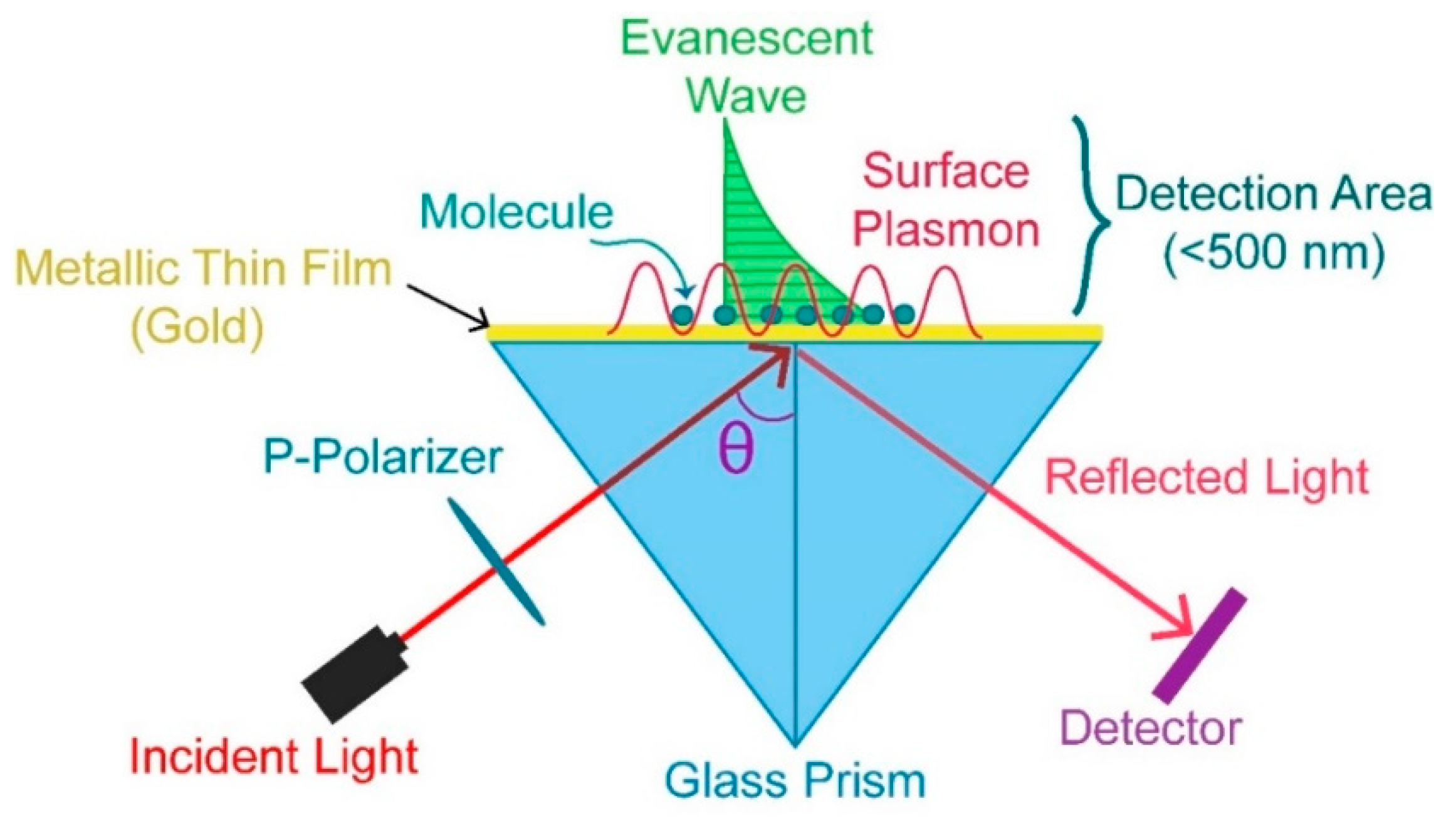
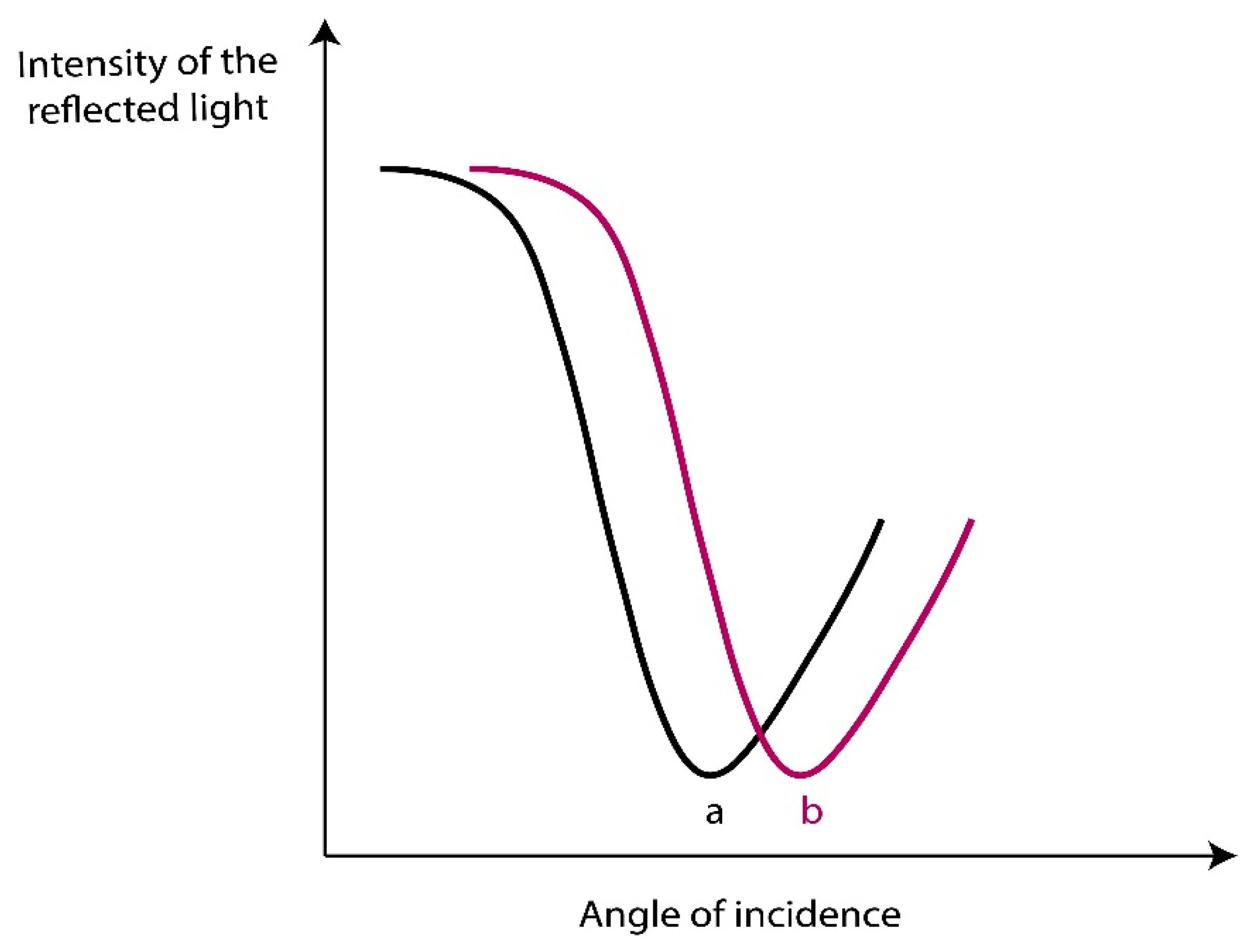
2. SPR Phenomena
3. SPR Based Sensor
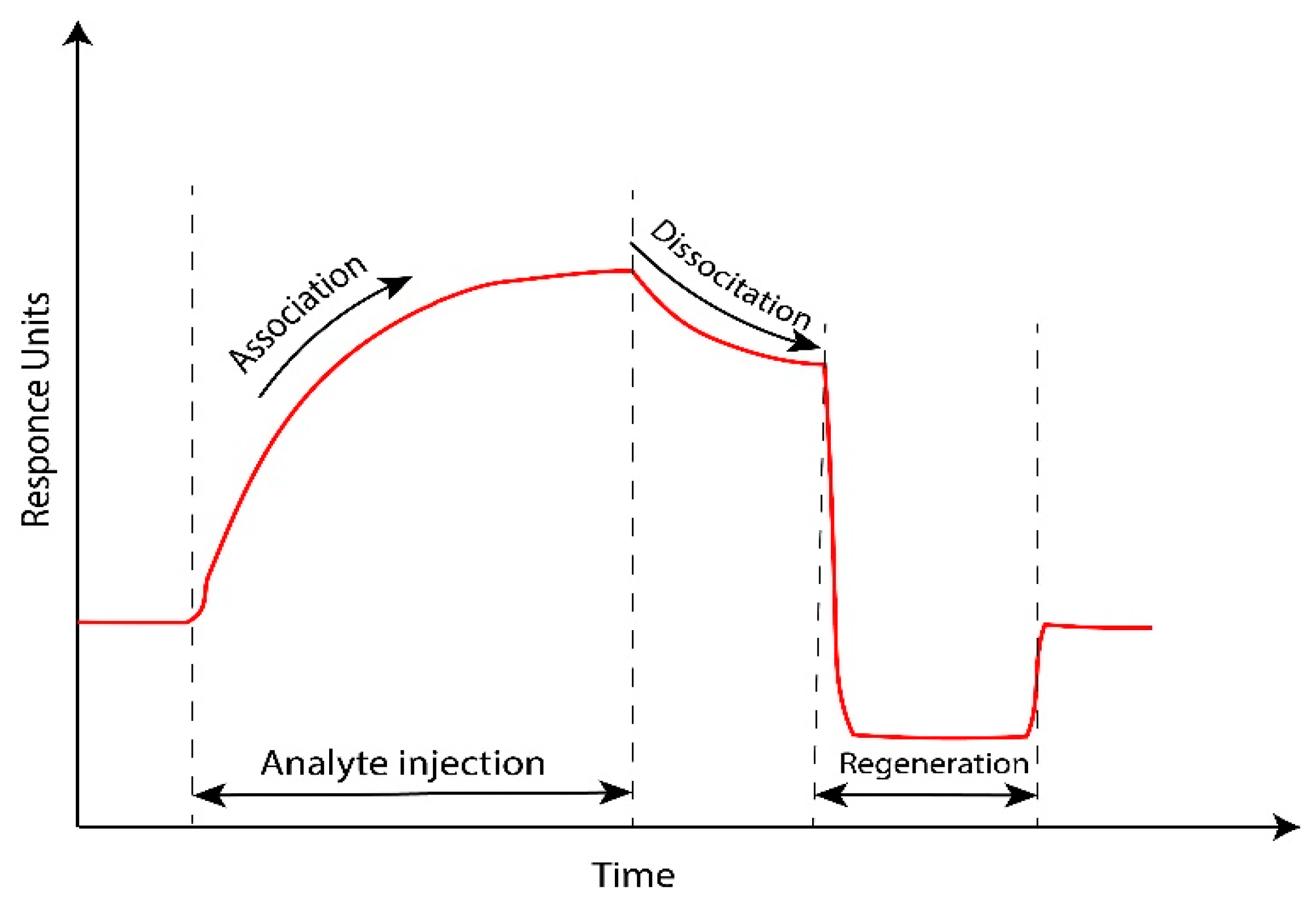
4. The Important Characteristics in SPR Sensor Performance
5. The Importance of SPR Biosensors in the Medical Diagnosis
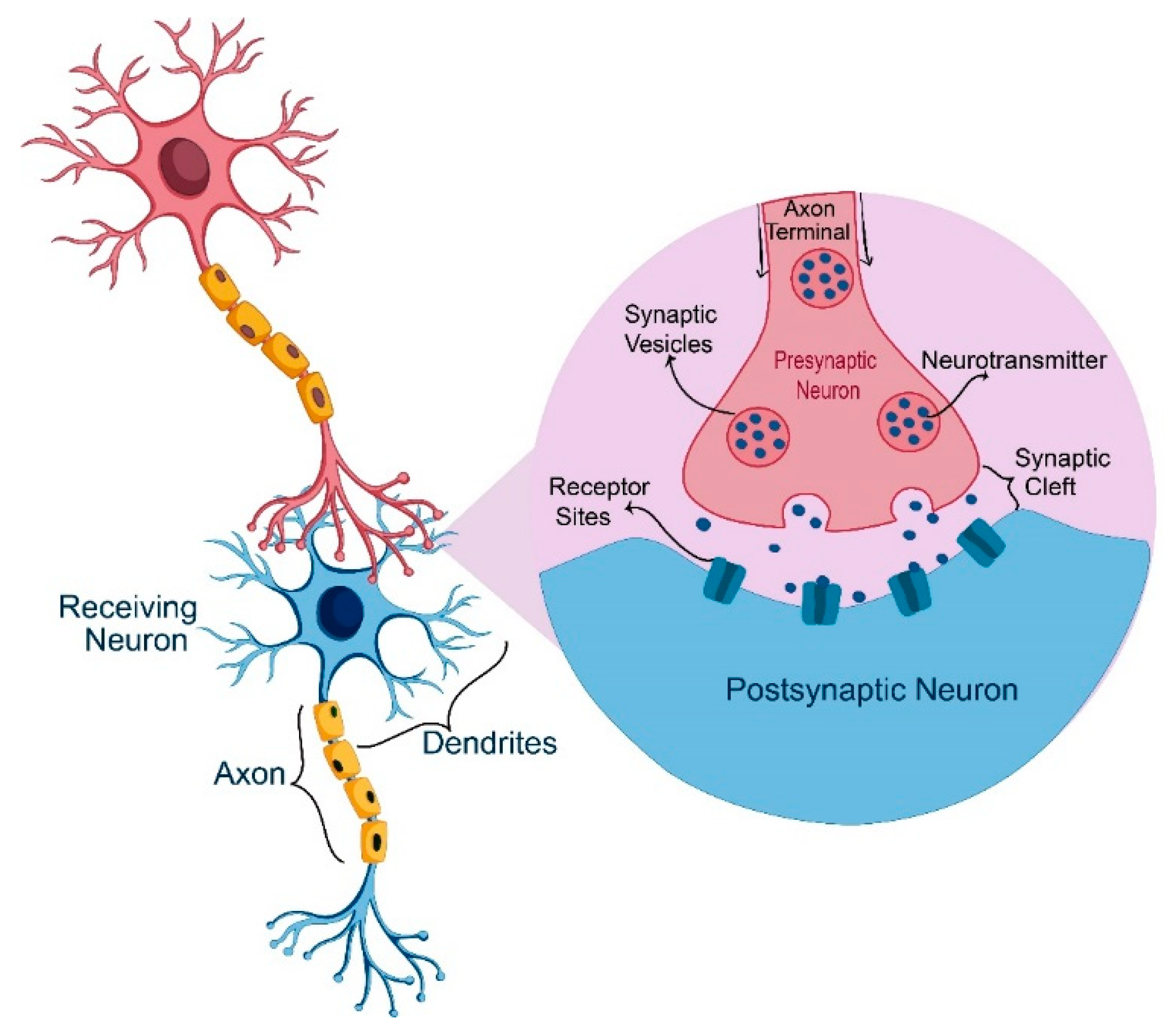
6. DA and Its Critical Role in the Human Body
7. DA Detection Using SPR Biosensors
8. The Advantages of DA Detection Using SPR Sensors
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kan, X.; Li, S.F.Y. Rapid Detection of Bacteria in Food by Surface Plasmon Resonance Sensors. Int. J. Adv. Sci. Eng. Technol. 2016, 1, 26–29. [Google Scholar]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Yanase, Y.; Sakamoto, K.; Kobayashi, K.; Hide, M. Diagnosis of Immediate-Type Allergy Using Surface Plasmon Resonance. Opt. Mater. Express 2016, 6, 1339. [Google Scholar] [CrossRef]
- Situ, C.; Mooney, M.H.; Elliott, C.T.; Buijs, J. Advances in Surface Plasmon Resonance Biosensor Technology towards High-Throughput, Food-Safety Analysis. TrAC Trends Anal. Chem. 2010, 29, 1305–1315. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Omar, N.A.S.; Anas, N.A.A.; Ramdzan, M.; Syahira, N. Highly Sensitive Surface Plasmon Resonance Optical Sensor for Detection of Copper, Zinc, and Nickel Ions. Sens. Lett. 2019, 17, 497–504. [Google Scholar] [CrossRef]
- Holzinger, M.; Goff, A.L.; Cosnier, S. Nanomaterials for Biosensing Applications: A Review. Front. Chem. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Ishimaru, A.; Jaruwatanadilok, S.; Kuga, Y. Generalized Surface Plasmon Resonance Sensors Using Metamaterials and Negative Index Materials. Prog. Electromagn. Res. 2006, 51, 139–152. [Google Scholar] [CrossRef]
- Pitarke, J.M.; Silkin, V.M.; Chulkov, E.V.; Echenique, P.M. Theory of Surface Plasmons and Surface-Plasmon Polaritons. Rep. Prog. Phys. 2007, 70, 1–87. [Google Scholar] [CrossRef]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface Plasmon Resonance Sensors. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Wood, R.W. On a Remarkable Case of Uneven Distribution of Light in A Diffraction Grating Spectrum. Proc. Phys. Soc. 1902, 18, 269–275. [Google Scholar]
- Fano, U. The Theory of Anomalous Diffraction Gratings and of Quasi-Stationary Waves on Metallic Surfaces (Sommerfeld’s Waves). JOSA 1941, 31, 213–222. [Google Scholar] [CrossRef]
- OTTO, A. Excitation of Nonradiative Surface Plasma Waves in Silver by the Method of Frustrated Total Reflection. Z. Phys. A Hadron. Nucl. 1968, 216, 398–410. [Google Scholar] [CrossRef]
- Kretschmann, E.; Raether, H. Radiative Decay of Non Radiative Surface Plasmons Excited by Light. Z. Naturforsch. A Phys. Sci. 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Rizal, C. Bio-Magnetoplasmonics, Emerging Biomedical Technologies and Beyond. J. Nanomed. Res. 2016, 3. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Surface Plasmon Resonance Spectroscopy as An Alternative for Sensing Heavy Metal Ions: A Review. Sens. Rev. 2013, 33, 305–314. [Google Scholar] [CrossRef]
- Homola, J.; Koudela, I.; Yee, S.S. Surface Plasmon Resonance Sensors Based on Diffraction Gratings and Prism Couplers: Sensitivity Comparison. Sens. Actuators B Chem. 1999, 54, 16–24. [Google Scholar] [CrossRef]
- Karlssonz, R.; Fält, A. Experimental Design for Kinetic Analysis of Protein-Protein Interactions with Surface Plasmon Resonance Biosensors. J. Immunol. Methods 1997, 200, 121–133. [Google Scholar] [CrossRef]
- Homola, J. Electromagnetic Theory of Surface Plasmons; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–44. [Google Scholar] [CrossRef]
- Mukhtar, W.M.; Halim, R.M.; Hassan, H. Optimization of SPR Signals: Monitoring the Physical Structures and Refractive Indices of Prisms. EPJ Web Conf. 2017, 162. [Google Scholar] [CrossRef]
- Al-qazwini, Y.; Noor, A.S.M.; Arasu, P.T.; Sadrolhosseini, A.R. Investigation of the Performance of an SPR-Based Optical Fiber Sensor Using Finite-Difference Time Domain. Curr. Appl. Phys. 2013, 13, 1354–1358. [Google Scholar] [CrossRef]
- Murat, N.F.; Mukhtar, W.M.; Rashid, A.R.A.; Dasuki, K.A.; Yussuf, A.A.R.A. Optimization of Gold Thin Films Thicknesses in Enhancing SPR Response. In Proceedings of the 2016 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 17–19 August 2016; pp. 244–247. [Google Scholar] [CrossRef]
- Michel, D.; Xiao, F.; Alameh, K. A Compact, Flexible Fiber-Optic Surface Plasmon Resonance Sensor with Changeable Sensor Chips. Sens. Actuators B Chem. 2017, 246, 258–261. [Google Scholar] [CrossRef]
- Nelson, B.P.; Frutos, A.G.; Brockman, J.M.; Corn, R.M. Near-Infrared Surface Plasmon Resonance Measurements of Ultrathin Films. 1. Angle Shift and SPR Imaging Experiments. Anal. Chem. 1999, 71, 3928–3934. [Google Scholar] [CrossRef]
- Patskovsky, S.; Kabashin, A.V.; Meunier, M.; Luong, J.H.T. Properties and Sensing Characteristics of Surface-Plasmon Resonance in Infrared Light. J. Opt. Soc. Am. A 2003, 20, 1644. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, W.M.; Murat, N.F.; Samsuri, N.D.; Dasuki, K.A. Maximizing the Response of SPR Signal: A Vital Role of Light Excitation Wavelength. AIP Conf. Proc. 2018, 2016. [Google Scholar] [CrossRef]
- Chung, J.W.; Kim, S.D.; Bernhardt, R.; Pyun, J.C. Application of SPR Biosensor for Medical Diagnostics of Human Hepatitis B Virus (HHBV). Sens. Actuators B Chem. 2005, 416–422. [Google Scholar] [CrossRef]
- Uzun, L.; Say, R.; Ünal, S.; Denizli, A. Production of Surface Plasmon Resonance Based Assay Kit for Hepatitis Diagnosis. Biosens. Bioelectron. 2009, 24, 2878–2884. [Google Scholar] [CrossRef]
- Uludag, Y.; Tothill, I.E. Cancer Biomarker Detection in Serum Samples Using Surface Plasmon Resonance and Quartz Crystal Microbalance Sensors with Nanoparticle Signal Amplification. Anal. Chem. 2012, 84, 5898–5904. [Google Scholar] [CrossRef]
- Ertürk, G.; Özen, H.; Tümer, M.A.; Mattiasson, B.; Denizli, A. Microcontact Imprinting Based Surface Plasmon Resonance (SPR) Biosensor for Real-Time and Ultrasensitive Detection of Prostate Specific Antigen (PSA) from Clinical Samples. Sens. Actuators B Chem. 2016, 224, 823–832. [Google Scholar] [CrossRef]
- He, L.; Pagneux, Q.; Larroulet, I.; Serrano, A.Y.; Pesquera, A.; Zurutuza, A.; Mandler, D.; Boukherroub, R.; Szunerits, S. Label-Free Femtomolar Cancer Biomarker Detection in Human Serum Using Graphene-Coated Surface Plasmon Resonance Chips. Biosens. Bioelectron. 2017, 89, 606–611. [Google Scholar] [CrossRef]
- Liang, R.P.; Yao, G.H.; Fan, L.X.; Qiu, J.D. Magnetic Fe 3O 4@Au Composite-Enhanced Surface Plasmon Resonance for Ultrasensitive Detection of Magnetic Nanoparticle-Enriched α-Fetoprotein. Anal. Chim. Acta 2012, 737, 22–28. [Google Scholar] [CrossRef]
- Osman, B.; Uzun, L.; Beşirli, N.; Denizli, A. Microcontact Imprinted Surface Plasmon Resonance Sensor for Myoglobin Detection. Mater. Sci. Eng. C 2013, 33, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Uzun, L.; Say, R.; Denizli, A. Use of Molecular Imprinted Nanoparticles as Biorecognition Element on Surface Plasmon Resonance Sensor. Sens. Actuators B Chem. 2011, 160, 791–799. [Google Scholar] [CrossRef]
- Bocková, M.; Chadtová Song, X.; Gedeonová, E.; Levová, K.; Kalousová, M.; Zima, T.; Homola, J. Surface Plasmon Resonance Biosensor for Detection of Pregnancy Associated Plasma Protein A2 in Clinical Samples. Anal. Bioanal. Chem. 2016, 408, 7265–7269. [Google Scholar] [CrossRef] [PubMed]
- Brun, A.P.L.; Soliakov, A.; Shah, D.S.H.; Holt, S.A.; Mcgill, A.; Lakey, J.H. Engineered Self-Assembling Monolayers for Label Free Detection of Influenza Nucleoprotein. Biomed. Microdevices 2015, 49. [Google Scholar] [CrossRef]
- Chang, Y.F.; Wang, W.H.; Hong, Y.W.; Yuan, R.Y.; Chen, K.H.; Huang, Y.W.; Lu, P.L.; Chen, Y.H.; Chen, Y.M.A.; Su, L.C.; et al. Simple Strategy for Rapid and Sensitive Detection of Avian Influenza A H7N9 Virus Based on Intensity-Modulated SPR Biosensor and New Generated Antibody. Anal. Chem. 2018, 90, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Huang, X.; Xu, J.; Li, G.; Ma, J.; Ji, H.F.; Zhu, S.; Chen, H. Rapid and Sensitive Detection of Maize Chlorotic Mottle Virus Using Surface Plasmon Resonance-Based Biosensor. Anal. Biochem. 2013, 440, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cairns, T.M.; Ditto, N.T.; Atanasiu, D.; Lou, H.; Brooks, B.D.; Saw, W.T.; Eisenberg, R.J.; Cohen, G.H. Surface Plasmon Resonance Reveals Direct Binding of Herpes Simplex Virus Glycoproteins GH/GL to GD and Locates a GH/GL Binding Site on GD. J. Virol. 2019, 93, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Anwar, S.; Rafya, R. Development of Surface Plasmon Resonance (SPR) Biosensors for Use in the Diagnostics of Malignant and Infectious Diseases. Laser Phys. Lett. 2018, 15. [Google Scholar] [CrossRef]
- Takemura, K.; Adegoke, O.; Suzuki, T.; Park, E.Y. A Localized Surface Plasmon Resonance-Amplified Immunofluorescence Biosensor for Ultrasensitive and Rapid Detection of Nonstructural Protein 1 of Zika Virus. PLoS ONE 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Yakes, B.J.; Papafragkou, E.; Conrad, S.M.; Neill, J.D.; Ridpath, J.F.; Burkhardt, W.; Kulka, M.; DeGrasse, S.L. Surface Plasmon Resonance Biosensor for Detection of Feline Calicivirus, a Surrogate for Norovirus. Int. J. Food Microbiol. 2013, 162, 152–158. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an Optical Sensor Based on Surface Plasmon Resonance Phenomenon for Diagnosis of Dengue Virus E-Protein. Sens. Bio-Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Mahdi, M.A. Structural, Optical and Sensing Properties of CdS-NH2GO Thin Film as a Dengue Virus E-Protein Sensing Material. Optik 2018, 171, 934–940. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Mustapha Kamil, Y.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive Detection of Dengue Virus Type 2 E-Proteins Signals Using Self-Assembled Monolayers/Reduced Graphene Oxide-PAMAM Dendrimer Thin Film-SPR Optical Sensor. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Mustapha Kamil, Y.; Fauzi, N.I.M.; Hashim, H.S.; Mahdi, M.A. Quantitative and Selective Surface Plasmon Resonance Response Based on a Reduced Graphene Oxide–Polyamidoamine Nanocomposite for Detection of Dengue Virus E-Proteins. Nanomaterials 2020, 10, 569. [Google Scholar] [CrossRef]
- Cenci, L.; Andreetto, E.; Vestri, A.; Bovi, M.; Barozzi, M.; Iacob, E.; Busato, M.; Castagna, A.; Girelli, D.; Bossi, A.M. Surface Plasmon Resonance Based on Molecularly Imprinted Nanoparticles for the Picomolar Detection of the Iron Regulating Hormone Hepcidin-25. J. Nanobiotechnol. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Zhang, Q.; Jing, L.; Wang, Y.; Zhang, J.; Ren, Y.; Wang, Y.; Wei, T.; Liedberg, B. Surface Plasmon Resonance Sensor for Femtomolar Detection of Testosterone with Water-Compatible Macroporous Molecularly Imprinted Film. Anal. Biochem. 2014, 463, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yockell-Lelièvre, H.; Bukar, N.; McKeating, K.S.; Arnaud, M.; Cosin, P.; Guo, Y.; Dupret-Carruel, J.; Mougin, B.; Masson, J.F. Plasmonic Sensors for the Competitive Detection of Testosterone. Analyst 2015, 140, 5105–5111. [Google Scholar] [CrossRef]
- Treviño, J.; Calle, A.; Rodríguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Single- and Multi-Analyte Determination of Gonadotropic Hormones in Urine by Surface Plasmon Resonance Immunoassay. Anal. Chim. Acta 2009, 647, 202–209. [Google Scholar] [CrossRef]
- Treviño, J.; Calle, A.; Rodríguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Surface Plasmon Resonance Immunoassay Analysis of Pituitary Hormones in Urine and Serum Samples. Clin. Chim. Acta 2009, 403, 56–62. [Google Scholar] [CrossRef]
- Sanghera, N.; Anderson, A.; Nuar, N.; Xie, C.; Mitchell, D.; Klein-Seetharaman, J. Insulin Biosensor Development: A Case Study. Int. J. Parallel Emerg. Distrib. Syst. 2017, 32, 119–138. [Google Scholar] [CrossRef]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-Free Imaging, Detection, and Mass Measurement of Single Viruses by Surface Plasmon Resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Siedhoff, D.; Strauch, M.; Shpacovitch, V.; Merhof, D. Unsupervised Data Analysis for Virus Detection with a Surface Plasmon Resonance Sensor. In Proceedings of the 2017 Seventh International Conference on Image Processing Theory, Tools and Applications (IPTA), Montreal, QC, Canada, 28 November–1 December 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Victoria, S. Application of Surface Plasmon Resonance (SPR) for the Detection of Single Viruses and Single Biological Nano-Objects. J. Bacteriol. Parasitol. 2012, 3. [Google Scholar] [CrossRef]
- Saylan, Y.; Yilmaz, F.; Özgür, E.; Derazshamshir, A.; Bereli, N.; Yavuz, H.; Denizli, A. Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis. In Surface Plasmon Resonance Sensors for Medical Diagnosis; Kumar, C.S.S.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 425–458. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting Polymer-Based Electrochemical Biosensors for Neurotransmitters: A Review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, J. Advanced Materials for Optical Sensing and Biosensing of Neurotransmitters. TrAC Trends Anal. Chem. 2015, 72, 27–44. [Google Scholar] [CrossRef]
- Krishna, V.M.; Somanathan, T.; Manikandan, E.; Tadi, K.K.; Uvarajan, S. Neurotransmitter Dopamine Enhanced Sensing Detection Using Fibre-Like Carbon Nanotubes by Chemical Vapor Deposition Technique. J. Nanosci. Nanotechnol. 2018, 18, 5380–5389. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Chen, W.; Wu, P. Electrocatalytic Oxidation and Determination of Dopamine in the Presence of Ascorbic Acid and Uric Acid at a Poly (p -Nitrobenzenazo Resorcinol) Modified Glassy Carbon Electrode. Sens. Actuators B Chem. 2007, 122, 309–314. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Cui, M.; Lin, L.; Jiang, S.; Jiao, L.; Zhang, L. A Promising Non-Aggregation Colorimetric Sensor of AuNRs–Ag + for Determination of Dopamine. Sens. Actuators B Chem. 2013, 176, 97–102. [Google Scholar] [CrossRef]
- Haven, N. Dopamine Synthesis, Uptake, Metabolism, and Receptors: Relevance to Gene Therapy of Parkinson’s Disease. Exp. Neurol. 1997, 9, 4–9. [Google Scholar] [CrossRef]
- Kim, J.-H.; Auerbach, J.M.; Rodríguez-Gómez, J.A.; Velasco, I.; Gavin, D.; Lumelsky, N.; McKay, R. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 2002, 418, 50–56. [Google Scholar] [CrossRef]
- Pezzella, A.; Ischia, M.; Napolitano, A.; Misuraca, G.; Prota, G. Iron-Mediated Generation of the Neurotoxin 6-Hydroxydopamine Quinone by Reaction of Fatty Acid Hydroperoxides with Dopamine: A Possible Contributory Mechanism for Neuronal Degeneration in Parkinson’s Disease. J. Med. Chem. 1997, 40, 2211–2216. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.; Van Hoesen, G.; Damasio, A.; Barnes, C. Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science 1984, 225, 1168–1170. [Google Scholar] [CrossRef] [PubMed]
- Wightman, M.; May, L.J.; Michael, A.C. Detection of Dopamine Dynamics in the Brain. Anal. Chem. 1988, 60, 769–779. [Google Scholar] [CrossRef]
- Kesby, J.P. Dopamine, Psychosis and Schizophrenia: The Widening Gap between Basic and Clinical Neuroscience. Transl. Psychiatry 2018, 8, 30. [Google Scholar] [CrossRef]
- Pandey, P.C.; Chauhan, D.S.; Singh, V. Effect of Processable Polyindole and Nanostructured Domain on the Selective Sensing of Dopamine. Mater. Sci. Eng. C 2012, 32, 1–11. [Google Scholar] [CrossRef]
- Yu, C.; Yan, J.; Tu, Y. Electrochemiluminescent Sensing of Dopamine Using CdTe Quantum Dots Capped with Thioglycolic Acid and Supported with Carbon Nanotubes. Microchim. Acta 2011, 175, 347–354. [Google Scholar] [CrossRef]
- Shankaran, D.R.; Iimura, K.; Kato, T. Simultaneous Determination of Ascorbic Acid and Dopamine at Sol–Gel Composite Electrode. Sens. Actuators B Chem. 2003, 94, 73–80. [Google Scholar] [CrossRef]
- Kurzatkowska, K.; Dolusic, E.; Dehaen, W.; Sieron, K.; Radecka, H. Gold Electrode Incorporating Corrole as an Ion-Channel Mimetic Sensor for Determination of Dopamine. Anal. Chem. 2009, 81, 7397–7405. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, P.; Yang, L. Determination of Dopamine in Rat Striatum by Microdialysis and High-Performance Liquid Chromatography with Electrochemical Detection on a Functionalized Multi-Wall Carbon Nanotube Electrode. Anal. Bioanal. Chem. 2006, 384, 1308–1313. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X. Electrochemical Behavior of a Covalently Modified Glassy Carbon Electrode with Aspartic Acid and Its Use for Voltammetric Differentiation of Dopamine and Ascorbic Acid. Anal. Bioanal. Chem. 2005, 382, 1669–1677. [Google Scholar] [CrossRef]
- Jagadeesh, J.S.; Natarajan, S. Schizophrenia: Interaction between Dopamine, Serotonin, Glutamate, GABA. RJPBCS 2013, 4, 1267–1271. [Google Scholar]
- Davis, K.L.; Kahn, R.S.; Ko, G.; Davidson, M. Dopamine in schizophrenia: A review and reconceptualization. Am. J. Psychiatry 1991, 148, 1474–1486. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Huang, W.; Chen, Y.; Zhang, K.; Cao, Y.; Tu, J. Facile Synthesis of Graphene / Polypyrrole 3D Composite for a High-Sensitivity Non-Enzymatic Dopamine Detection. J. Appl. Polym. Sci. 2017, 134, 44840. [Google Scholar] [CrossRef]
- Roy, A.; Pickar, D.; De Jong, J.; Karoum, F.; Linnoila, M. Norepinephrine and its metabolites in cerebrospinal fluid, plasma, and urine: Relationship to hypothalamic-pituitary-adrenal axis function in depression. Arch. Gen. Psychiatry 1988, 45, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Okumura, T.; Nakajima, Y.; Matsuoka, M.; Takamatsu, T. Study of Salivary Catecholamines Using Fully Automated Column-Switching High-Performance Liquid Chromatography. J. Chromatogr. B Biomed. Appl. 1997, 694, 305–316. [Google Scholar] [CrossRef]
- Cao, Y.; Mcdermott, M.T. Femtomolar and Selective Dopamine Detection by a Gold Nanoparticle Enhanced Surface Plasmon Resonance Aptasensor. bioRxiv 2018, 1–24. [Google Scholar] [CrossRef]
- Yoshitake, T.; Yoshitake, S.; Fujino, K.; Nohta, H.; Yamaguchi, M.; Kehr, J. High-Sensitive Liquid Chromatographic Method for Determination of Neuronal Release of Serotonin, Noradrenaline and Dopamine Monitored by Microdialysis in the Rat Prefrontal Cortex. J. Neurosci. Methods 2004, 140, 163–168. [Google Scholar] [CrossRef]
- Carrera, V.; Sabater, E.; Vilanova, E.; Sogorb, M.A. A Simple and Rapid HPLC-MS Method for the Simultaneous Determination of Epinephrine, Norepinephrine, Dopamine and 5-Hydroxytryptamine: Application to the Secretion of Bovine Chromaffin Cell Cultures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 847, 88–94. [Google Scholar] [CrossRef]
- Muzzi, C.; Bertocci, E.; Terzuoli, L.; Porcelli, B.; Ciari, I.; Pagani, R.; Guerranti, R. Simultaneous Determination of Serum Concentrations of Levodopa, Dopamine, 3-O-Methyldopa and α-Methyldopa by HPLC. Biomed. Pharmacother. 2008, 62, 253–258. [Google Scholar] [CrossRef]
- Woolley, A.T.; Lao, K.; Glazer, A.N.; Mathies, R.A. Capillary Electrophoresis Chips with Integrated Electrochemical Detection. Anal. Chem. 1998, 70, 684–688. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Xie, H.; Fu, Z. Trivalent Copper Chelate-Luminol Chemiluminescence System for Highly Sensitive CE Detection of Dopamine in Biological Sample after Clean-up Using SPE. Electrophoresis 2012, 33, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, S.; Huang, J.; Ye, F. Quantum Dot-Enhanced Chemiluminescence Detection for Simultaneous Determination of Dopamine and Epinephrine by Capillary Electrophoresis. Talanta 2011, 85, 2650–2654. [Google Scholar] [CrossRef] [PubMed]
- Thabano, J.R.E.; Breadmore, M.C.; Hutchinson, J.P.; Johns, C.; Haddad, P.R. Silica Nanoparticle-Templated Methacrylic Acid Monoliths for in-Line Solid-Phase Extraction-Capillary Electrophoresis of Basic Analytes. J. Chromatogr. A 2009, 1216, 4933–4940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, B.; Lin, X. In-Situ FTIR Spectroelectrochemical Study of Dopamine at a Glassy Carbon Electrode in a Neutral Solution. Anal. Sci. 2002, 18, 931–933. [Google Scholar] [CrossRef]
- Abaidur, S.M.; Alothman, Z.A.; Alam, S.M.; Lee, S.H. Flow Injection-Chemiluminescence Determination of Dopamine Using Potassium Permanganate and Formaldehyde System. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 221–225. [Google Scholar] [CrossRef]
- Fritzen-Garcia, M.B.; Monteiro, F.F.; Cristofolini, T.; Acuña, J.J.S.; Zanetti-Ramos, B.G.; Oliveira, I.R.W.Z.; Soldi, V.; Pasa, A.A.; Creczynski-Pasa, T.B. Characterization of Horseradish Peroxidase Immobilized on PEGylated Polyurethane Nanoparticles and Its Application for Dopamine Detection. Sens. Actuators B Chem. 2013, 182, 264–272. [Google Scholar] [CrossRef]
- Liu, S.; Sun, W.; Hu, F. Graphene Nano Sheet-Fabricated Electrochemical Sensor for the Determination of Dopamine in the Presence of Ascorbic Acid Using Cetyltrimethylammonium Bromide as the Discriminating Agent. Sens. Actuators B Chem. 2012, 173, 497–504. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically Modified Electrodes for Electrochemical Detection of Dopamine in the Presence of Uric Acid and Ascorbic Acid: A Review. TrAC Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kim, K.-J.; Yoon, J.; Jo, J.; El-Said, W.A.; Choi, J.-W. Silver Nanoparticle Modified Electrode Covered by Graphene Oxide for the Enhanced Electrochemical Detection of Dopamine. Sensors 2017, 17, 2771. [Google Scholar] [CrossRef]
- Hows, M.E.P.; Lacroix, L.; Heidbreder, C.; Organ, A.J.; Shah, A.J. High-Performance Liquid Chromatography/Tandem Mass Spectrometric Assay for the Simultaneous Measurement of Dopamine, Norepinephrine, 5-Hydroxytryptamine and Cocaine in Biological Samples. J. Neurosci. Methods 2004, 138, 123–132. [Google Scholar] [CrossRef]
- Moini, M.; Schultz, C.L.; Mahmood, H. CE/Electrospray Ionization-MS Analysis of Underivatized D/L-Amino Acids and Several Small Neurotransmitters at Attomole Levels through the Use of 18-Crown-6-Tetracarboxylic Acid as a Complexation Reagent/Background Electrolyte. Anal. Chem. 2003, 75, 6282–6287. [Google Scholar] [CrossRef] [PubMed]
- Syslová, K.; Rambousek, L.; Kuzma, M.; Najmanová, V.; Bubeníková-Valešová, V.; Šlamberová, R.; Kačer, P. Monitoring of Dopamine and Its Metabolites in Brain Microdialysates: Method Combining Freeze-Drying with Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2011, 1218, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Reza Hormozi Nezhad, M.; Tashkhourian, J.; Khodaveisi, J.; Reza Khoshi, M. Simultaneous Colorimetric Determination of Dopamine and Ascorbic Acid Based on the Surface Plasmon Resonance Band of Colloidal Silver Nanoparticles Using Artificial Neural Networks. Anal. Methods 2010, 2, 1263–1269. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hui, Q.S.; Xu, L.X.; Jiang, J.G.; Sun, Y. Fluorimetric Determination of Dopamine in Pharmaceutical Products and Urine Using Ethylene Diamine as the Fluorigenic Reagent. Anal. Chim. Acta 2003, 497, 93–99. [Google Scholar] [CrossRef]
- Kruss, S.; Landry, M.P.; Vander Ende, E.; Lima, B.M.A.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter Detection Using Corona Phase Molecular Recognition on Fluorescent Single-Walled Carbon Nanotube Sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Kim, J. Fabrication of a Dopamine Sensor Based on Carboxyl Quantum Dots. J. Nanosci. Nanotechnol. 2015, 15, 7871–7875. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Salem, D.P.; Vuković, L.; Lima, B.; Vander Ende, E.; Boyden, E.S.; Strano, M.S. High-Resolution Imaging of Cellular Dopamine Efflux Using a Fluorescent Nanosensor Array. Proc. Natl. Acad. Sci. USA 2017, 114, 1789–1794. [Google Scholar] [CrossRef]
- Qi, H.; Peng, Y.; Gao, Q.; Zhang, C. Applications of Nanomaterials in Electrogenerated Chemiluminescence Biosensors. Sensors 2009, 9, 674–695. [Google Scholar] [CrossRef]
- Bu, Y.; Lee, S. Influence of Dopamine Concentration and Surface Coverage of Au Shell on the Optical Properties of Au, Ag, and Ag CoreAu Shell Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 3923–3931. [Google Scholar] [CrossRef]
- Bu, Y.; Lee, S.-W. Optical Properties of Dopamine Molecules with Silver Nanoparticles as Surface-Enhanced Raman Scattering (SERS) Substrates at Different PH Conditions. J. Nanosci. Nanotechnol. 2013, 13, 5992–5996. [Google Scholar] [CrossRef]
- Ranc, V.; Markova, Z.; Hajduch, M.; Prucek, R.; Kvitek, L.; Kaslik, J.; Safarova, K.; Zboril, R. Magnetically Assisted Surface-Enhanced Raman Scattering Selective Determination of Dopamine in an Artificial Cerebrospinal Fluid and a Mouse Striatum Using Fe3O4/Ag Nanocomposite. Anal. Chem. 2014, 86, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Choi, D.K.; Lee, K.J.; Choi, J.W. Surface-Enhanced Raman Spectroscopy Detection of Dopamine by DNA Targeting Amplification Assay in Parkisons’s Model. Biosens. Bioelectron. 2015, 67, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xia, M.; Liang, O.; Sun, K.; Cipriano, A.F.; Schroeder, T.; Liu, H.; Xie, Y.H. Label-Free SERS Selective Detection of Dopamine and Serotonin Using Graphene-Au Nanopyramid Heterostructure. Anal. Chem. 2015, 87, 10255–10261. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, C.; Nan, H.; Zhu, Q.; Qin, F.; Manohari, A.G.; Wei, M.; Zhu, Z.; Shi, Z.; Ni, Z. SERS-Active ZnO/Ag Hybrid WGM Microcavity for Ultrasensitive Dopamine Detection. Appl. Phys. Lett. 2016, 109. [Google Scholar] [CrossRef]
- Deftereos, N.T.; Calokerinos, A.C.; Efstathiou, C.E. Flow Injection Chemiluminometric Determination of Epinephrine, Norepinephrine, Dopamine and L-DOPA. Analyst 1993, 118, 627–632. [Google Scholar] [CrossRef]
- Dutta, P.; Pernites, R.B.; Danda, C.; Advincula, R.C. SPR Detection of Dopamine Using Cathodically Electropolymerized, Molecularly Imprinted Poly-p-Aminostyrene Thin Films. Macromol. Chem. Phys. 2011, 212, 2439–2451. [Google Scholar] [CrossRef]
- Jia, K.; Khaywah, M.Y.; Li, Y.; Bijeon, J.L.; Adam, P.M.; Déturche, R.; Guelorget, B.; François, M.; Louarn, G.; Ionescu, R.E. Strong Improvements of Localized Surface Plasmon Resonance Sensitivity by Using Au/Ag Bimetallic Nanostructures Modified with Polydopamine Films. ACS Appl. Mater. Interfaces 2014, 6, 219–227. [Google Scholar] [CrossRef]
- Sebők, D.; Csapó, E.; Preočanin, T.; Bohus, G.; Kallay, N.; Dékány, I. Adsorption of Ibuprofen and Dopamine on Functionalized Gold Using Surface Plasmon Resonance Spectroscopy at Solid-Liquid Interface. Croat. Chem. Acta 2013, 86, 287–295. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.-H.; Oh, B.-K.; Choi, J.-W. Localized Surface Plasmon Resonance-Based Label-Free Biosensor for Highly Sensitive Detection of Dopamine. J. Nanosci. Nanotechnol. 2014, 14, 5658–5661. [Google Scholar] [CrossRef]
- Su, R.; Pei, Z.; Huang, R.; Qi, W.; Wang, M.; Wang, L.; He, Z. Polydopamine-Assisted Fabrication of FiberOptic Localized Surface Plasmon Resonance Sensor Based on Gold Nanoparticles. Trans. Tianjin Univ. 2015, 21, 412–419. [Google Scholar] [CrossRef]
- Kamal Eddin, F.B.; Fen, Y.W. Recent Advances in Electrochemical and Optical Sensing of Dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W. Recent Development of SPR Spectroscopy as Potential Method for Diagnosis of Dengue Virus E-Protein. Sens. Rev. 2018, 38, 106–116. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a Graphene-Based Surface Plasmon Resonance Optical Sensor Chip for Potential Biomedical Application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of Adulterated Honey by Surface Plasmon Resonance Optical Sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Rashid, S.A.; Jamaludin, N.; Noor, A.S.M. Surface Plasmon Resonance Sensor Using Polypyrrole-Chitosan/Graphene Quantum Dots Layer for Detection of Sugar. Mater. Res. Express 2019, 6, 075028. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and Optical Properties of Chitosan–Poly(Amidoamine) Dendrimer Composite Thin Film for Potential Sensing Pb2+ Using an Optical Spectroscopy. Optik 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-Free Optical Spectroscopy for Characterizing Binding Properties of Highly Sensitive Nanocrystalline Cellulose-Graphene Oxide Based Nanocomposite towards Nickel Ion. Spectrochim. Acta A 2019, 212, 25–31. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M. Optical Studies of Graphene Oxide/Poly(Amidoamine) Dendrimer Composite Thin Film and Its Potential for Sensing Hg2+ Using Surface Plasmon Resonance Spectroscopy. Sens. Mater. 2019, 31, 1147. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of Surface Plasmon Resonance with Novel Valinomycin Doped Chitosan-Graphene Oxide Thin Film for Sensing Potassium Ion. Spectrochim. Acta A 2018, 191, 111–115. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Naseri, M.; Rashid, S.A. Polypyrrole-Chitosan/Nickel-Ferrite Nanoparticle Composite Layer for Detecting Heavy Metal Ions Using Surface Plasmon Resonance Technique. Opt. Laser Technol. 2017, 93, 216–223. [Google Scholar] [CrossRef]
- Alwahib, A.A.; Sadrolhosseini, A.R.; An’Amt, M.N.; Lim, H.N.; Yaacob, M.H.; Abu Bakar, M.H.; Ming, H.N.; Mahdi, M.A. Reduced Graphene Oxide/Maghemite Nanocomposite for Detection of Hydrocarbon Vapor Using Surface Plasmon Resonance. IEEE Photonics J. 2016, 8, 1–9. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of Surface Plasmon Resonance Sensor for Determining Zinc Ion Using Novel Active Nanolayers as Probe. Spectrochim. Acta A 2015, 134, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR Sensor Chip for Detection of Small Molecules Using Molecularly Imprinted Polymer with Embedded Gold Nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Akamatsu, K.; Nishiguchi, S.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. Composite of Au Nanoparticles and Molecularly Imprinted Polymer as a Sensing Material. Anal. Chem. 2004, 76, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Kumbhat, S.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Joshi, V.; Miura, N. Surface Plasmon Resonance Biosensor for Dopamine Using D3 Dopamine Receptor as a Biorecognition Molecule. Biosens. Bioelectron. 2007, 23, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh Kamali, K.; Pandikumar, A.; Sivaraman, G.; Lim, H.N.; Wren, S.P.; Sun, T.; Huang, N.M. Silver@graphene Oxide Nanocomposite-Based Optical Sensor Platform for Biomolecules. RSC Adv. 2015, 5, 17809–17816. [Google Scholar] [CrossRef]
- Rithesh Raj, D.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface Plasmon Resonance Based Fiber Optic Dopamine Sensor Using Green Synthesized Silver Nanoparticles. Sens. Actuators B Chem. 2016, 224, 600–606. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Thakur, G.; Kotsuchibashi, Y.; Naicker, S.; Narain, R.; Thundat, T. Rapid and Highly Sensitive Detection of Dopamine Using Conjugated Oxaborole-Based Polymer and Glycopolymer Systems. ACS Appl. Mater. Interfaces 2017, 9, 15225–15231. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.H.; Yang, H.; Park, C.S.; Lee, C.S.; Kwon, O.S.; Park, T.H.; Jang, J. Human Dopamine Receptor-Conjugated Multidimensional Conducting Polymer Nanofiber Membrane for Dopamine Detection. ACS Appl. Mater. Interfaces 2016, 8, 28897–28903. [Google Scholar] [CrossRef]
- Manaf, A.A.; Ghadiry, M.; Soltanian, R.; Ahmad, H.; Lai, C.K. Picomole Dopamine Detection Using Optical Chips. Plasmonics 2017, 12, 1505–1510. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, B.D. Surface Plasmon Resonance Based Highly Selective Fiber Optic Dopamine Sensor Fabricated Using Molecular Imprinted GNP/SnO2 Nanocomposite. J. Light. Technol. 2018, 36, 5956–5962. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, S.; Xu, J.; Li, Z.; Li, C.; Jing, Y.; Zhao, X.; Pan, J.; Zhang, C. and Man, B. Sensitive and Selective SPR Sensor Employing Gold-Supported Graphene Composite Film/D-Shaped Fiber for Dopamine Detection. J. Phys. D Appl. Phys. 2019, 29, 465705. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Xu, Z.; Chen, Y. Investigation of Dopamine Immobilized on Gold by Surface Plasmon Resonance. AIP Adv. 2019, 9, 035028. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Davis, J.J.; Luo, X. Ultrasensitive and Selective Voltammetric Aptasensor for Dopamine Based on a Conducting Polymer Nanocomposite Doped with Graphene Oxide. Microchim. Acta 2015, 182, 1123–1129. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cui, H.; Lai, C.Z.; Liu, L.J. Gold Nanoparticle-Catalyzed Luminol Chemiluminescence and Its Analytical Applications. Anal. Chem. 2005, 77, 3324–3329. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, J.Y.; Yan, Y.; Zhao, Y.S.; Yao, J. Electrogenerated Chemiluminescence of Metal-Organic Complex Nanowires: Reduced Graphene Oxide Enhancement and Biosensing Application. Adv. Mater. 2012, 24, 4745–4749. [Google Scholar] [CrossRef]
- Liu, X.; Hu, X.; Xie, Z.; Chen, P.; Sun, X.; Yan, J.; Zhou, S. In Situ Bifunctionalized Carbon Dots with Boronic Acid and Amino Groups for Ultrasensitive Dopamine Detection. Anal. Methods 2016, 8, 3236–3241. [Google Scholar] [CrossRef]
- Liang, W.; He, S.; Fang, J. Self-Assembly of J-Aggregate Nanotubes and Their Applications for Sensing Dopamine. Langmuir 2014, 30, 805–811. [Google Scholar] [CrossRef]
- Fang, X.; Ren, H.; Zhao, H.; Li, Z. Ultrasensitive Visual and Colorimetric Determination of Dopamine Based on the Prevention of Etching of Silver Nanoprisms by Chloride. Microchim. Acta 2017, 184, 415–421. [Google Scholar] [CrossRef]
- Tang, L.; Li, S.; Han, F.; Liu, L.; Xu, L.; Ma, W.; Kuang, H.; Li, A.; Wang, L.; Xu, C. SERS-Active Au@Ag Nanorod Dimers for Ultrasensitive Dopamine Detection. Biosens. Bioelectron. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Dong, J.X.; Li, N.B.; Luo, H.Q. The Formation of Zirconium Hexacyanoferrate(II) Nanoparticles and Their Application in the Highly Sensitive Determination of Dopamine Based on Enhanced Resonance Rayleigh Scattering. Anal. Methods 2013, 5, 5541–5548. [Google Scholar] [CrossRef]
- Qin, W.W.; Wang, S.P.; Li, J.; Peng, T.H.; Xu, Y.; Wang, K.; Shi, J.Y.; Fan, C.H.; Li, D. Visualizing Dopamine Released from Living Cells Using a Nanoplasmonic Probe. Nanoscale 2015, 7, 15070–15074. [Google Scholar] [CrossRef] [PubMed]
- Taghdiri, M.; Mohamadipour-taziyan, A. Application of Sephadex LH-20 for Microdetermination of Dopamine by Solid Phase Spectrophotometry. ISRN Pharm. 2012, 2012, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, C. High-Sensitive Sensor of Dopamine Based on Photoluminescence Quenching of Hierarchical CdS Spherical Aggregates. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Zeng, Z.; Cui, B.; Wang, Y.; Sun, C.; Zhao, X.; Cui, H. Dual Reaction-Based Multimodal Assay for Dopamine with High Sensitivity and Selectivity Using Functionalized Gold Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 16518–16524. [Google Scholar] [CrossRef]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A Photoelectrochemical Sensor for Ultrasensitive Dopamine Detection Based on Single-Layer NanoMoS2 Modified Gold Electrode. Sens. Actuators B Chem. 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-Time and Label-Free Bio-Sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar]
- Li, Z.Y.; Xia, Y. Metal Nanoparticles with Gain toward Single-Molecule Detection by Surface-Enhanced Raman Scattering. Nano Lett. 2010, 10, 243–249. [Google Scholar] [CrossRef]

| Material | LOD | Detection Range | References |
|---|---|---|---|
| MIP-Au electrode | 1 pM | - | [110] |
| DA-RC | 0.085 ng/mL | 0.085 ng/mL–700 ng/mL | [129] |
| DA antibodies/Au NPs/ITO | 1 nM | 0.001–100 µM | [113] |
| Ag@GO | 30 nM | 100 nM–2 µM | [130] |
| Ag NPs | 0.2 µM | 0.2–30 µM | [131] |
| Conjugated polymer P(NIPAAm149-st-MAAmBO19) and P(LAEMA21) | 1 nM | 1 nM–0.1 mM | [132] |
| Pt | 50 pM | 0.1 nM–32 µM | [134] |
| DAAPT-AuNPs | 200 fM | 100 µM–2 mM 200 fM–20 nM | [80] |
| Molecular Imprinted GNP/SnO2 Nanocomposite | 31 nM | 0–100 µM | [135] |
| Au/graphene/DBA D-POF | - | 0.1 nM–1 µM | [136] |
| Method | Lowest Detection Limit | References |
|---|---|---|
| EC | 78 fM | [138] |
| CL | 0.19 nM | [139] |
| ECL | 0.31 pM | [140] |
| Fluorescence | 0.1 pM | [141] |
| Spectrophotometry | 0.4 nM | [142] |
| Colorimetry | 0.16 nM | [143] |
| SERS | 0.006 pM | [144] |
| RRS | 0.392 ng/mL | [145] |
| PRRS | 0.1 pM | [146] |
| SPS | 1.7 µM | [147] |
| PL | 10 nM | [148] |
| Absorption | 1.2 nM | [149] |
| PEC | 2.3 pM | [150] |
| SPR | 200 fM | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamal Eddin, F.B.; Fen, Y.W. The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection. Molecules 2020, 25, 2769. https://doi.org/10.3390/molecules25122769
Kamal Eddin FB, Fen YW. The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection. Molecules. 2020; 25(12):2769. https://doi.org/10.3390/molecules25122769
Chicago/Turabian StyleKamal Eddin, Faten Bashar, and Yap Wing Fen. 2020. "The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection" Molecules 25, no. 12: 2769. https://doi.org/10.3390/molecules25122769
APA StyleKamal Eddin, F. B., & Fen, Y. W. (2020). The Principle of Nanomaterials Based Surface Plasmon Resonance Biosensors and Its Potential for Dopamine Detection. Molecules, 25(12), 2769. https://doi.org/10.3390/molecules25122769







