Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution
Abstract
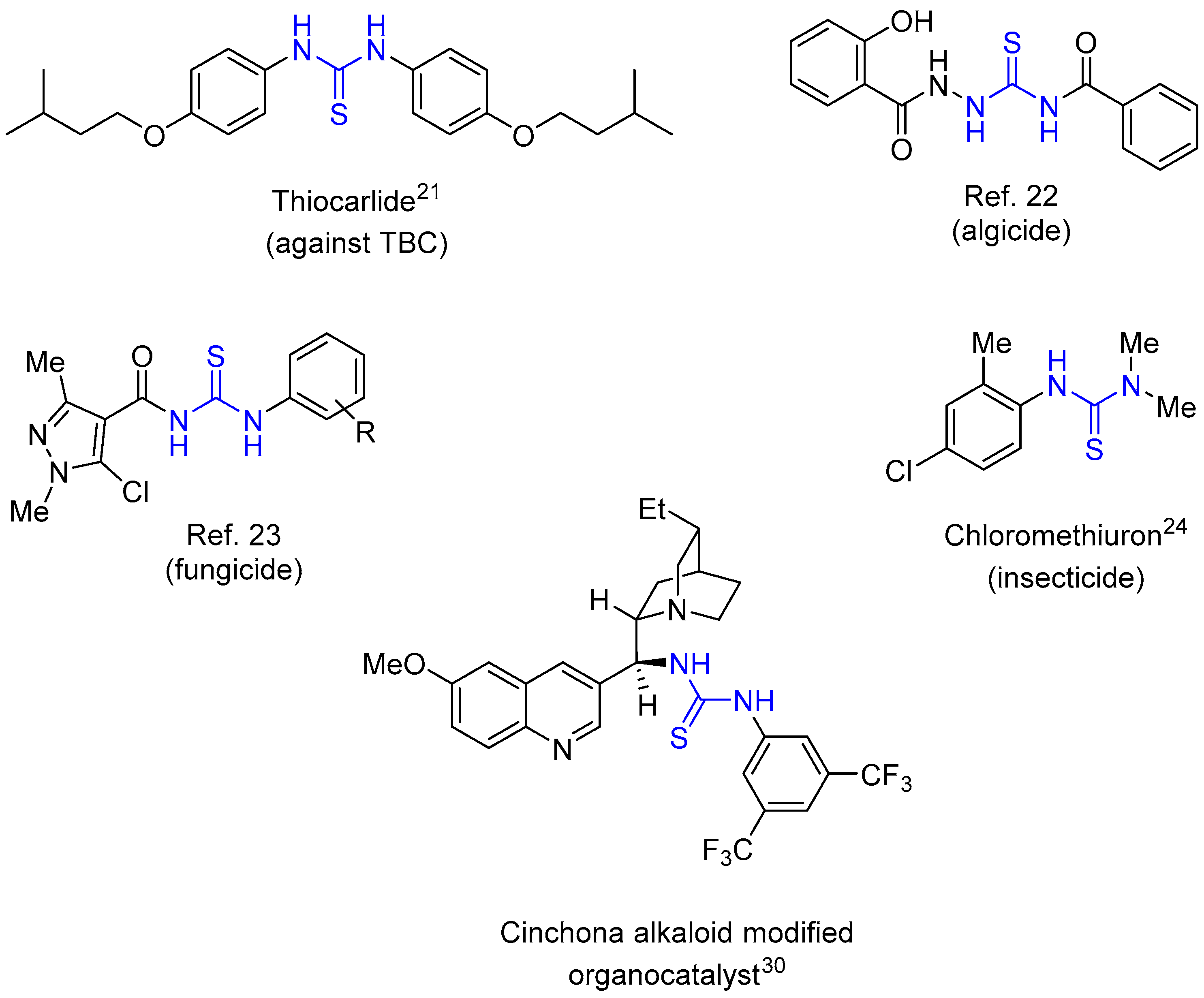
1. Introduction
2. Results
3. Materials and Methods
3.1. General
3.2. General Procedure for the Preparation of the Aqueous Solution of Polysulfide Anions
3.3. General Procedure for the CF Synthesis of Thioureas 11–16
3.4. General Procedure for the CF Synthesis of Thioureas 26–36
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akwi, F.M.; Watts, P. Continuous flow chemistry: Where are we now? Recent applications, challenges and limitations. Chem. Commun. 2018, 54, 13894–13928. [Google Scholar] [CrossRef] [PubMed]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Gilmore, K. The Hitchhiker’s Guide to Flow Chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.; Stevens, C. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology-A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef] [PubMed]
- Cantillo, D.; Sheibani, H.; Kappe, C.O. Flash Flow Pyrolysis: Mimicking Flash Vacuum Pyrolysis in a High-Temperature/High-Pressure Liquid-Phase Microreactor Environment. J. Org. Chem. 2012, 77, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Calmanti, R.; Galvan, M.; Amadio, E.; Perosa, A.; Selva, M. High-Temperature Batch and Continuous-Flow Transesterification of Alkyl and Enol Esters with Glycerol and Its Acetal Derivatives. ACS Sustain. Chem. Eng. 2018, 6, 3964–3973. [Google Scholar] [CrossRef]
- Adeyemi, A.; Bergman, J.; Brånalt, J.; Sävmarker, J.; Larhed, M. Continuous Flow Synthesis under High-Temperature/High-Pressure Conditions Using a Resistively Heated Flow Reactor. Org. Process. Res. Dev. 2017, 21, 947–955. [Google Scholar] [CrossRef]
- De Angelis, S.; Celestini, P.; Purgatorio, R.; DeGennaro, L.; Rebuzzini, G.; Luisi, R.; Carlucci, C. Development of a continuous flow synthesis of propranolol: Tackling a competitive side reaction. J. Flow Chem. 2019, 9, 231–236. [Google Scholar] [CrossRef]
- Ollivier, N.; Toupy, T.; Hartkoorn, R.C.; Desmet, R.; Monbaliu, J.-C.M.; Melnyk, O. Accelerated microfluidic native chemical ligation at difficult amino acids toward cyclic peptides. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Tadele, K.; Verma, S.; Nadagouda, M.N.; Gonzalez, M.A.; Varma, R.S. A rapid flow strategy for the oxidative cyanation of secondary and tertiary amines via C-H activation. Sci. Rep. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Wegner, J.; Ceylan, S.; Kirschning, A. Flow Chemistry—A Key Enabling Technology for (Multistep) Organic Synthesis. Adv. Synth. Catal. 2012, 354, 17–57. [Google Scholar] [CrossRef]
- Porta, R.; Benaglia, M.; Puglisi, A. Flow Chemistry: Recent Developments in the Synthesis of Pharmaceutical Products. Org. Process. Res. Dev. 2016, 20, 2–25. [Google Scholar] [CrossRef]
- Snead, D.R.; Jamison, T.F. A Three-Minute Synthesis and Purification of Ibuprofen: Pushing the Limits of Continuous-Flow Processing. Angew. Chem. Int. Ed. 2015, 54, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Coley, C.W.; Thomas, D.A.; Lummiss, J.A.M.; Jaworski, J.N.; Breen, C.P.; Schultz, V.; Hart, T.; Fishman, J.S.; Rogers, L.; Gao, H.; et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 2019, 365, eaax1566. [Google Scholar] [CrossRef]
- Bédard, A.-C.; Adamo, A.; Aroh, K.C.; Russell, M.G.; Bedermann, A.A.; Torosian, J.; Yue, B.; Jensen, K.F.; Jamison, T.F. Reconfigurable system for automated optimization of diverse chemical reactions. Science 2018, 361, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Cao, W.; Dai, F.; Hu, R.; Tang, B.Z. Economic Sulfur Conversion to Functional Polythioamides through Catalyst-Free Multicomponent Polymerizations of Sulfur, Acids, and Amines. J. Am. Chem. Soc. 2019, 142, 978–986. [Google Scholar] [CrossRef]
- Chen, L.; Xia, P.; Du, T.; Deng, Y.; Xiao, Y. Catalyst-Free One-Pot Synthesis of Unsymmetrical Five- and Six-Membered Sulfur-Annulated Heterocyclic Perylene Diimides for Electron-Transporting Property. Org. Lett. 2019, 21, 5529–5532. [Google Scholar] [CrossRef]
- Phetsuksiri, B.; Jackson, M.; Scherman, H.; McNeil, M.; Besra, G.S.; Baulard, A.; Slayden, A.R.; DeBarber, A.E.; Barry, C.E., III; Baird, M.S.; et al. Unique Mechanism of Action of the Thiourea Drug Isoxyl onMycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 53123–53130. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wei, L.; Hong, Z.; Rao, L.; Ren, Y.; Wan, J.; Feng, L. Design, synthesis and algicides activities of thiourea derivatives as the novel scaffold aldolase inhibitors. Bioorg. Med. Chem. 2019, 27, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shi, Q.; Chen, Z.; He, M.; Jin, L.; Pan, T. Synthesis and Bioactivity of Pyrazole Acyl Thiourea Derivatives. Molecules 2012, 17, 5139–5150. [Google Scholar] [CrossRef] [PubMed]
- Worthing, C.R.; Hance, R.J. The Pesticide Manual: A World Compendium, 9th ed.; British Crop Protection Council: Surrey, UK, 1991; ISBN 9780948404429. [Google Scholar]
- Biswas, K.; Greaney, M.F. Insertion of Arynes into Thioureas: A New Amidine Synthesis. Org. Lett. 2011, 13, 4946–4949. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, H.; Wu, W.; Chen, H.-J.; Jiang, H. Metal-Free Synthesis of 2-Aminobenzothiazoles via Aerobic Oxidative Cyclization/Dehydrogenation of Cyclohexanones and Thioureas. Org. Lett. 2013, 15, 2604–2607. [Google Scholar] [CrossRef]
- Batey, R.A.; Powell, D.A. A general synthetic method for the formation of substituted 5-aminotetrazoles from thioureas: A strategy for diversity amplification. Org. Lett. 2000, 2, 3237–3240. [Google Scholar] [CrossRef]
- Ghodse, S.M.; Telvekar, V.N. Synthesis of 2-aminothiazole derivatives from easily available thiourea and alkyl/aryl ketones using aqueous NaICl2. Tetrahedron Lett. 2015, 56, 472–474. [Google Scholar] [CrossRef]
- Maddani, M.R.; Prabhu, K.R. A Concise Synthesis of Substituted Thiourea Derivatives in Aqueous Medium. J. Org. Chem. 2010, 75, 2327–2332. [Google Scholar] [CrossRef]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly Enantioselective Conjugate Addition of Nitromethane to Chalcones Using Bifunctional Cinchona Organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef]
- Madarasz, A.; Dósa, Z.; Varga, S.; Soós, T.; Csampai, A.; Pápai, I. Thiourea Derivatives as Brønsted Acid Organocatalysts. ACS Catal. 2016, 6, 4379–4387. [Google Scholar] [CrossRef]
- Schreiner, P.R. Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem. Soc. Rev. 2003, 32, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef] [PubMed]
- Nickisch, R.; Gabrielsen, S.M.; Meier, M.A. Novel Access to Known and Unknown Thiourea Catalyst via a Multicomponent-Reaction Approach. Chem. Select 2020, 5, 11915–11920. [Google Scholar] [CrossRef]
- Nguyen, T.B. Recent Advances in Organic Reactions Involving Elemental Sulfur. Adv. Synth. Catal. 2017, 359, 1066–1130. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Retailleau, P.; Al-Mourabit, A. Elemental Sulfur Disproportionation in the Redox Condensation Reaction betweeno-Halonitrobenzenes and Benzylamines. Angew. Chem. Int. Ed. 2014, 53, 13808–13812. [Google Scholar] [CrossRef]
- Xie, H.; Cai, J.; Wang, Z.; Huang, H.; Deng, G.-J. A Three-Component Approach to 3,5-Diaryl-1,2,4-thiadiazoles under Transition-Metal-Free Conditions. Org. Lett. 2016, 18, 2196–2199. [Google Scholar] [CrossRef]
- Liao, Y.; Peng, Y.; Qi, H.; Deng, G.-J.; Gong, H.; Li, C. Palladium-catalyzed benzothieno[2,3-b]indole formation via dehydrative–dehydrogenative double C–H sulfuration using sulfur powder, indoles and cyclohexanones. Chem. Commun. 2015, 51, 1031–1034. [Google Scholar] [CrossRef]
- Chen, J.; Li, G.; Xie, Y.; Liao, Y.; Xiao, F.; Deng, G.-J. Four-Component Approach to N-Substituted Phenothiazines under Transition-Metal-Free Conditions. Org. Lett. 2015, 17, 5870–5873. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, P.; Xu, F.; Deng, Z.; Long, L.; Luo, G.; Ye, M. Metal-Free Aminothiation of Alkynes: Three-Component Tandem Annulation toward Indolizine Thiones from 2-Alkylpyridines, Ynals, and Elemental Sulfur. J. Org. Chem. 2019, 84, 12639–12647. [Google Scholar] [CrossRef]
- Kozlov, M.; Komkov, A.; Losev, T.; Tyurin, A.; Dmitrenok, A.; Zavarzin, I.; Volkova, Y.A. Flexible Synthesis of Phosphoryl-Substituted Imidazolines, Tetrahydropyrimidines, and Thioamides by Sulfur-Mediated Processes. J. Org. Chem. 2019, 84, 11533–11541. [Google Scholar] [CrossRef]
- Szabó, T.; Milen, M. Recent application of elemental sulfur in the synthesis of S-heterocycles (microreview). Chem. Heterocycl. Compd. 2019, 55, 126–128. [Google Scholar] [CrossRef]
- Zhu, T.-H.; Xu, X.-P.; Cao, J.-J.; Wei, T.-Q.; Wang, S.-Y.; Ji, S.-J. Cobalt(II)-Catalyzed Isocyanide Insertion Reaction with Amines under Ultrasonic Conditions: A Divergent Synthesis of Ureas, Thioureas and Azaheterocycles. Adv. Synth. Catal. 2014, 356, 509–518. [Google Scholar] [CrossRef]
- Tan, W.; Wei, J.; Jiang, X. Thiocarbonyl Surrogate via Combination of Sulfur and Chloroform for Thiocarbamide and Oxazolidinethione Construction. Org. Lett. 2017, 19, 2166–2169. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Al-Mourabit, A.; Ermolenko, L. Three-Component Reaction between Isocyanides, Aliphatic Amines and Elemental Sulfur: Preparation of Thioureas under Mild Conditions with Complete Atom Economy. Synthesis 2014, 46, 3172–3179. [Google Scholar] [CrossRef]
- Shavel, A.; Cadavid, D.; Ibáñez, M.; Carrete, A.; Cabot, A. Continuous Production of Cu2ZnSnS4 Nanocrystals in a Flow Reactor. J. Am. Chem. Soc. 2012, 134, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Németh, A.G.; Szabó, R.; Domján, A.; Keserű, G.M.; Ábrányi-Balogh, P. Chromatography-Free Multicomponent Synthesis of Thioureas Enabled by Aqueous Solution of Elemental Sulfur. ChemistryOpen 2021, 10, 16–27. [Google Scholar] [CrossRef]
- Isley, N.A.; Linstadt, R.T.H.; Kelly, S.M.; Gallou, F.; Lipshutz, B.H. Nucleophilic Aromatic Substitution Reactions in Water Enabled by Micellar Catalysis. Org. Lett. 2015, 17, 4734–4737. [Google Scholar] [CrossRef]
- Goncalves, R.S.U.; Abdelnur, P.V.; Santos, V.G.; Simas, R.C.; Eberlin, M.N.; Magalhães, A.; Gonzalez, E.R.U.P. Synthesis of potentially bioactive PABA-related N-(aminoalkyl)lactamic amino acids and esters via selective SNAr reactions. Amino Acids 2011, 40, 197–204. [Google Scholar] [CrossRef]
- Gierczyk, B.; Schroeder, G.; Brzezinski, B. Reaction of Some Strong N-Bases with Chloropentafluorobenzene in the Presence of Water Molecules. J. Org. Chem. 2003, 68, 3139–3144. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Shemyakina, O.A.; Volostnykh, O.G.; Stepanov, A.V.; Mal’Kina, A.G.; Ushakov, I.A. Synthesis of Acetylenic Amides with Propyllactam Moieties by In Situ DBU or DBN Ring-Opening Rearrangement in the Presence of Acetylenic Esters. Synthesis 2017, 50, 853–858. [Google Scholar] [CrossRef]
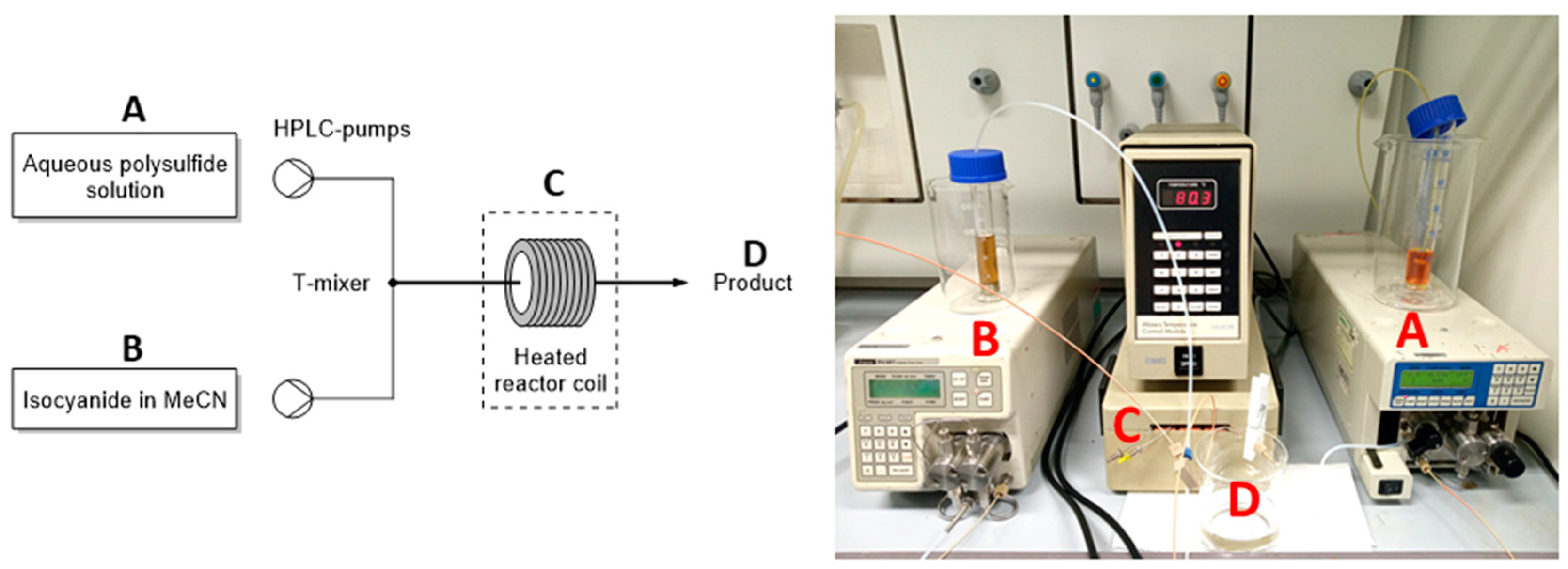
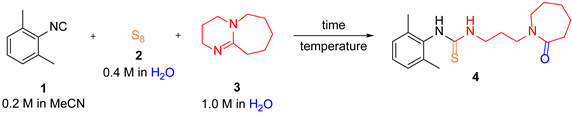 | ||||
|---|---|---|---|---|
| Entry a | T [°C] | Flow Rate [mL min−1] | Residence Time | HPLC Conversion 4/1 b,c [%] |
| 1 | 60 | 1.0 | 26 s | 36/64 |
| 2 | 60 | 0.4 | 1 min 6 s | 50/50 |
| 3 | 80 | 0.4 | 1 min 6 s | 62/38 |
| 4 | 80 | 0.2 | 2 min 12 s | 84/16 |
| 5 | 80 | 0.6 | 3 min 16 s | 93/7 |
| 6 | 80 | 0.4 | 4 min 54 s | 98/2 |
| 7 | 80 | 0.3 | 6 min 32 s | 99/1 (88) |
 | ||||
|---|---|---|---|---|
| Entry a | Isocyanide | Amine | Product | Yield [%] b |
| 1 |  1 1 |  9 9 | 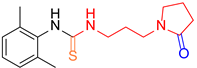 11 11 | 90 |
| 2 | 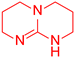 10 10 | 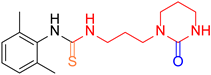 12 12 | 56 | |
| 3 | 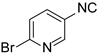 5 5 | 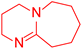 3 3 | 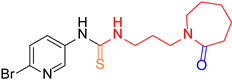 13 13 | 67 |
| 4 | 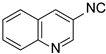 6 6 | 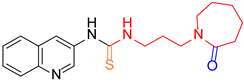 14 14 | 68 | |
| 5 |  7 7 | 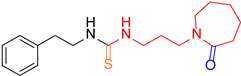 15 15 | 40 | |
| 6 | 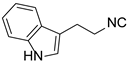 8 8 | 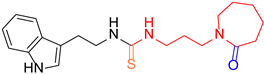 16 16 | 54 | |
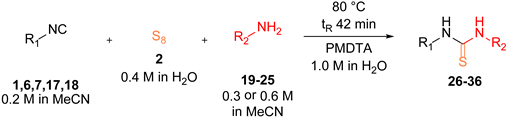 | ||||
|---|---|---|---|---|
| Entry a | Isocyanide | Amine | Product | Yield [%] d |
| 1 |  1 1 | 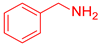 19 19 |  26 26 | 96 |
| 2 | 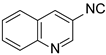 6 6 | 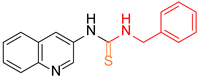 27 27 | 92 | |
| 3 | 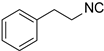 7 7 | 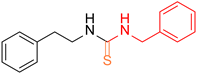 28 28 | 49 | |
| 4 | 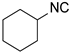 17 17 |  29 29 | 42 | |
| 5 |  18 18 |  30 30 | 42 | |
| 6 | 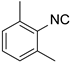 1 1 | 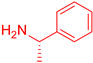 20 20 | 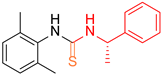 31 31 | 96 |
| 7 b | 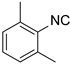 1 1 | 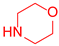 21 21 |  32 b 32 b | 76 |
| 8 b,c | 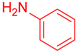 22 22 |  33 b,c 33 b,c | 70 | |
| 9 b | 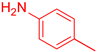 23 23 |  34 b 34 b | 79 | |
| 10 b | 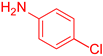 24 24 |  35 b 35 b | 39 | |
| 11 b | 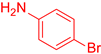 25 25 | 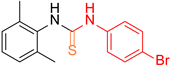 36 b 36 b | 36 | |
| Amine | Sulfur [mg, mmol] | Amine [µL or mg, mmol] | Water [mL] | T [°C] |
|---|---|---|---|---|
| 1,8-diazabicyclo[5.4.0]undec-7-ene | 32, 1.00 | 373 µL, 2.50 | 2.13 | 60 |
| 1,5-Diazabicyclo[4.3.0]non-5-ene | 310 µL, 2.50 | 2.19 | 60 | |
| 1,5,7-Triazabicyclo[4.4.0]dec-5-ene | 348 mg, 2.50 | 2.50 | 60 | |
| 7-Methyl-1,5,7-triazabicyclo[4.4.0]dec-5-ene | 360 µL, 2.50 | 2.17 | 60 | |
| N,N,N′,N″,N″-Pentamethyldiethylenetriamine | 522 µL, 2.50 | 1.98 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, A.G.; Szabó, R.; Orsy, G.; Mándity, I.M.; Keserű, G.M.; Ábrányi-Balogh, P. Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution. Molecules 2021, 26, 303. https://doi.org/10.3390/molecules26020303
Németh AG, Szabó R, Orsy G, Mándity IM, Keserű GM, Ábrányi-Balogh P. Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution. Molecules. 2021; 26(2):303. https://doi.org/10.3390/molecules26020303
Chicago/Turabian StyleNémeth, András Gy., Renáta Szabó, György Orsy, István M. Mándity, György M. Keserű, and Péter Ábrányi-Balogh. 2021. "Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution" Molecules 26, no. 2: 303. https://doi.org/10.3390/molecules26020303
APA StyleNémeth, A. G., Szabó, R., Orsy, G., Mándity, I. M., Keserű, G. M., & Ábrányi-Balogh, P. (2021). Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution. Molecules, 26(2), 303. https://doi.org/10.3390/molecules26020303







