Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function
Abstract
1. Introduction
2. Results
2.1. Chrysoeriol Protected ARPE-19 Cells against H2O2-Induced Death
2.2. Chrysoeriol Suppressed H2O2-Induced Oxidative Stress in ARPE-19 Cells
2.3. Chrysoeriol Enhanced Mitochondrial Function by Upregulating Mitochondrion-Related Genes
2.4. Chrysoeriol Regulated Mitochondrial Process Proteins
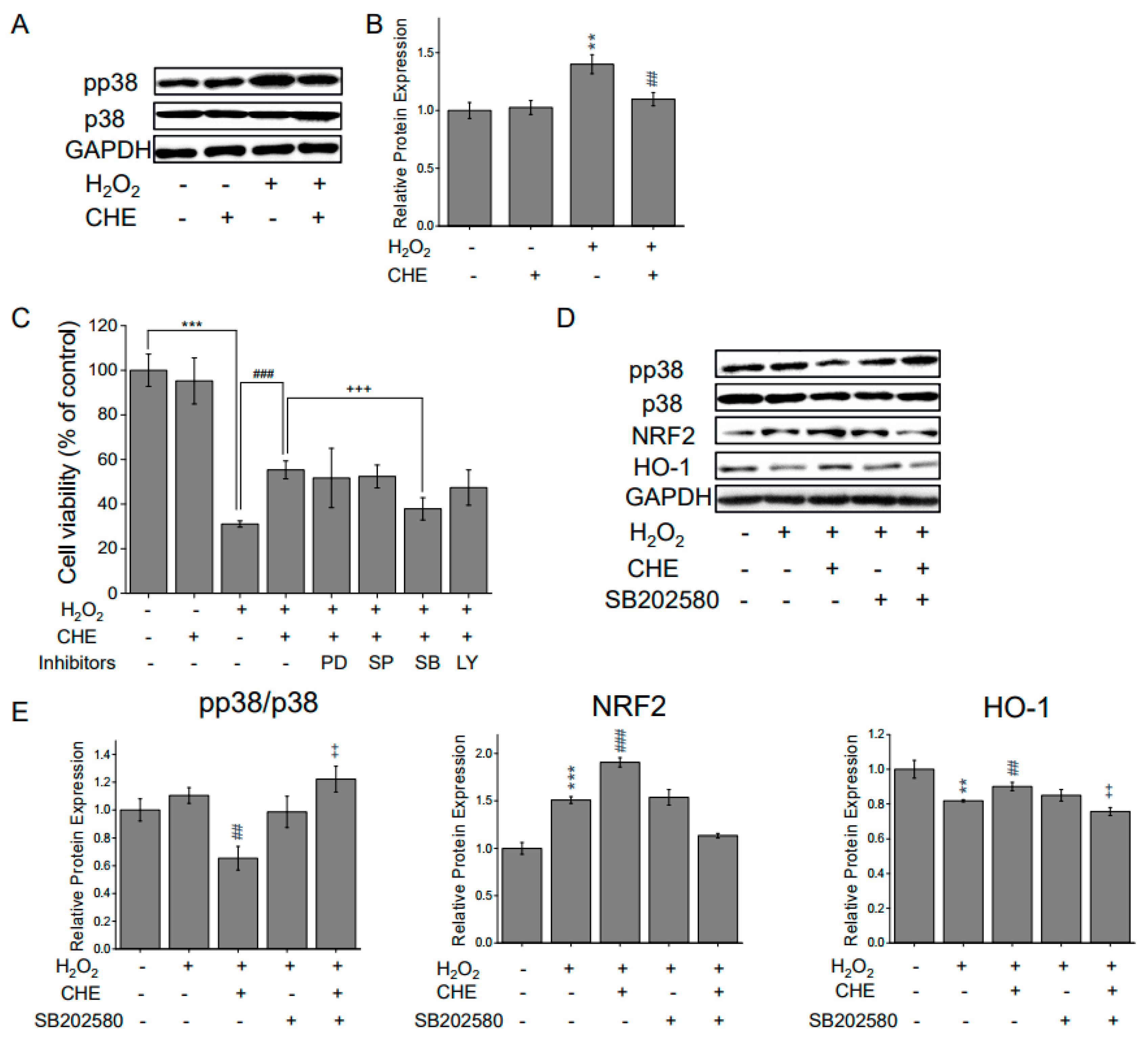
2.5. Chrysoeriol-Mediated Activation of p38 and Mitochondrial-Related Genes
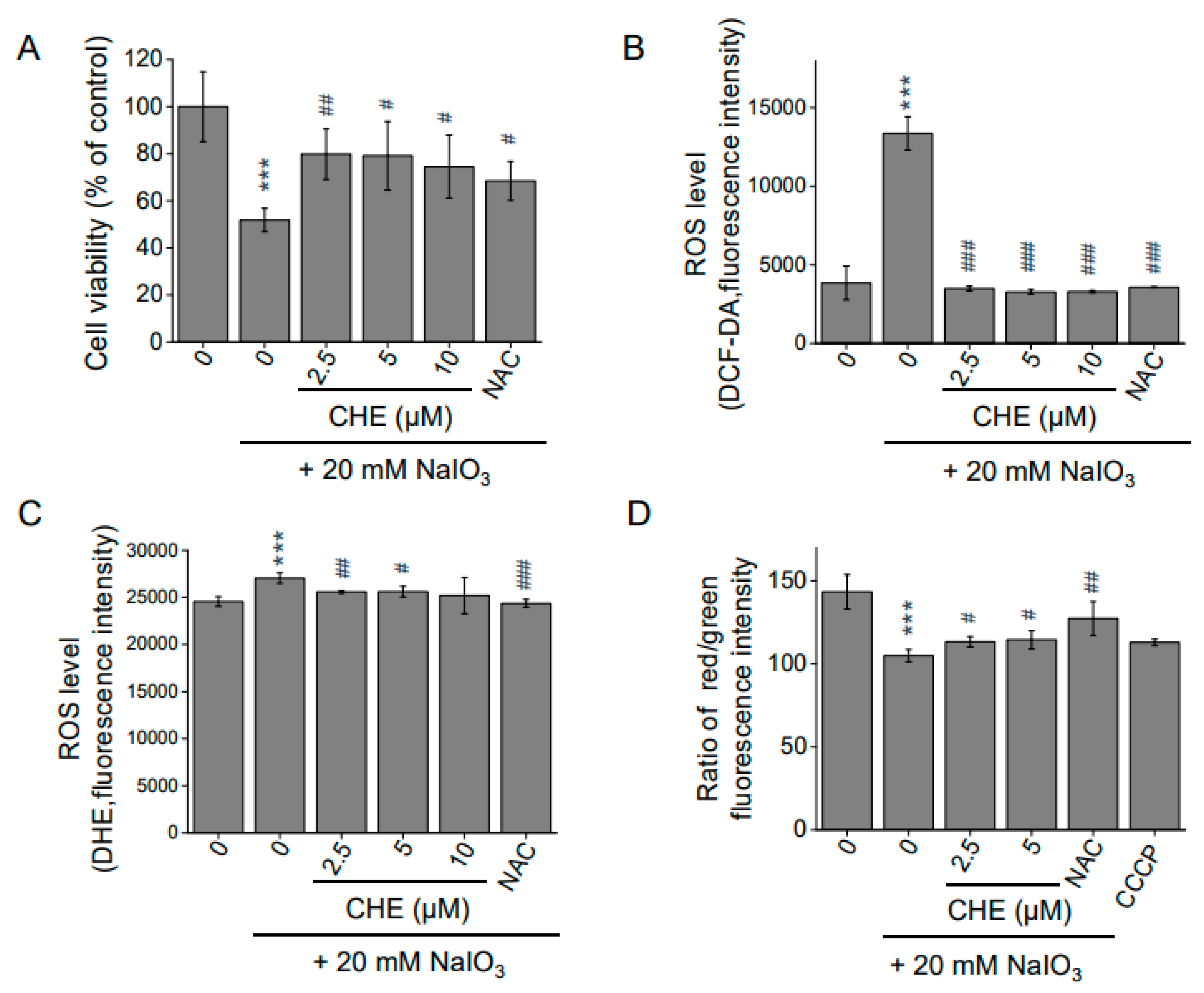
2.6. Chrysoeriol Protected ARPE-19 Cells from Sodium Iodate-Induced Oxidative Damage
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chrisoeriol
4.2.1. The Source of Chrysoeriol
4.2.2. Purification Protocol and Purity Analysis of Chrysoeriol
4.3. Cell Culture and Cell Viability Assay
4.4. ROS Measurement
4.5. Mitochondrial Membrane Potential (MMP) Assay
4.6. RNA Collection and Quantitative PCR
4.7. Western Blotting
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
References
- Miller, J.W.; Bagheri, S.; Vavvas, D.G. Advances in Age-related Macular Degeneration Understanding and Therapy. US Ophthalmic Rev. 2017, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Schmitz-Valckenberg, S.; Fleckenstein, M. Recent developments in the treatment of age-related macular degeneration. J. Clin. Investig. 2014, 124, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54. [Google Scholar] [CrossRef] [PubMed]
- Nebbioso, M.; Lambiase, A.; Cerini, A.; Limoli, P.G.; La Cava, M.; Greco, A. Therapeutic Approaches with Intravitreal Injections in Geographic Atrophy Secondary to Age-Related Macular Degeneration: Current Drugs and Potential Molecules. Int. J. Mol. Sci. 2019, 20, 1693. [Google Scholar] [CrossRef]
- Hernández-Zimbrón, L.F.; Zamora-Alvarado, R.; Ochoa-De la Paz, L.; Velez-Montoya, R.; Zenteno, E.; Gulias-Cañizo, R.; Quiroz-Mercado, H.; Gonzalez-Salinas, R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Hollyfield, J.G.; Bonilha, V.L.; Rayborn, M.E.; Yang, X.; Shadrach, K.G.; Lu, L.; Ufret, R.L.; Salomon, R.G.; Perez, V.L. Oxidative damageinduced inflammation initiates age-related macular degeneration. Nat. Med. 2008, 14, 194–198. [Google Scholar] [CrossRef]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Viiri, J.; Kaarniranta, K.; Błasiak, J. Mitochondrial quality control in AMD: Does mitophagy play a pivotal role? Cell. Mol. Life Sci. 2018, 75, 2991–3008. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.I.; Kumar, M.S.; Unnikrishnan, M.K.; Mohan, H. Effect of O-glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorganic Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Poór, M. Inhibition of xanthine oxidase-catalyzed xanthine and 6-mercaptopurine oxidation by flavonoid aglycones and some of their conjugates. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-directed analysis for the antioxidant compound in Salvia verticillata. Iran. J. Pharm. Res. 2016, 15, 241–246. [Google Scholar]
- Choi, D.Y.; Jeong, Y.L.; Kim, M.R.; Woo, E.R.; Yoon, G.K.; Keon, W.K. Chrysoeriol potently inhibits the induction of nitric oxide synthase by blocking AP-1 activation. J. Biomed. Sci. 2005, 12, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ikejima, T.; Li, L.; Wu, R.; Yuan, X.; Zhao, J.; Wang, Y.; Peng, S. Impairment of mitochondrial biogenesis and dynamics involved in isoniazid-induced apoptosis of HepG2 cells was alleviated by p38 MAPK pathway. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Liu, Z.; Song, X.D.; Xin, Y.; Wang, X.J.; Yu, H.; Bai, Y.Y.; Liu, J.H.; Zhang, C.N.; Hui, R.T. Protective effect of chrysoeriol against doxorubicin-induced cardiotoxicity in vitro. Chin. Med. J. (Engl). 2009, 122, 2652–2656. [Google Scholar]
- Min, D.Y.; Jung, E.; Ahn, S.S.; Lee, Y.H.; Lim, Y.; Shin, S.Y. Chrysoeriol prevents TNFα-induced Cyp19 gene expression via Egr-1 downregulation in Mcf7 breast cancer cells. Int. J. Mol. Sci. 2020, 21, 7523. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, Z.; Min, Q.; Palida, A.; Zhang, Y.Y.; Tang, R.; Chen, L.; Li, H. 8-Chrysoeriol, as a potential BCL-2 inhibitor triggers apoptosis of SW1990 pancreatic cancer cells. Bioorg. Chem. 2018, 77, 478–484. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, Y.S.; Choi, E.M. Chrysoeriol isolated from Eurya cilliata leaves protects MC3T3-E1 cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. J. Appl. Toxicol. 2010, 30, 666–673. [Google Scholar] [CrossRef]
- Abo-Qotb, S.M.S.; Hassanein, A.M.M.; Desoukey, S.Y.; Wanas, A.S.; Tawfik, H.M.; Orabi, M.A.A. In vivo anti-inflammatory and hepatoprotective activities of Orobanche crenata (Forssk.) aerial parts in relation to its phytomolecules. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 antioxidant transcription factor in age-related macular degeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2016; Volume 854, pp. 67–72. [Google Scholar]
- Bellezza, I. Oxidative stress in age-related macular degeneration: NRF2 as therapeutic target. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Vera, J.; Wolkenhauer, O. The systems biology of mitochondrial fission and fusion and implications for disease and aging. Biogerontology 2014, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kowald, A.; Klipp, E. Mathematical models of mitochondrial aging and dynamics. In Progress in Molecular Biology and Translational Science, 1st ed.; Elsevier B.V: Amsterdam, The Netherlands, 2014; Volume 127, pp. 63–92. [Google Scholar]
- Bereiter-Hahn, J. Mitochondrial dynamics in aging and disease. In Progress in Molecular Biology and Translational Science, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; Volume 127, pp. 93–131. [Google Scholar]
- Gui, C.; Ren, Y.; Chen, J.; Wu, X.; Mao, K.; Li, H.; Yu, H.; Zou, F.; Li, W. p38 MAPK-DRP1 signaling is involved in mitochondrial dysfunction and cell death in mutant A53T α-synuclein model of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2020, 388. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, L.; Wang, G.; Niu, W.; He, Z.; Ding, L.; Jia, J. Suppression of mitochondrial fission in experimental cerebral ischemia: The potential neuroprotective target of p38 MAPK inhibition. Neurochem. Int. 2015, 90, 1–8. [Google Scholar] [CrossRef]
- Debattisti, V.; Gerencser, A.A.; Saotome, M.; Das, S.; Hajnóczky, G. ROS Control Mitochondrial Motility through p38 and the Motor Adaptor Miro/Trak. Cell Rep. 2017, 21, 1667–1680. [Google Scholar] [CrossRef]
- Mazumder, S.; De, R.; Debsharma, S.; Bindu, S.; Maity, P.; Sarkar, S.; Saha, S.J.; Siddiqui, A.A.; Banerjee, C.; Nag, S.; et al. Indomethacin impairs mitochondrial dynamics by activating the PKCζ-p38-DRP1 pathway and inducing apoptosis in gastric cancer and normal mucosal cells. J. Biol. Chem. 2019, 294, 8238–8258. [Google Scholar] [CrossRef]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26. [Google Scholar] [CrossRef]
- Xiao, J.; Yao, J.; Jia, L.; Lin, C.; Zacks, D.N. Protective effect of Met12, a small peptide inhibitor of fas, on the retinal pigment epithelium and photoreceptor after sodium iodate injury. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1801–1810. [Google Scholar] [CrossRef]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular Anatomy of the Developing Human Retina. Dev. Cell 2017, 43, 763–779.e4. [Google Scholar] [CrossRef]
- Machalińska, A.; Kawa, M.P.; Pius-Sadowska, E.; Rogińska, D.; Kłos, P.; Baumert, B.; Wiszniewska, B.; Machaliński, B. Endogenous regeneration of damaged retinal pigment epithelium following low dose sodium iodate administration: An insight into the role of glial cells in retinal repair. Exp. Eye Res. 2013, 112, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin. Experiment. Ophthalmol. 2018, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye. Am. J. Ther. 2016, 23, 98–117. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Hu, H.; Hao, L.; Tang, C.; Zhu, Y.; Jiang, Q.; Yao, J. Activation of KGFR-Akt-mTOR-Nrf2 signaling protects human retinal pigment epithelium cells from Ultra-violet. Biochem. Biophys. Res. Commun. 2018, 495, 2171–2177. [Google Scholar] [CrossRef]
- Felszeghy, S.; Viiri, J.; Paterno, J.J.; Hyttinen, J.M.; Koskela, A.; Chen, M.; Leinonen, H.; Tanila, H.; Kivinen, N.; Koistinen, A.; et al. Loss of NRF-2 and PGC-1α genes leads to retinal pigment epithelium damage resembling dry age-related macular degeneration. Redox Biol. 2018, 20, 1–12. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Chang, S.-F.; Chau, S.-F.; Chiu, S.-C. The Protective Effect of Hispidin against Hydrogen Peroxide-Induced Oxidative Stress in ARPE-19 Cells via Nrf2 Signaling Pathway. Biomolecules 2019, 9, 380. [Google Scholar] [CrossRef]
- Hu, X.; Liang, Y.; Zhao, B.; Wang, Y. Thymoquinone protects human retinal pigment epithelial cells against hydrogen peroxide induced oxidative stress and apoptosis. J. Cell. Biochem. 2019, 120, 4514–4522. [Google Scholar] [CrossRef]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar]
- Zhao, H.; Wang, R.; Ye, M.; Zhang, L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2018, 936–944. [Google Scholar]
- Barot, M.; Gokulgandhi, M.R.; Mitra, A.K. Mitochondrial Dysfunction in Retinal Diseases. Curr. Eye Res. 2011, 36, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J.; Kaarniranta, K.; Pawlowska, E.; Szczepanska, J.; Jablkowska, A.; Blasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Ploumi, C.; Daskalaki, I.; Tavernarakis, N. Mitochondrial biogenesis and clearance: A balancing act. FEBS J. 2017, 284, 183–195. [Google Scholar] [CrossRef]
- Ito, Y.A.; Di Polo, A. Mitochondrial dynamics, transport, and quality control: A bottleneck for retinal ganglion cell viability in optic neuropathies. Mitochondrion 2017, 36, 186–192. [Google Scholar] [CrossRef]
- McBride, H.; Scorrano, L. Mitochondrial dynamics and physiology. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 148–149. [Google Scholar] [CrossRef]
- Kotiadis, V.N.; Duchen, M.R.; Osellame, L.D. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1254–1265. [Google Scholar] [CrossRef]
- Stotland, A.; Gottlieb, R.A. Mitochondrial quality control: Easy come, easy go. Biochim. Biophys. Acta. Mol. Cell Res. 2015, 1853, 2802–2811. [Google Scholar] [CrossRef]
- McBride, H.; Soubannier, V. Mitochondrial Function: OMA1 and OPA1, the Grandmasters of Mitochondrial Health. Curr. Biol. 2010, 20, 274–276. [Google Scholar] [CrossRef]
- MacVicar, T.; Langer, T. OPA1 processing in cell death and disease—The long and short of it. J. Cell Sci. 2016, 129, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive oxygen species and mitochondrial dynamics: The yin and yang of mitochondrial dysfunction and cancer progression. Antioxidants 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Latimer, H.R.; Veal, E.A. Peroxiredoxins in regulation of MAPK signalling pathways; sensors and barriers to signal transduction. Mol. Cells 2016, 39, 40–45. [Google Scholar] [PubMed]
- Serras, F. The benefits of oxidative stress for tissue repair and regeneration. Fly (Austin). 2016, 10, 128–133. [Google Scholar] [CrossRef]
- Miranda, S.; González-Rodríguez, Á.; García-Ramírez, M.; Revuelta-Cervantes, J.; Hernández, C.; Simó, R.; Valverde, Á.M. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J. Cell. Physiol. 2012, 227, 2352–2362. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Lee, M.H.; Kim, H.T.; Seo, W.D.; Kim, J.Y.; Baek, I.Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef]
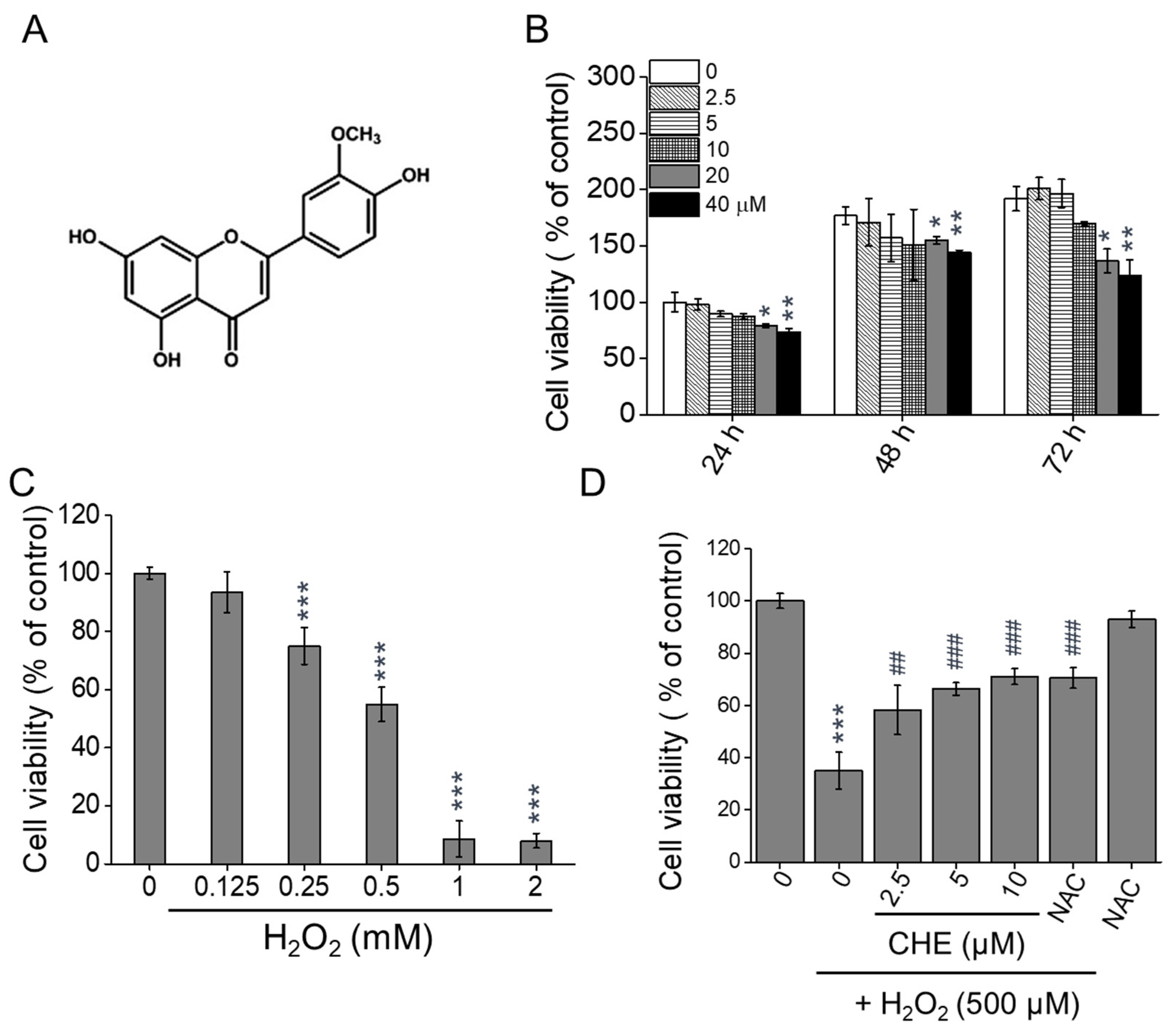
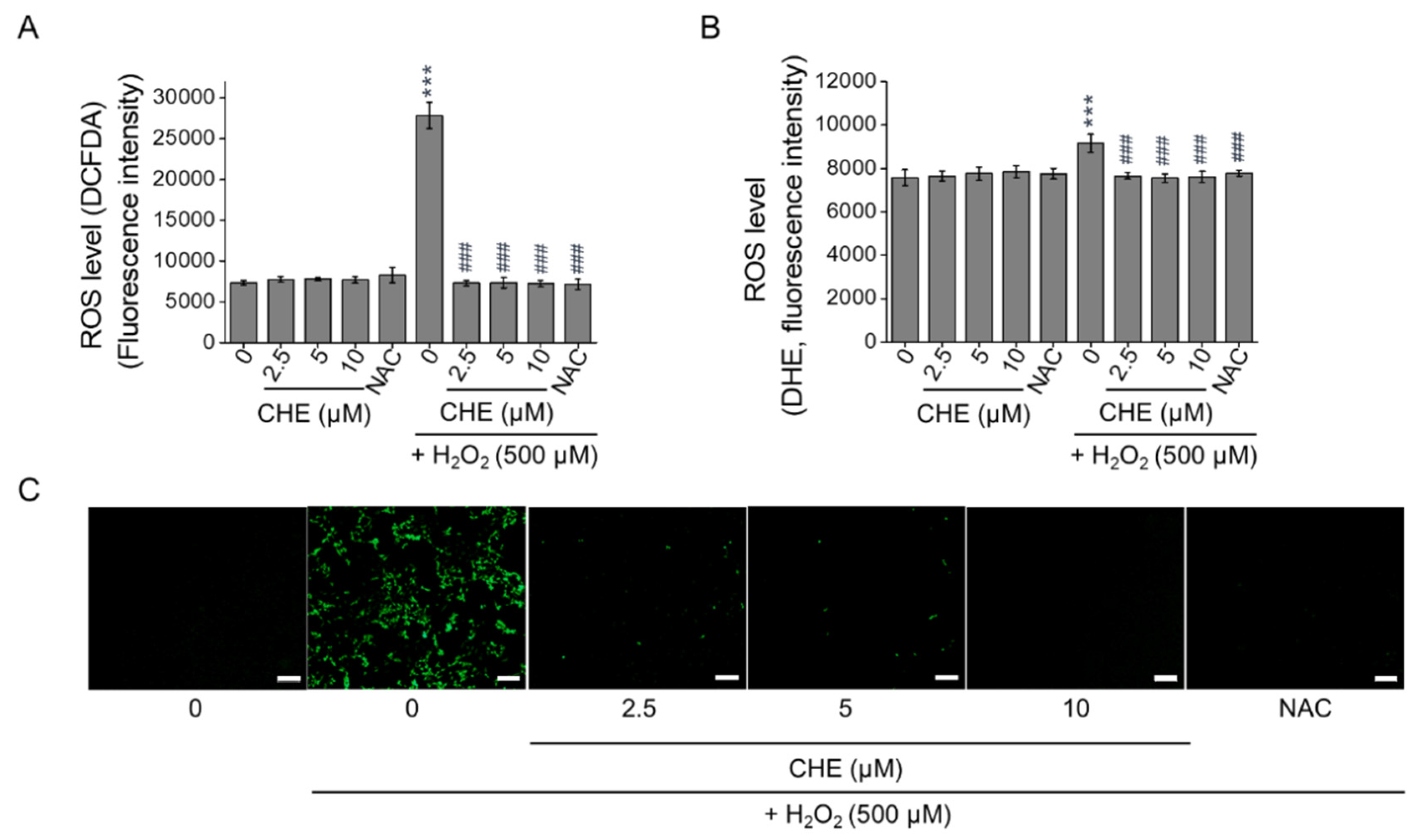

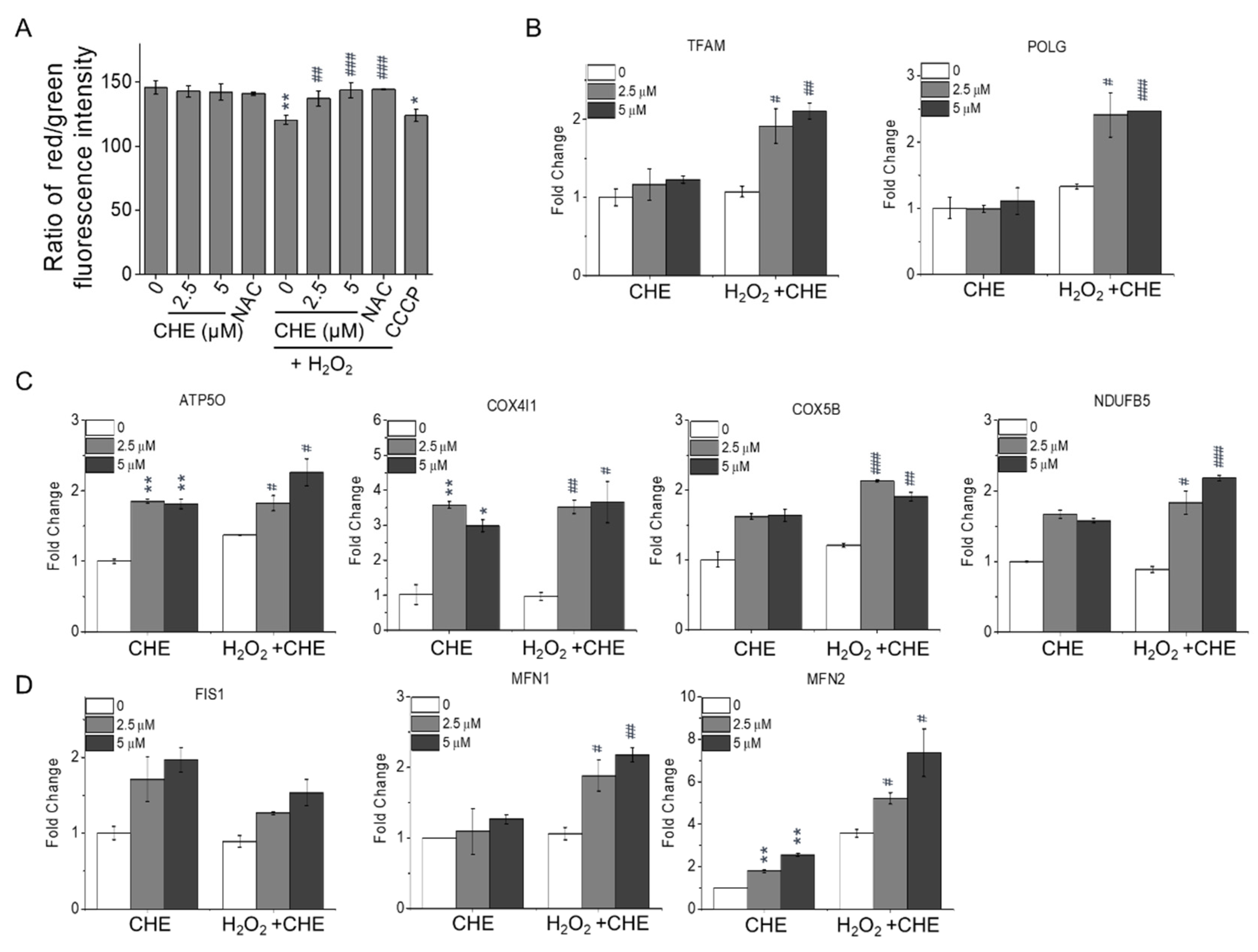
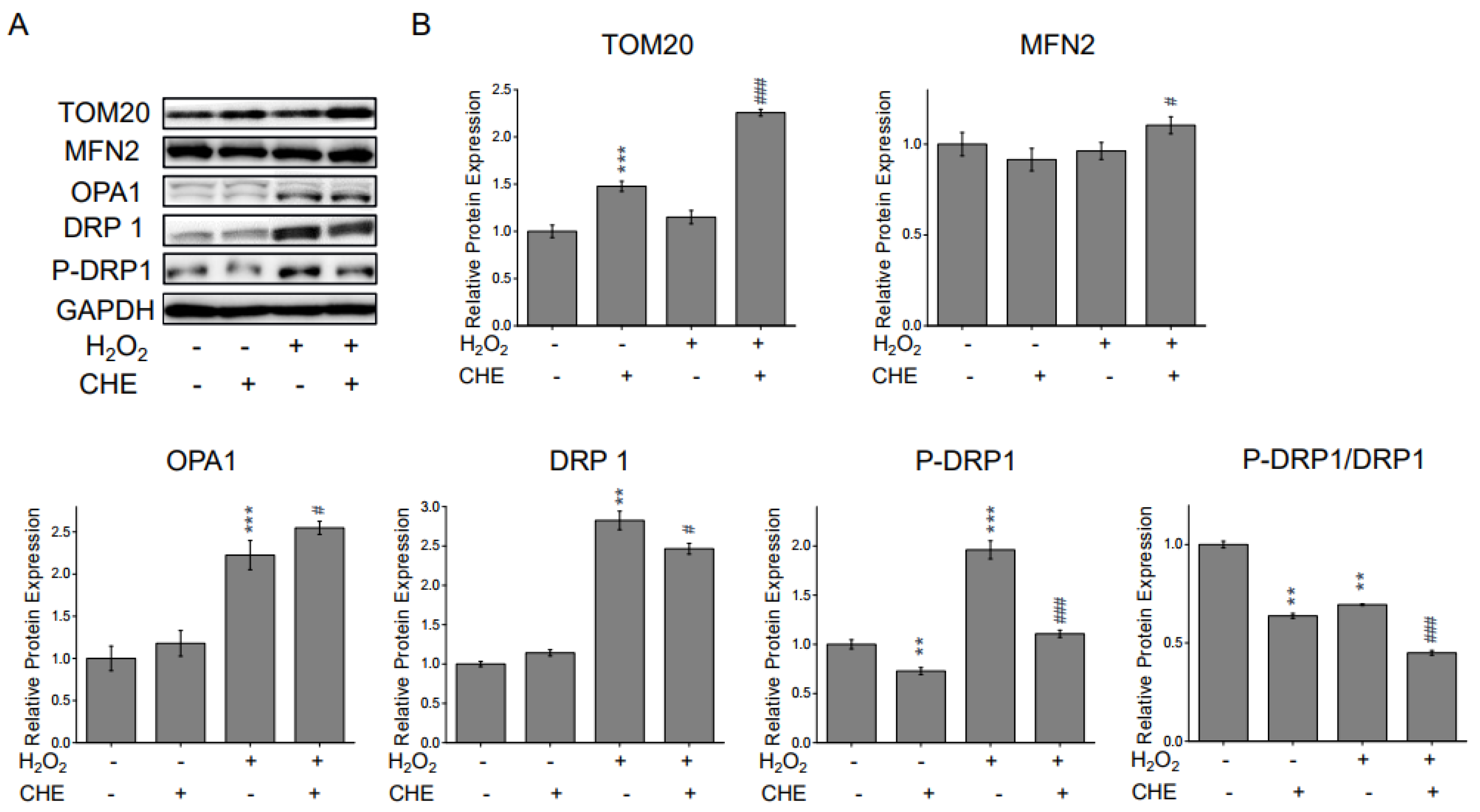
| Target Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| ATP5O | CGCTATGCCACAGCTCTTTA | AAGGCAGAAACGACTCCTTG |
| COX4I1 | GGCATTGAAGGAGAAGGAGA | TCATGTCCAGCATCCTCTTG |
| COX5B | GAGGTGGTGTTCCCACTGAT | CAGACGACGCTGGTATTGTC |
| NDUFB5 | CTTCCTCACTCGTGGCTTTC | TCTGGGACATAGCCTTCTGG |
| FIS1 | GACATCCGTAAAGGCATCGT | ACAGCAAGTCCGATGAGTCC |
| MFN1 | TGCCCTCTTGAGAGATGACC | TCTTTCCATGTGCTGTCTGC |
| MFN2 | ATGCATCCCCACTTAAGCAC | GCAGAACTTTGTCCCAGAGC |
| TFAM | TAAGACTGCAAGCAGCGAAG | TTCTCAGTTTCCCAGGTGCT |
| POLG | TGCAGTGAGGAGGAGGAGTT | CCCAGGTAAGTGCCATGAGT |
| SOD1 | GAAGGTGTGGGGAAGCATTA | CTTTGCCCAAGTCATCTGCT |
| SOD2 | AAACCTCAGCCCTAACGGTG | GCCTGTTGTTCCTTGCAGTG |
| CAT | GATAGCCTTCGACCCAAGCA | AGAAGGCTGTTGTTCCGGAG |
| GPX1 | AGTCGGTGTATGCCTTCTCG | CAAACTGGTTGCACGGGAAG |
| HO-1 | AGTCTTCGCCCCTGTCTACT | GCTTGAACTTGGTGGCACTG |
| NQO-1 | AAAGGACCCTTCCGGAGTAA | CGTTTCTTCCATCCTTCCAG |
| NRF2 | GCGACGGAAAGAGTATGAGC | ACGTAGCCGAAGAAACCTCA |
| GAPDH | ACCCAGAAGACTGTGGATGG | TTCTAGACGGCAGGTCAGGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Kwon, S.Y.; Woo, S.-Y.; Seo, W.D.; Kim, D.Y. Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function. Molecules 2021, 26, 313. https://doi.org/10.3390/molecules26020313
Kim MH, Kwon SY, Woo S-Y, Seo WD, Kim DY. Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function. Molecules. 2021; 26(2):313. https://doi.org/10.3390/molecules26020313
Chicago/Turabian StyleKim, Myung Hee, So Yeon Kwon, So-Yeun Woo, Woo Duck Seo, and Dae Yu Kim. 2021. "Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function" Molecules 26, no. 2: 313. https://doi.org/10.3390/molecules26020313
APA StyleKim, M. H., Kwon, S. Y., Woo, S.-Y., Seo, W. D., & Kim, D. Y. (2021). Antioxidative Effects of Chrysoeriol via Activation of the Nrf2 Signaling Pathway and Modulation of Mitochondrial Function. Molecules, 26(2), 313. https://doi.org/10.3390/molecules26020313





