Peretinoin, an Acyclic Retinoid, for the Secondary Prevention of Hepatocellular Carcinoma
Abstract
1. Introduction
2. Peretinoin
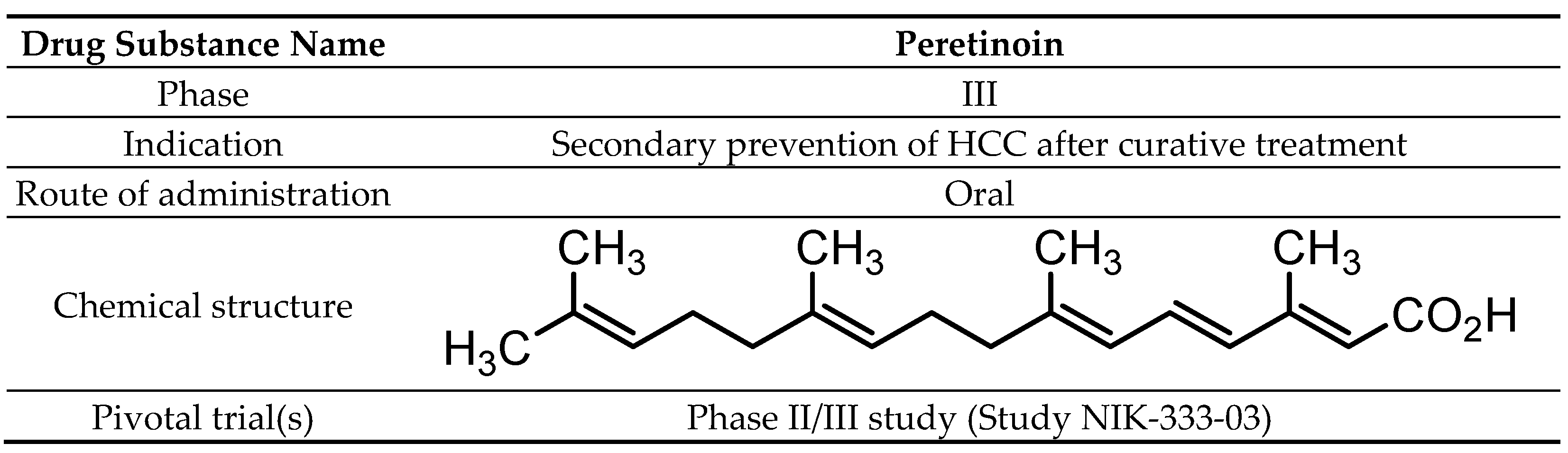
2.1. Chemistry
2.2. Pharmacodynamics
2.3. Pharmacokinetics and Metabolism
2.4. Clinical Efficacy
2.4.1. Phase I Study (Study NIK-333-01 Conducted in Japan)
2.4.2. Clinical Pharmacology Study (Study NIK-333-02 Conducted in Japan)
2.4.3. Phase II/III Study (Study NIK-333-03 Conducted in Japan, Confirmatory Trial)
2.4.4. Ongoing Trials
Phase III study (Study NIK-333-05 conducted in Japan, Confirmatory Trial) (ClinicalTrials.gov identifier NCT01640808)
Phase III study (Study K-333-06 conducted in Japan, Exploratory Trial)
K-333-3.01AS
2.5. Safety and Tolerability
3. Discussion and Future Perspectives
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Agency for Research on Cancer. Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; International Agency for Research on Cancer: Lyon, France, 2010; Volume 93. [Google Scholar]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- El–Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1752–1763. [Google Scholar] [CrossRef] [PubMed]
- Ebara, M.; Okabe, S.; Kita, K.; Sugiura, N.; Fukuda, H.; Yoshikawa, M.; Kondo, F.; Saisho, H. Percutaneous ethanol injection for small hepatocellular carcinoma: Therapeutic efficacy based on 20-year observation. J. Hepatol. 2005, 43, 458–464. [Google Scholar] [CrossRef]
- Matsui, O.; Kadoya, M.; Yoshikawa, J.; Gabata, T.; Arai, K.; Demachi, H.; Miyayama, S.; Takashima, T.; Unoura, M.; Kogayashi, K. Small hepatocellular carcinoma: Treatment with subsegmental transcatheter arterial embolization. Radiolohy 1993, 188, 79–83. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kurokawa, F.; Shirahashi, H.; Kusano, N.; Hironaka, K.; Okita, K. Percutaneous radiofrequency ablation therapy with combined angiography and computed tomography assistance for patients with hepatocellular carcinoma. Cancer 2001, 91, 1342–1348. [Google Scholar] [CrossRef]
- Todo, S.; Furukawa, H. Living donor liver transplantation for adult patients with hepatocellular carcinoma: Experience in Japan. Ann. Surg. 2004, 240, 451–461. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; De Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Romito, R.; Schiavo, M.; Mariani, L.; Camerini, T.; Bhoori, S.; Capussotti, L.; Calise, F.; Pellicci, R.; Belli, G.; et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006, 44, 1543–1554. [Google Scholar] [CrossRef]
- Kuzuya, T.; Katano, Y.; Kumada, T.; Toyoda, H.; Nakano, I.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Hayashi, K.; Honda, T.; et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2007, 22, 1929–1935. [Google Scholar] [CrossRef]
- Breitenstein, S.; Dimitroulis, D.; Petrowsky, H.; Puhan, M.A.; Müllhaupt, B.; Clavien, P. Systematic review and meta-analysis of interferon after curative treatment of hepatocellular carcinoma in patients with viral hepatitis. BJS 2009, 96, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Loria, P.; Lonardo, A.; Anania, F. Liver and diabetes. A vicious circle. Hepatol. Res. 2013, 43, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Moriwaki, H.; Omori, M. In vitro binding affinity of novel synthetic polyprenoids (polyprenoic acids) to cellular retinoid-binding proteins. Gan 1981, 72, 974–977. [Google Scholar] [PubMed]
- Muto, Y.; Moriwaki, H.; Ninomiya, M.; Adachi, S.; Saito, A.; Takasaki, K.T.; Tanaka, T.; Tsurumi, K.; Okuno, M.; Tomita, E.; et al. Prevention of Second Primary Tumors by an Acyclic Retinoid, Polyprenoic Acid, in Patients with Hepatocellular Carcinoma. N. Engl. J. Med. 1996, 334, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Moriwaki, H.; Saito, A.M. Prevention of Second Primary Tumors by an Acyclic Retinoid in Patients with Hepatocellular Carcinoma. N. Engl. J. Med. 1999, 340, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Yamashita, T.; Yamashita, T.; Arai, K.; Sakai, Y.; Sakai, A.; Nakamura, M.; Mizukoshi, E.; Kaneko, S. Peretinoin, an acyclic retinoid, improves the hepatic gene signature of chronic hepatitis C following curative therapy of hepatocellular carcinoma. BMC Cancer 2013, 13, 191. [Google Scholar] [CrossRef]
- Funaki, M.; Kitabayashi, J.; Shimakami, T.; Nagata, N.; Kai, T.; Takegoshi, K.; Okada, H.; Murai, K.; Shirasaki, T.; Oyama, T.; et al. Peretinoin, an acyclic retinoid, inhibits hepatocarcinogenesis by suppressing sphingosine kinase 1 expression in vitro and in vivo. Sci. Rep. 2017, 7, 16978. [Google Scholar] [CrossRef]
- Okada, H.; Honda, M.; Campbell, J.S.; Sakai, Y.; Yamashita, T.; Takebuchi, Y.; Hada, K.; Shirasaki, T.; Takabatake, R.; Nakamura, M.; et al. Acyclic Retinoid Targets Platelet-Derived Growth Factor Signaling in the Prevention of Hepatic Fibrosis and Hepatocellular Carcinoma Development. Cancer Res. 2012, 72, 4459–4471. [Google Scholar] [CrossRef]
- Tan, C.-K. Peretinoin as an adjuvant therapy for hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1201–1210. [Google Scholar] [CrossRef]
- di Martino, O.; Welch, J.S. Retinoic acid receptors in acute myeloid leukemia therapy. Cancers 2019, 11, 1915. [Google Scholar] [CrossRef] [PubMed]
- Uray, I.P.; Dmitrovsky, E.; Brown, P.H. Retinoids and rexinoids in cancer prevention: From laboratory to clinic. Semin. Oncol. 2016, 43, 49–64. [Google Scholar] [CrossRef]
- Kagawa, M.; Sano, T.; Ishibashi, N.; Hashimoto, M.; Okuno, M.; Moriwaki, H.; Suzuki, R.; Kohno, H.; Tanaka, T. An acyclic retinoid, nik-333, inhibits n-diethylnitrosamine-induced rat hepatocarcinogenesis through suppression of tgf-alpha expression and cell proliferation. Carcinogenesis 2004, 25, 979–985. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Tanabe, K.K. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr. Cancer Drug Targets. 2012, 12, 1129–1159. [Google Scholar] [PubMed]
- Sano, T.; Kagawa, M.; Okuno, M.; Ishibashi, N.; Hashimoto, M.; Yamamoto, M.; Suzuki, R.; Kohno, H.; Matsushima-Nishiwaki, R.; Takano, Y.; et al. Prevention of Rat Hepatocarcinogenesis by Acyclic Retinoid Is Accompanied by Reduction in Emergence of Both TGF-α-Expressing Oval-Like Cells and Activated Hepatic Stellate Cells. Nutr. Cancer 2005, 51, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-B.; Liu, C. Role of liver stem cells in hepatocarcinogenesis. World J. Stem Cells 2014, 6, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Bi, Y.; Zhu, G.-H.; He, Y.; Su, Y.; He, B.-C.; Wang, Y.; Kang, Q.; Chen, L.; Zuo, G.-W.; et al. Retinoic acid signalling induces the differentiation of mouse fetal liver-derived hepatic progenitor cells. Liver Int. 2009, 29, 1569–1581. [Google Scholar] [CrossRef]
- Shimizu, M.; Sakai, H.; Shirakami, Y.; Iwasa, J.; Yasuda, Y.; Kubota, M.; Takai, K.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Acyclic retinoid inhibits diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BLKS/J- +Leprdb/+Leprdb mice. Cancer Prev. Res. 2011, 4, 128. [Google Scholar] [CrossRef]
- Tomaru, Y.; Nakanishi, M.; Miura, H.; Kimura, Y.; Ohkawa, H.; Ohta, Y.; Hayashizaki, Y.; Suzuki, M. Identification of an inter-transcription factor regulatory network in human hepatoma cells by Matrix RNAi. Nucleic Acids Res. 2009, 37, 1049–1060. [Google Scholar] [CrossRef]
- Nakanishi, M.; Tomaru, Y.; Miura, H.; Hayashizaki, Y.; Suzuki, M. Identification of transcriptional regulatory cascades in retinoic acid-induced growth arrest of HepG2 cells. Nucleic Acids Res. 2008, 36, 3443–3454. [Google Scholar] [CrossRef][Green Version]
- Uray, I.P.; Shen, Q.; Seo, H.S.; Kim, H.; Lamph, W.W.; Bissonnette, R.P.; Brown, P.H. Rexinoid-induced expression of IGFBP-6 requires RARbeta-dependent permissive cooperation of retinoid receptors and ap-1. J. Biol. Chem. 2009, 284, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Koza-Taylor, P.H.; DiMattia, D.A.; Hames, L.; Fu, H.; Dragnev, K.H.; Turi, T.; Beebe, J.S.; Freemantle, S.J.; Dmitrovsky, E. Microarray analysis uncovers retinoid targets in human bronchial epithelial cells. Oncogene 2003, 22, 4924–4932. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Zender, L.; Villanueva, A.; Tovar, V.; Sia, D.; Chiang, D.Y.; Llovet, J.M. Cancer gene discovery in hepatocellular carcinoma. J. Hepatol. 2010, 52, 921–929. [Google Scholar] [CrossRef]
- Huang, H.; Fujii, H.; Sankila, A.; Mahler-Araujo, B.M.; Matsuda, M.; Cathomas, G.; Ohgaki, H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis c virus infection. Am. J. Pathol. 1999, 155, 1795–1801. [Google Scholar] [CrossRef]
- Roskams, T.; Yang, S.Q.; Koteish, A.; Durnez, A.; Devos, R.; Huang, X.; Achten, R.; Verslype, C.; Diehl, A.M. Oxidative Stress and Oval Cell Accumulation in Mice and Humans with Alcoholic and Nonalcoholic Fatty Liver Disease. Am. J. Pathol. 2003, 163, 1301–1311. [Google Scholar] [CrossRef]
- Zhaohui, F.; Feng, Z.; Eveleigh, J.; Iyer, G.; Pan, J.; Amin, S.; Chung, F.-L.; Tang, M.-S. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis 2002, 23, 1781–1789. [Google Scholar] [CrossRef]
- Okada, H.; Takabatake, R.; Honda, M.; Takegoshi, K.; Yamashita, T.; Nakamura, M.; Shirasaki, T.; Sakai, Y.; Shimakami, T.; Nagata, N.; et al. Peretinoin, an acyclic retinoid, suppresses steatohepatitis and tumorigenesis by activating autophagy in mice fed an atherogenic high-fat diet. Oncotarget 2017, 8, 39978–39993. [Google Scholar] [CrossRef]
- Okusaka, T.; Ueno, H.; Ikeda, M.; Morizane, C. Phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. Hepatol. Res. 2011, 41, 542–552. [Google Scholar] [CrossRef]
- Okusaka, T.; Ueno, H.; Ikeda, M.; Morizane, C.; Ishiguro, Y.; Kosuge, T.; Shimada, K.; Sano, T.; Sakamoto, Y. A phase I and pharmacokinetic clinical trial of oral administration of the acyclic retinoid NIK-333. J. Clin. Oncol. 2005, 23, 3108. [Google Scholar] [CrossRef]
- Okita, K.; Peretinoin Study Group; Izumi, N.; Matsui, O.; Tanaka, K.; Kaneko, S.; Moriwaki, H.; Ikeda, K.; Osaki, Y.; Numata, K.; et al. Peretinoin after curative therapy of hepatitis C-related hepatocellular carcinoma: A randomized double-blind placebo-controlled study. J. Gastroenterol. 2015, 50, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; The Peretinoin Study Group; Izumi, N.; Ikeda, K.; Osaki, Y.; Numata, K.; Ikeda, M.; Kokudo, N.; Imanaka, K.; Nishiguchi, S.; et al. Survey of survival among patients with hepatitis C virus-related hepatocellular carcinoma treated with peretinoin, an acyclic retinoid, after the completion of a randomized, placebo-controlled trial. J. Gastroenterol. 2014, 50, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Crowell, J.A.; Boone, C.W.; Steele, V.E.; Lubet, R.A.; Greenwald, P.; Alberts, D.S.; Covey, J.M.; Doody, L.A.; Knapp, G.G.; et al. Clinical development plan: N-(4-hydroxyphenyl)retinamide. J. Cell. Biochem. Suppl. 1994, 20, 176–196. [Google Scholar] [PubMed]
- Kamm, J.J. Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J. Am. Acad. Dermatol. 1982, 6, 652–659. [Google Scholar] [CrossRef]
- Lindamood, C., 3rd; Dillehay, D.L.; Lamon, E.W.; Giles, H.D.; Shealy, Y.F.; Sani, B.P.; Hill, D.L. Toxicologic and immunologic evaluations of n-(all-trans-retinoyl)-dl-leucine and n-(all-trans-retinoyl)glycine. Toxicol. Appl. Phamracol. 1988, 96, 279–295. [Google Scholar] [CrossRef]
- Hough, S.; Avioli, L.V.; Muir, H.; Gelderblom, D.; Jenkins, G.; Kurasi, H.; Slatopolsky, E.; Bergfeld, M.A.; Teitelbaum, S.L. Effects of Hypervitaminosis A on the Bone and Mineral Metabolism of the Rat*. Endocrinology 1988, 122, 2933–2939. [Google Scholar] [CrossRef]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef]
- Shimizu, M.; Imai, K.; Moriwaki, H.; Shirakami, Y.; Takai, K. Acyclic retinoid in chemoprevention of hepatocellular carcinoma: Targeting phosphorylated retinoid X receptor-α for prevention of liver carcinogenesis. J. Carcinog. 2012, 11, 11. [Google Scholar] [CrossRef]
- Wilson, C.; Mann, D.A.; Borthwick, L. Epigenetic reprogramming in liver fibrosis and cancer. Adv. Drug Deliv. Rev. 2017, 121, 124–132. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Lee, M.S.; Ryoo, B.-Y.; Stein, S.; Lee, K.-H.; Verret, W.; Spahn, J.; Shao, H.; Liu, B.; Iizuka, K.; et al. LBA26Updated safety and clinical activity results from a phase ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Hasegawa, K.; Takayama, T.; Ijichi, M.; Matsuyama, Y.; Imamura, H.; Sano, K.; Sugawara, Y.; Kokudo, N.; Makuuchi, M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: A randomized trial. Hepatology 2006, 44, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Kudchadkar, R.; Gonzalez, R.; Lewis, K. PI-88: A novel inhibitor of angiogenesis. Expert Opin. Investig. Drugs 2008, 17, 1769–1776. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.-S.; Sheen, I.-S.; Wang, Y.-C.; Gu, S.-L.; Chu, C.-M.; Shih, S.-C.; Wang, P.-C.; Chang, W.-H.; Wang, H.-Y. Is the vascular endothelial growth factor messenger RNA expression in resectable hepatocellular carcinoma of prognostic value after resection. World J. Gastroenterol. 2004, 10, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Kaji, K.; Kitade, M.; Aihara, Y.; Sato, S.; Seki, K.; Sawada, Y.; Takaya, H.; Okura, Y.; Kawaratani, H.; et al. Acyclic retinoid and angiotensin-II receptor blocker exert a combined protective effect against diethylnitrosamine-induced hepatocarcinogenesis in diabetic OLETF rats. BMC Cancer 2018, 18, 1164. [Google Scholar] [CrossRef] [PubMed]

| Protocol | Phase | Endpoints | Subjects | Design | Number of Subjects |
|---|---|---|---|---|---|
| Muto et al. (1996) | Post-curative treatment of HCC (HBV, HCV, NBNC) | Randomized, double blinded, placebo control | 89 | ||
| NIK-333-01 (Japan) | I | PK, safety | Post-curative treatment of HCC (HBV, HCV, B+C, NBNC) | Open label | 33 |
| NIK-333-02 (Japan) | I | Gene expression, PK, safety | Post-curative treatment of HCC (HCV) | Randomized, parallel group, open label | 12 |
| NIK-333-03 (Japan) | II/III | Efficacy, safety | Post-curative treatment of HCC (HCV) | Randomized, double blinded, parallel group, placebo control | 401 |
| K-333-1.01EU (UK) | I | PK, safety | Japanese and Caucasian males volunteer | Open, crossover, single administration/ Double-blind, randomized, parallel-group, placebo-controlled, repeated administration | 41 |
| K-333-07 (JAPAN) | I | PK, Safety on QT/QTc interval | Healthy male volunteer | double-blind, randomized, placebo- and positive-control | 56 |
| NIK-333-05 (Japan) | III (ongoing) | Efficacy, safety | Post-curative treatment of HCC (HCV) | Randomized, double blinded, parallel group, placebo control | 600 |
| K-333-06 (Japan) | III (ongoing) | Efficacy, safety | Post-curative treatment of HCC (HBV) | Randomized, double blinded, parallel group, placebo control | 100 |
| K-333-3.01A (KR/TW/SG) | III (ongoing) | Efficacy, safety | Post-curative treatment of HCC (HBV or HCV) | Randomized, double blinded, parallel group, placebo control | 600 |
| SOC (MedDRA ver.12.0) PT (MedDRA ver.12.0) | Adverse Events | Adverse Drug Reactions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Group | 300 mg/day Peretinoin Group | 600 mg/day Peretinoin Group | Placebo Group | 300 mg/day Peretinoin Group | 600 mg/day Peretinoin Group | |||||||
| N = 129 | N = 131 | N = 132 | N = 129 | N = 131 | N = 132 | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Gastrointestinal disorders | ||||||||||||
| Ascites * | 8 | 6.2 | 15 | 11.5 | 21 | 15.9 | 0 | 0.0 | 3 | 2.3 | 9 | 6.8 |
| Diarrhea * | 7 | 5.4 | 10 | 7.6 | 16 | 12.1 | 1 | 0.8 | 1 | 0.8 | 2 | 1.5 |
| Varices esophageal * | 11 | 8.5 | 15 | 11.5 | 13 | 9.8 | 2 | 1.6 | 1 | 0.8 | 0 | 0.0 |
| Constipation | 5 | 3.9 | 10 | 7.6 | 8 | 6.1 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 |
| Abdominal discomfort | 2 | 1.6 | 4 | 3.1 | 8 | 6.1 | 1 | 0.8 | 0 | 0.0 | 2 | 1.5 |
| Stomatitis | 2 | 1.6 | 5 | 3.8 | 7 | 5.3 | 0 | 0.0 | 1 | 0.8 | 2 | 1.5 |
| Cheilitis | 0 | 0.0 | 1 | 0.8 | 7 | 5.3 | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 |
| Gastritis | 2 | 1.6 | 7 | 5.3 | 4 | 3.0 | 0 | 0.0 | 1 | 0.8 | 1 | 0.8 |
| Reflux esophagitis | 0 | 0.0 | 5 | 3.8 | 3 | 2.3 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 |
| Infections and infestations | ||||||||||||
| Nasopharyngitis * | 46 | 35.7 | 57 | 43.5 | 50 | 37.9 | 3 | 2.3 | 1 | 0.8 | 1 | 0.8 |
| Cystitis | 4 | 3.1 | 6 | 4.6 | 9 | 6.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Urinary tract infection | 0 | 0.0 | 6 | 4.6 | 8 | 6.1 | 0 | 0.0 | 1 | 0.8 | 2 | 1.5 |
| Musculoskeletal and connective tissue disorders | ||||||||||||
| Back pain * | 10 | 7.8 | 11 | 8.4 | 17 | 12.9 | 2 | 1.6 | 1 | 0.8 | 1 | 0.8 |
| Osteoarthritis | 0 | 0.0 | 1 | 0.8 | 4 | 3.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Blood and lymphatic system disorders | ||||||||||||
| Anemia | 2 | 1.6 | 1 | 0.8 | 7 | 5.3 | 2 | 1.6 | 0 | 0.0 | 2 | 1.5 |
| Iron deficiency anemia | 0 | 0.0 | 0 | 0.0 | 4 | 3.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Vascular disorders | ||||||||||||
| Hypertension | 4 | 3.1 | 10 | 7.6 | 12 | 9.1 | 4 | 3.1 | 10 | 7.6 | 11 | 8.3 |
| Respiratory, thoracic and mediastinal disorders | ||||||||||||
| Pleural effusion | 1 | 0.8 | 0 | 0.0 | 5 | 3.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Injury, poisoning and procedural complication | ||||||||||||
| Lumbar vertebral fracture | 1 | 0.8 | 1 | 0.8 | 5 | 3.8 | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 |
| Nervous system disorders | ||||||||||||
| Headache * | 11 | 8.5 | 15 | 11.5 | 17 | 12.9 | 4 | 3.1 | 9 | 6.9 | 11 | 8.3 |
| Dizziness | 4 | 3.1 | 5 | 3.8 | 9 | 6.8 | 0 | 0.0 | 1 | 0.8 | 1 | 0.8 |
| General disorders and administration site conditions | ||||||||||||
| Edema peripheral* | 11 | 8.5 | 11 | 8.4 | 16 | 12.1 | 1 | 0.8 | 3 | 2.3 | 8 | 6.1 |
| Pyrexia | 8 | 6.2 | 13 | 9.9 | 12 | 9.1 | 1 | 0.8 | 1 | 0.8 | 2 | 1.5 |
| Edema | 4 | 3.1 | 3 | 2.3 | 10 | 7.6 | 1 | 0.8 | 0 | 0.0 | 3 | 2.3 |
| Thirst | 2 | 1.6 | 1 | 0.8 | 4 | 3.0 | 0 | 0.0 | 1 | 0.8 | 4 | 3.0 |
| Chest discomfort | 0 | 0.0 | 4 | 3.1 | 1 | 0.8 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 |
| Metabolism and nutrition disorders | ||||||||||||
| Hypokalemia | 1 | 0.8 | 5 | 3.8 | 3 | 2.3 | 0 | 0.0 | 2 | 1.5 | 0 | 0.0 |
| Skin and subcutaneous tissue disorders | ||||||||||||
| Pruritus | 9 | 7.0 | 12 | 9.2 | 11 | 8.3 | 0 | 0.0 | 6 | 4.6 | 6 | 4.5 |
| Nail disorder | 2 | 1,6 | 7 | 5.3 | 4 | 3.0 | 2 | 1.6 | 6 | 4.6 | 4 | 3.0 |
| Dermatitis | 0 | 0.0 | 2 | 1.5 | 4 | 3.0 | 0 | 0.0 | 1 | 0.8 | 3 | 2.3 |
| Onychoclasis | 0 | 0.0 | 1 | 0.8 | 4 | 3.0 | 0 | 0.0 | 1 | 0.8 | 4 | 3.0 |
| Investigation | ||||||||||||
| Albumin urine present * | 8 | 6.2 | 14 | 10.7 | 29 | 22.0 | 5 | 3.9 | 11 | 8.4 | 26 | 19.7 |
| Blood pressure increased * | 19 | 14.7 | 20 | 15.3 | 26 | 19.7 | 16 | 12.4 | 16 | 12.2 | 21 | 15.9 |
| Transaminases increased * | 15 | 11.6 | 10 | 7.6 | 23 | 17.4 | 7 | 5.4 | 5 | 3.8 | 11 | 8.3 |
| Protein urine present | 0 | 0.0 | 2 | 1.5 | 8 | 6.1 | 0 | 0.0 | 0 | 0.0 | 8 | 6.1 |
| Blood urine present | 3 | 2.3 | 7 | 5.3 | 5 | 3.8 | 1 | 0.8 | 3 | 2.3 | 3 | 2.3 |
| SOC (MedDRA ver.12.0) PT (MedDRA ver.12.0) | Adverse Events | Adverse Drug Reactions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo Group | 300 mg/day Peretinoin Group | 600 mg/day Peretinoin Group | Placebo Group | 300 mg/day Peretinoin Group | 600 mg/day Peretinoin Group | |||||||
| N = 129 | N = 131 | N = 132 | N = 129 | N = 131 | N = 132 | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Gastrointestinal disorders | ||||||||||||
| Ascites | 1 | 0.8 | 1 | 0.8 | 8 | 6.1 | 0 | 0.0 | 1 | 0.8 | 5 | 3.8 |
| Varices esophageal | 4 | 3.1 | 7 | 5.3 | 5 | 3.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Gastric ulcer hemorrhage | 0 | 0.0 | 0 | 0.0 | 3 | 2.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Gastric varices | 0 | 0.0 | 2 | 1.5 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Infections and infestations | ||||||||||||
| Urinary tract infection | 0 | 0.0 | 3 | 2.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hepatobiliary disorders | ||||||||||||
| Hepatic failure | 0 | 0.0 | 2 | 1.5 | 1 | 0.8 | 0 | 0.0 | 1 | 0.8 | 1 | 0.8 |
| Cardiac disorders | ||||||||||||
| Cardio-respiratory arrest | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 | 2 | 1.5 |
| Nervous system disorders | ||||||||||||
| Hepatic encephalopathy | 0 | 0.0 | 2 | 1.5 | 2 | 1.5 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 |
| General disorders and administration site conditions | ||||||||||||
| Pyrexia | 0 | 0.0 | 1 | 0.8 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Neoplasms benign, malignant and unspecified (incl. cysts and polyps) | ||||||||||||
| Gastric cancer stage 0 | 0 | 0.0 | 1 | 0.8 | 3 | 2.3 | 0 | 0.0 | 1 | 0.8 | 0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, H.Y.; Yoo, S.Y.; Heo, J. Peretinoin, an Acyclic Retinoid, for the Secondary Prevention of Hepatocellular Carcinoma. Molecules 2021, 26, 295. https://doi.org/10.3390/molecules26020295
Woo HY, Yoo SY, Heo J. Peretinoin, an Acyclic Retinoid, for the Secondary Prevention of Hepatocellular Carcinoma. Molecules. 2021; 26(2):295. https://doi.org/10.3390/molecules26020295
Chicago/Turabian StyleWoo, Hyun Young, So Young Yoo, and Jeong Heo. 2021. "Peretinoin, an Acyclic Retinoid, for the Secondary Prevention of Hepatocellular Carcinoma" Molecules 26, no. 2: 295. https://doi.org/10.3390/molecules26020295
APA StyleWoo, H. Y., Yoo, S. Y., & Heo, J. (2021). Peretinoin, an Acyclic Retinoid, for the Secondary Prevention of Hepatocellular Carcinoma. Molecules, 26(2), 295. https://doi.org/10.3390/molecules26020295








