A 3D Plasmonic Crossed-Wire Nanostructure for Surface-Enhanced Raman Scattering and Plasmon-Enhanced Fluorescence Detection
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Apparatus
2.3. Synthesis of Ag Nanowires (Ag NWs)
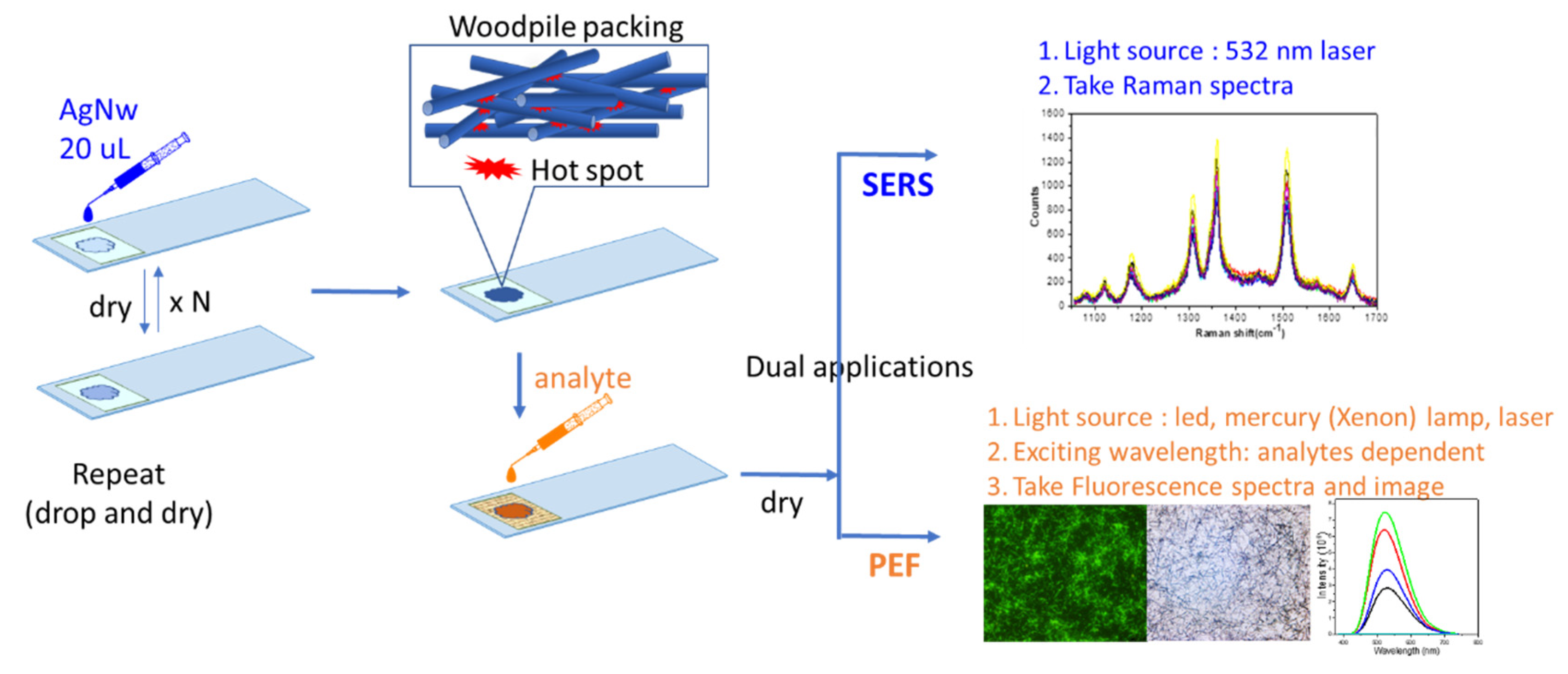
2.4. Construction of a 3D Nanowire Chip and Measurement
2.5. Raman Measurement
3. Result and Discussion
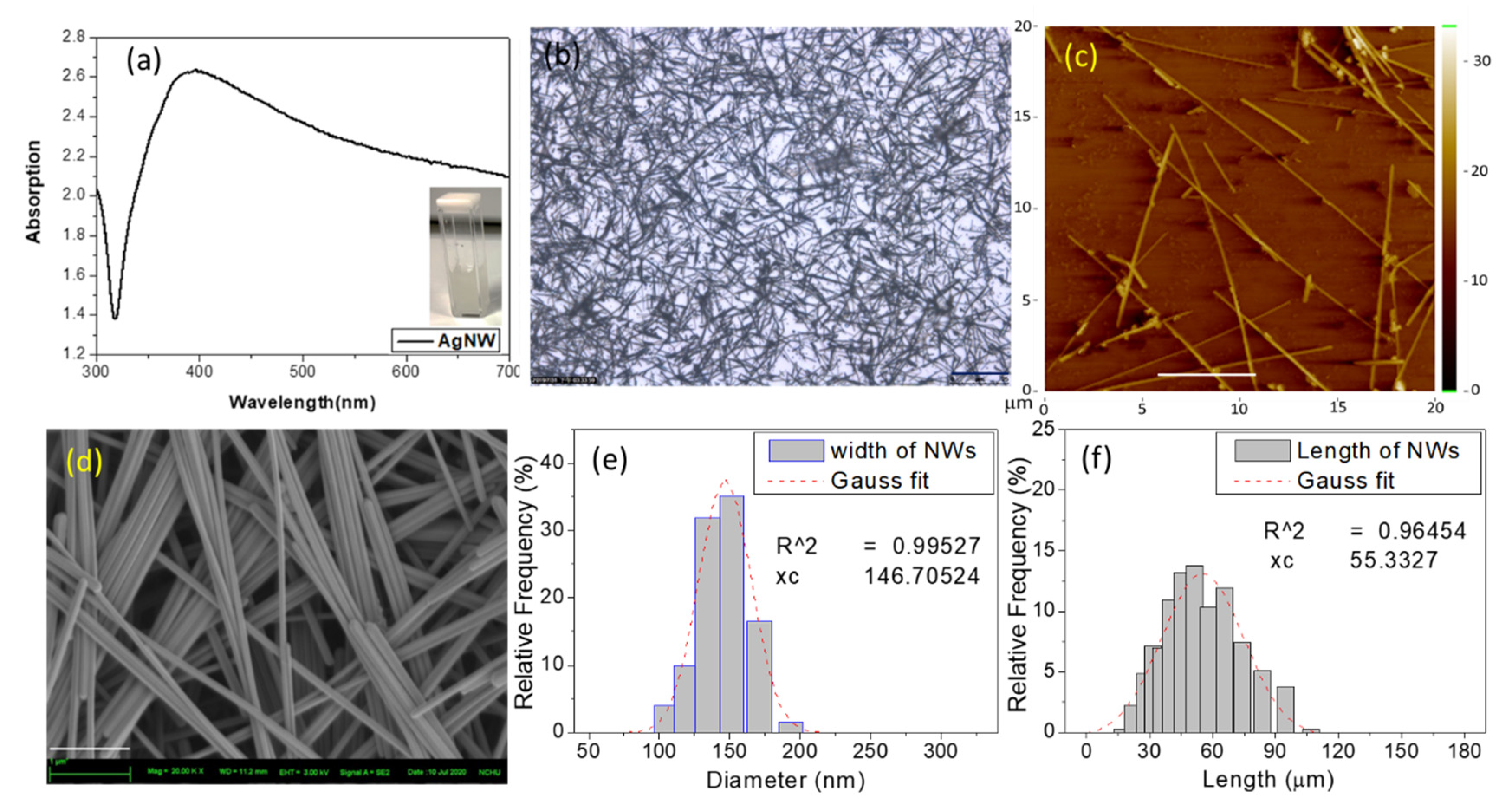
3.1. Characterization of Ag NWs
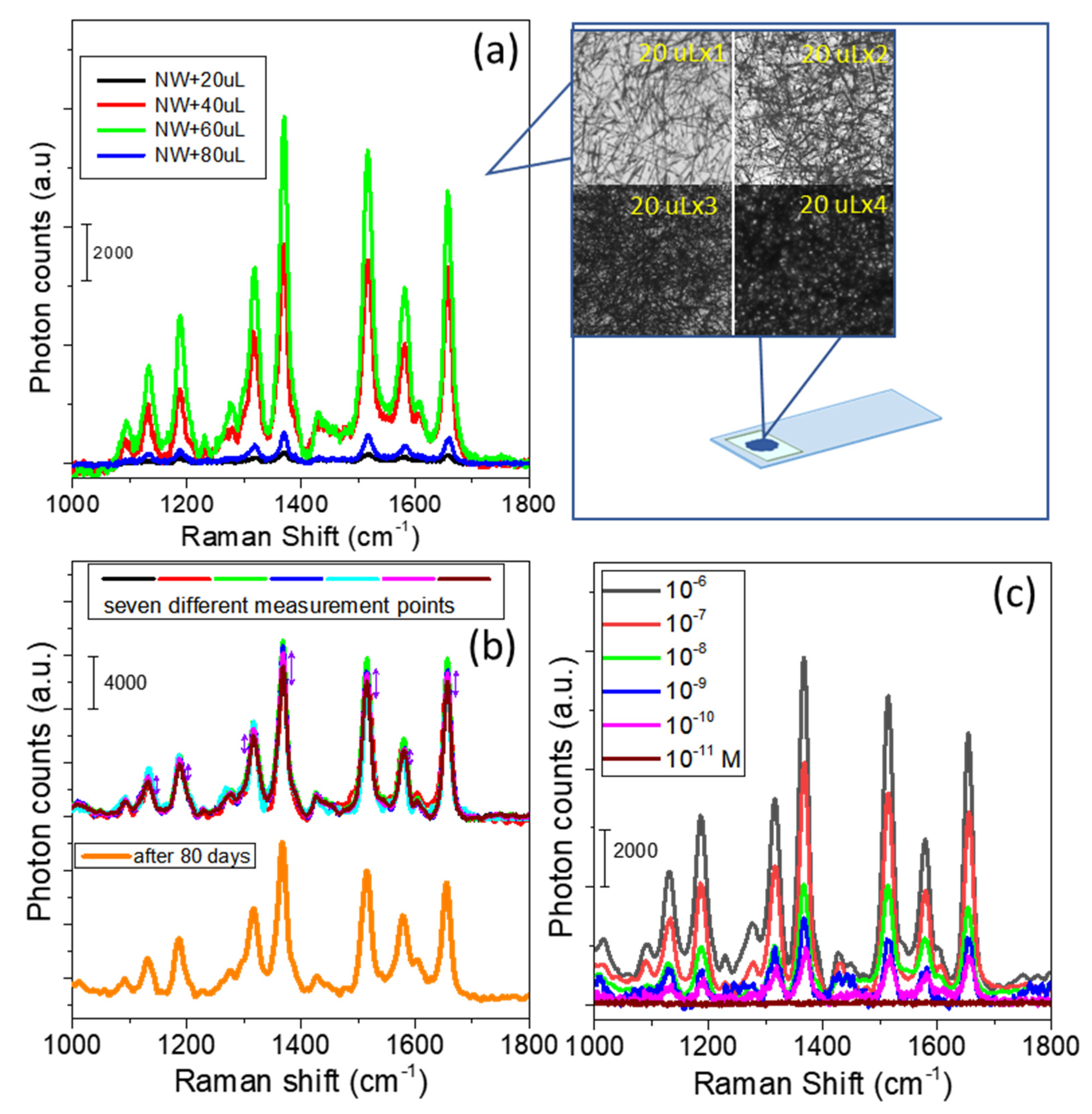
3.2. Construction of an Ag NW 3D Nanostructure
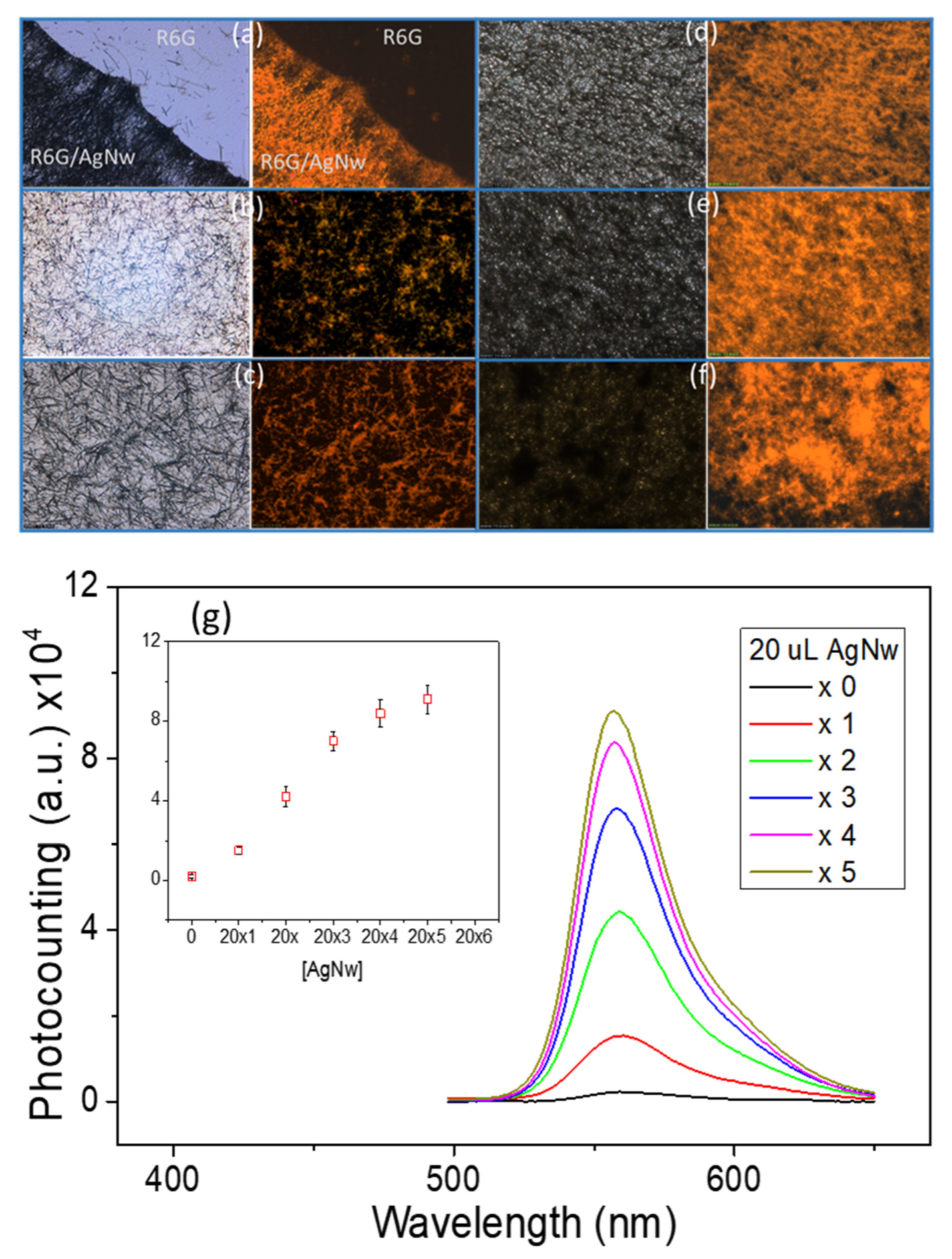
3.3. Plasmon-Enhanced Fluorescence
3.4. Appearance of 3D-RCW
3.5. Application of 3D-RCW
- Most used Au, while we used a very stable Ag.
- Most used nanoparticles, while we used nanowires.
- A 3D substrate was constructed in our chip.
- Our chip and analytes were prepared simply.
- Most detected just one or two pesticides, while we detected four pesticides that belong to three types of pesticides (carbamate, paraquat, and fipronil).
- Fipronil is hard to detect by SERS, but our chip detected it.
- We detected fluorescence and Raman using the same chip.
3.6. Further Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jones, M.R.; Osberg, K.D.; Macfarlane, R.J.; Langille, M.R.; Mirkin, C.A. Templated techniques for the synthesis and assembly of plasmonic nanostructures. Chem. Rev. 2011, 111, 3736–3827. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Nallathamby, P.D.; Huang, T.; Xu, X.H. Design and characterization of optical nanorulers of single nanoparticles using optical microscopy and spectroscopy. Nanoscale 2010, 2, 1715–1722. [Google Scholar] [CrossRef]
- Cushing, S.K.; Wu, N. Progress and Perspectives of Plasmon-Enhanced Solar Energy Conversion. J. Phys. Chem. Lett. 2016, 7, 666–675. [Google Scholar] [CrossRef]
- Ding, D.; Liu, K.; He, S.; Gao, C.; Yin, Y. Ligand-exchange assisted formation of Au/TiO2 Schottky contact for visible-light photocatalysis. Nano Lett. 2014, 14, 6731–6736. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Cao, Y.C.; Jin, R.; Mirkin, C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef]
- Beeram, S.R.; Zamborini, F.P. Selective attachment of antibodies to the edges of gold nanostructures for enhanced localized surface plasmon resonance biosensing. J. Am. Chem. Soc. 2009, 131, 11689–11691. [Google Scholar] [CrossRef]
- Masetti, M.; Xie, H.N.; Krpetić, Ž.; Recanatini, M.; Alvarez-Puebla, R.A.; Guerrini, L. Revealing DNA interactions with exogenous agents by surface-enhanced Raman scattering. J. Am. Chem. Soc. 2015, 137, 469–476. [Google Scholar] [CrossRef]
- Freeman, R.G.; Grabar, K.C.; Allison, K.J.; Bright, R.M.; Davis, J.A.; Guthrie, A.P.; Hommer, M.B.; Jackson, M.A.; Smith, P.C.; Walter, D.G.; et al. Self-Assembled Metal Colloid Monolayers: An Approach to SERS Substrates. Science 1995, 267, 1629–1632. [Google Scholar] [CrossRef]
- Gryczynski, I.; Malicka, J.; Gryczynski, Z.; Lakowicz, J.R. Radiative decay engineering 4. Experimental studies of surface plasmon-coupled directional emission. Anal. Biochem. 2004, 324, 170–182. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, K.; Sobhani, H.; Bao, K.; Nordlander, P. Geometric Dependence of the Line Width of Localized Surface Plasmon Resonances. J. Phys. Chem. Lett. 2013, 4, 1352–1357. [Google Scholar] [CrossRef]
- Shiohara, A.; Wang, Y.; Liz-Marzán, L.M. Recent approaches toward creation of hot spots for SERS detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Glass, A.M.; Liao, P.F.; Bergman, J.G.; Olson, D.H. Interaction of metal particles with adsorbed dye molecules: Absorption and luminescence. Opt. Lett. 1980, 5, 368. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Rao, W.; Li, Q.; Wang, Y.; Li, T.; Wu, L. Comparison of photoluminescence quantum yield of single gold nanobipyramids and gold nanorods. ACS Nano 2015, 9, 2783–2791. [Google Scholar] [CrossRef]
- Geddes, C.D.; Lakowicz, J.R. Editorial: Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, T.; Li, W.; Hou, L.; Liu, J.; Gong, Q. Single-Molecule Spontaneous Emission in the Vicinity of an Individual Gold Nanorod. J. Phys. Chem. C 2011, 115, 15822–15828. [Google Scholar] [CrossRef]
- Li, J.F.; Li, C.Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef]
- Yun, S.; Niu, X.; Yu, Z.; Hu, W.; Brochu, P.; Pei, Q. Compliant silver nanowire-polymer composite electrodes for bistable large strain actuation. Adv. Mater. 2012, 24, 1321–1327. [Google Scholar] [CrossRef]
- Park, S.G.; Mun, C.; Lee, M.; Jeon, T.Y.; Shim, H.S.; Lee, Y.J.; Kwon, J.D.; Kim, C.S.; Kim, D.H. 3D Hybrid Plasmonic Nanomaterials for Highly Efficient Optical Absorbers and Sensors. Adv. Mater. 2015, 27, 4290–4295. [Google Scholar] [CrossRef]
- Nam, S.; Song, M.; Kim, D.H.; Cho, B.; Lee, H.M.; Kwon, J.D.; Park, S.G.; Nam, K.S.; Jeong, Y.; Kwon, S.H.; et al. Ultrasmooth, extremely deformable and shape recoverable Ag nanowire embedded transparent electrode. Sci. Rep. 2014, 4, 4788. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Zhang, Z.; Zhu, L.; Sun, M. Nanowire-supported plasmonic waveguide for remote excitation of surface-enhanced Raman scattering. Light Sci. Appl. 2014, 3, e199. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.S.; Brolo, A.G. A review on recent advances in the applications of surface-enhanced Raman scattering in analytical chemistry. Anal. Chim. Acta 2020, 1097, 1–29. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Huang, C.T.; Wang, W.H.; Chang, C.C. A dual photoluminescence enhancement system: Stabilization of a water soluble AIEE fluorogen using silver nanowire. Faraday Discuss. 2017, 196, 55–69. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, P.; Gao, Q.; Zhang, Y.; Tang, Y. High-Concentration Preparation of Silver Nanowires: Restraining in Situ Nitric Acidic Etching by Steel-Assisted Polyol Method. Chem. Mater. 2008, 20, 1699–1704. [Google Scholar] [CrossRef]
- Li, X.; Choy, W.C.H.; Ren, X.; Zhang, D.; Lu, H. Highly Intensified Surface Enhanced Raman Scattering by Using Monolayer Graphene as the Nanospacer of Metal Film—Metal Nanoparticle Coupling System. Adv. Funct. Mater. 2014, 24, 3114–3122. [Google Scholar] [CrossRef]
- Jeong, J.W.; Arnob, M.M.; Baek, K.M.; Lee, S.Y.; Shih, W.C.; Jung, Y.S. 3D Cross-Point Plasmonic Nanoarchitectures Containing Dense and Regular Hot Spots for Surface-Enhanced Raman Spectroscopy Analysis. Adv. Mater. 2016, 28, 8695–8704. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef]
- Li, X.; Lee, H.K.; Phang, I.Y.; Lee, C.K.; Ling, X.Y. Superhydrophobic-Oleophobic Ag Nanowire Platform: An Analyte-Concentrating and Quantitative Aqueous and Organic Toxin Surface-Enhanced Raman Scattering Sensor. Anal. Chem. 2014, 86, 10437–10444. [Google Scholar] [CrossRef]
- Chen, M.; Phang, I.Y.; Lee, M.R.; Yang, J.K.; Ling, X.Y. Layer-by-layer assembly of Ag nanowires into 3D woodpile-like structures to achieve high density “hot spots” for surface-enhanced Raman scattering. Langmuir 2013, 29, 7061–7069. [Google Scholar] [CrossRef]
- Sharma, V.K.; Aulakh, J.S.; Malik, A.K. Thiram: Degradation, applications and analytical methods. J. Environ. Monit. 2003, 5, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Meng, G.; Huang, Z.; Ke, Y.; Zhou, Q.; Hu, X. A flexible transparent Ag-NC@PE film as a cut-and-paste SERS substrate for rapid in situ detection of organic pollutants. Analyst 2016, 141, 5864–5869. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis. LWT Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Alsammarraie, F.K.; Lin, M. Using Standing Gold Nanorod Arrays as Surface-Enhanced Raman Spectroscopy (SERS) Substrates for Detection of Carbaryl Residues in Fruit Juice and Milk. J. Agric. Food Chem. 2017, 65, 666–674. [Google Scholar] [CrossRef]
- Bo, H. Determination of paraquat residue in plant-derived foodstuffs by high performance liquid chromatography-tandem mass spectrometry. Se Pu 2011, 29, 180–183. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, X.; Zhang, S.J.; Liu, L.; Zhao, Y.M.; Xu, H.J. Ultrasensitive and quantitative detection of paraquat on fruits skins via surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2015, 213, 452–456. [Google Scholar] [CrossRef]
- Vidau, C.; González-Polo, R.A.; Niso-Santano, M.; Gómez-Sánchez, R.; Bravo-San Pedro, J.M.; Pizarro-Estrella, E.; Blasco, R.; Brunet, J.L.; Belzunces, L.P.; Fuentes, J.M. Fipronil is a powerful uncoupler of oxidative phosphorylation that triggers apoptosis in human neuronal cell line SHSY5Y. Neurotoxicology 2011, 32, 935–943. [Google Scholar] [CrossRef]
- Tu, Q.; Hickey, M.E.; Yang, T.; Gao, S.; Zhang, Q.; Qu, Y.; Du, X.; Wang, J.; He, L. A simple and rapid method for detecting the pesticide fipronil on egg shells and in liquid eggs by Raman microscopy. Food Control 2019, 96, 16–21. [Google Scholar] [CrossRef]
- Ly, N.H.; Nguyen, T.H.; Nghi, N.; Kim, Y.H.; Joo, S.W. Surface-Enhanced Raman Scattering Detection of Fipronil Pesticide Adsorbed on Silver Nanoparticles. Sensors 2019, 19, 1355. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface Enhanced Raman Scattering with Gold Nanoparticles: Effect of Particle Shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef]
- Hatab, N.A.; Eres, G.; Hatzinger, P.B.; Gu, B. Detection and Analysis of Cyclotrimethylene-trinitramine (RDX) in Environmental Samples by Surface Enhanced Raman Spectroscopy. J. Raman Spectrosc. 2010, 41, 1131–1136. [Google Scholar] [CrossRef]
- Andreou, C.; Hoonejani, M.R.; Barmi, M.R.; Moskovits, M.; Meinhart, C.D. Rapid Detection of Drugs of Abuse in Saliva using Surface Enhanced Raman Spectroscopy and Microfluidics. ACS Nano 2013, 7, 7157–7164. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dai, X.; Stogin, B.B.; Wong, T.-S. Ultrasensitive Surface-Enhanced Raman Scattering Detection in Common Fluids. Proc. Natl. Acad. Sci. USA 2016, 113, 268–273. [Google Scholar] [CrossRef]
- Péron, O.; Rinnert, E.; Lehaitre, M.; Crassous, P.; Compère, C. Detection of Polycyclic Aromatic Hydrocarbon (PAH) Compounds in Artificial Sea-Water using Surface-Enhanced Raman Scattering (SERS). Talanta 2009, 79, 199–204. [Google Scholar] [CrossRef]
- Fierro-Mercado, P.M.; Hernández-Rivera, S.P. Highly Sensitive Filter Paper Substrate for SERS Trace Explosives Detection. Int. J. Spectrosc. 2012, 2012, 716527. [Google Scholar] [CrossRef]
- Wang, J.F.; Wu, X.Z.; Xiao, R.; Dong, P.T.; Wang, C.G. Performance-Enhancing Methods for Au Film over Nanosphere Surface-Enhanced Raman Scattering Substrate and Melamine Detection Application. PLoS ONE 2014, 9, e97967. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Abu Hatab, N.A.; Oran, J.M.; Sepaniak, M.J. Surface-Enhanced Raman Spectroscopy Substrates Created via Electron Beam Lithography and Nanotransfer Printing. ACS Nano 2008, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; McNay, G.; McInroy, A.; Fitchett, K. New Developments in SERS for the Pharmaceutical Industry. 2017, 2. Available online: http://www.iptonline.com/pdfviewarticle.asp?cat=3&article=689 (accessed on 7 January 2021).
- Yu, W.W.; White, I.M. Inkjet-Printed Paper-Based SERS Dipsticks and Swabs for Trace Chemical Detection. Analyst 2013, 138, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.M.; Hatzinger, P.B.; Gu, B. Trace-Level Perchlorate Analysis of Impacted Groundwater by Elevated Gold Ellipse Dimer Nanoantenna Surface-Enhanced Raman Scattering. J. Raman Spectrosc. 2017, 48, 518–524. [Google Scholar] [CrossRef]
- Lu, G.; Forbes, T.Z.; Haes, A.J. SERS Detection of Uranyl using Functionalized Gold Nanostars Promoted by Nanoparticle Shape and Size. Analyst 2016, 141, 5137–5143. [Google Scholar] [CrossRef] [PubMed]
- Schmit, V.L.; Martoglio, R.; Scott, B.; Strickland, A.D.; Carron, K.T. Lab-on-a-Bubble: Synthesis, Characterization, and Evaluation of Buoyant Gold Nanoparticle-Coated Silica Spheres. J. Am. Chem. Soc. 2012, 134, 59–62. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A.; Putnam, M.D. Detection of Hexavalent Chromium using Gold/4-(2-Mercaptoethyl)pyridinium Surface Enhanced Raman Scattering-Active Capture Matrices. Anal. Chim. Acta 2013, 801, 70–77. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Miliutina, E.; Svorcik, V.; Lyutakov, O. Metal-organic framework (MOF-5) coated SERS active gold gratings: A platform for the selective detection of organic contaminants in soil. Anal. Chim. Acta 2019, 1068, 70–79. [Google Scholar] [CrossRef]
- Weng, S.; Li, M.; Chen, C.; Gao, X.; Zheng, S.; Zeng, X. Fast and accurate determination of organophosphate pesticides using surface-enhanced Raman scattering and chemometrics. Anal. Methods 2015, 7, 2563–2567. [Google Scholar] [CrossRef]



| Thiram | Carbaryl | Paraquat | Fipronil | ||||
|---|---|---|---|---|---|---|---|
| SERS | Mode | SERS | Mode | SERS | Mode | SERS | Mode |
| 567 | C–S stretching | 837 | C–N stretching | ||||
| 1150 | N=C=S stretching | 1191 | C=C bending | ||||
| 1386 | C–N stretching | 1382 | symmetric vibration (naphthalene ring) | 1293 | C–C structural distortion | ||
| 1516 | C–H wagging | 1442 | C–H wagging of naphthalene ring | ||||
| 1578 | stretching of C=C double | 1642 | C=N stretching | ||||
| 2253 | nitrile (−C≣N) | ||||||
| Analytes | LODs | Substrates | Nobel Metal Nanostructure (Exciting Laser) | Refs |
|---|---|---|---|---|
| rhodamine 6G (R6G) | - | SERS signal: nanostars > nanotriangles > nanospheres | Gold nanostructures (785 nm lasering) | [43] |
| cyclotrimethylenetrinitramine (RDX) | 0.15 mg/L | glass slide | Au NP suspension (785 nm lasering) | [44] |
| methamphetamine | - | flow-focusing microfluidic (PDMS) | controlled Ag-NP/salts aggregation (633 nm lasering) | [45] |
| DNA bases 4-aminothiophenol (4-ATP) | 1 fM- 10 aM | slippery liquid-infused porous surface-enhanced Raman scattering (SLIPSERS) | Au NPs + analyte (633 nm lasering) | [46] |
| polychlorinated biphenyls (PCBs) | 1PPb | |||
| naphthalene | 25 ppm | “chemical tether” to anchor NPs on a quartz substrate | Au NPs (633 nm lasering) | [47] |
| 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (2,4-DNT), 1,3,5-trinitrobenzene (TNB) | 0.89~ 94 pg. | clusters of NPs on the cellulose fibers of the paper. | Au NPs (785 nm lasering) | [48] |
| melamine | 1 ppb | physical vapor deposition and then electrochemical deposition | (formation of Ag or Au metal film over nanosphere (FON) surface) (785 nm lasering) | [49] |
| parathion-methyl thiram chlorpyrifos | 2.60 0.24 3.51 ng/cm2 | nanoparticle solution was dropped uniformly on the sticky side of adhesive tape | Au NPs (paste and peel off’ sampling approach.) (633 nm lasering) | [50] |
| crystal violet mitoxantrone | 10 nM 1 nM | electron beam lithography and nanotransfer printing | Au nanopattern (633 nm lasering) | [51] |
| melamine | 33 ppb | lithographically, substrate is composed of an array of pyramidal-shaped pits etched into silicon. | Ag/Au NPs pyramidal Klarite@ substrates | [52] |
| organophosphate malathion, heroin, cocaine | 413 pg, 9 ng, 15 ng | inkjet-printed paper-based dipsticks and swabs | Au nanoclusters (785 nm lasering) | [53] |
| perchlorate | 0.343 ± 0.025 mg/L | electron beam lithography | Au ellipse dimer array (785 nm lasering) | [54] |
| uranyl solution | 120 nM. | chemical modification on nanostructure surface | Au nanostars. (785 nm lasering) | [55] |
| cyanide | 173 ppt | lab-on-a-bubble (LoB) assay | Au NP-coated LoBs | [56] |
| hexavalent chromium | 59 ppb | a capture matrix with self-assembled monolayer on immobilizing nanocluster | Au/MEPH + substrates (785 nm lasering) | [57] |
| paraoxon fenitrothion | 10−12 M | metal-organic framework (MOF-5) | Au-grating (785 nm lasering) | [58] |
| methyl parathion, edifenphos, ethyl paraxon | * | spectra collection, then chemometric methods (standard normal variate variance (SNV) method) | Ag sol gel (532 nm lasering) | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-T.; Jan, F.-J.; Chang, C.-C. A 3D Plasmonic Crossed-Wire Nanostructure for Surface-Enhanced Raman Scattering and Plasmon-Enhanced Fluorescence Detection. Molecules 2021, 26, 281. https://doi.org/10.3390/molecules26020281
Huang C-T, Jan F-J, Chang C-C. A 3D Plasmonic Crossed-Wire Nanostructure for Surface-Enhanced Raman Scattering and Plasmon-Enhanced Fluorescence Detection. Molecules. 2021; 26(2):281. https://doi.org/10.3390/molecules26020281
Chicago/Turabian StyleHuang, Chun-Ta, Fuh-Jyh Jan, and Cheng-Chung Chang. 2021. "A 3D Plasmonic Crossed-Wire Nanostructure for Surface-Enhanced Raman Scattering and Plasmon-Enhanced Fluorescence Detection" Molecules 26, no. 2: 281. https://doi.org/10.3390/molecules26020281
APA StyleHuang, C.-T., Jan, F.-J., & Chang, C.-C. (2021). A 3D Plasmonic Crossed-Wire Nanostructure for Surface-Enhanced Raman Scattering and Plasmon-Enhanced Fluorescence Detection. Molecules, 26(2), 281. https://doi.org/10.3390/molecules26020281






