Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction
Abstract
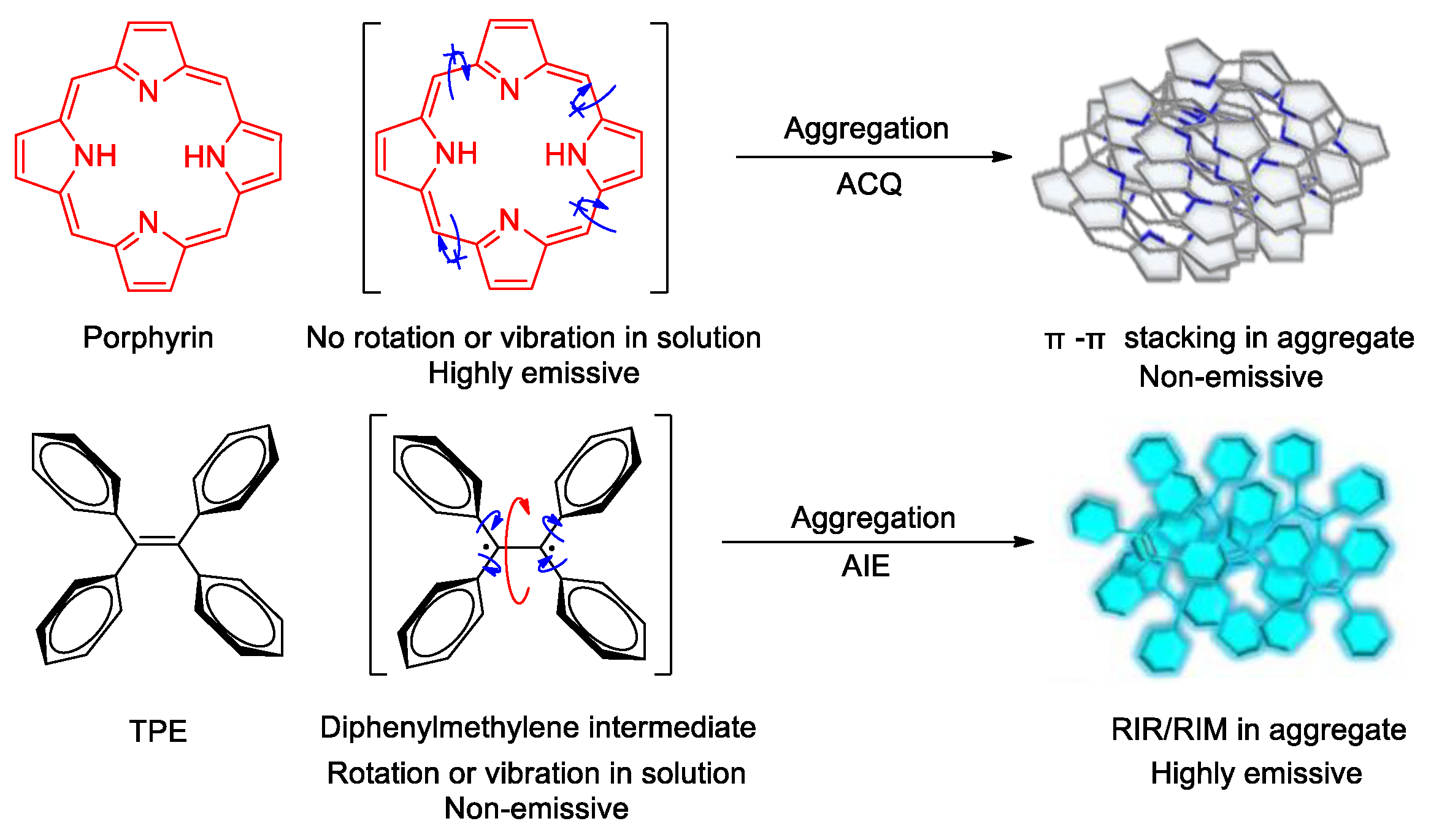
1. Introduction
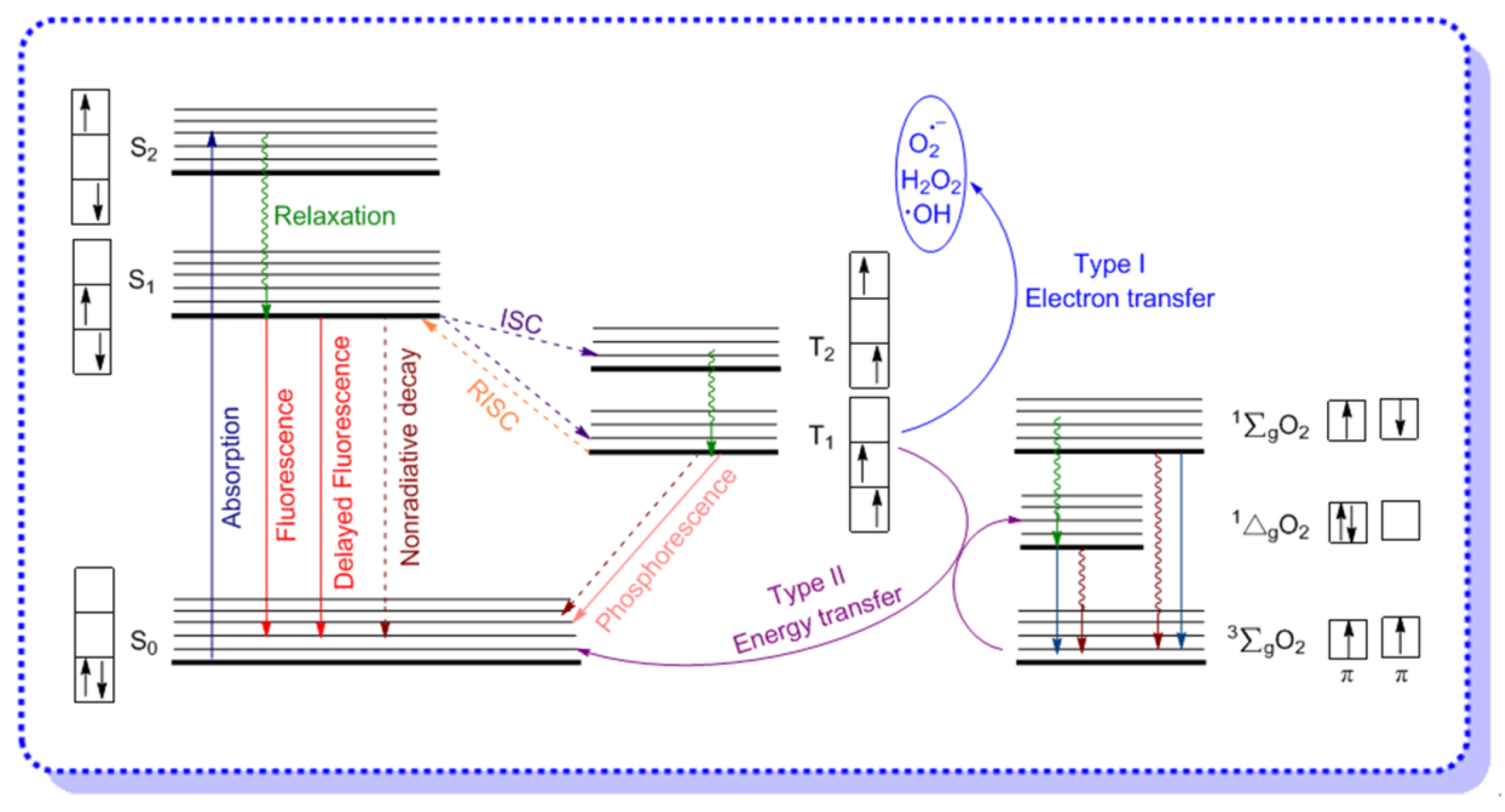
2. Aggregation-Induced ROS Generation Mechanism
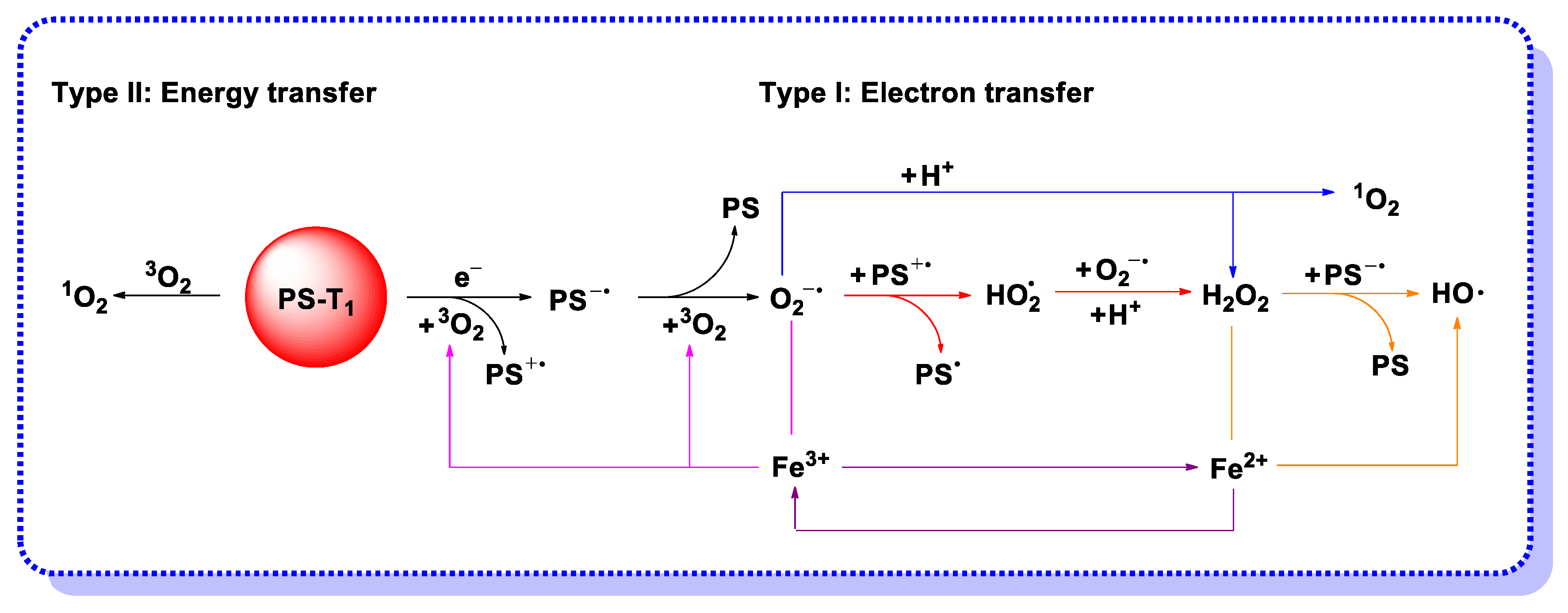
2.1. Electron Transfer (Type I) Mechanism
2.2. Energy Transfer (Type II) Mechanism
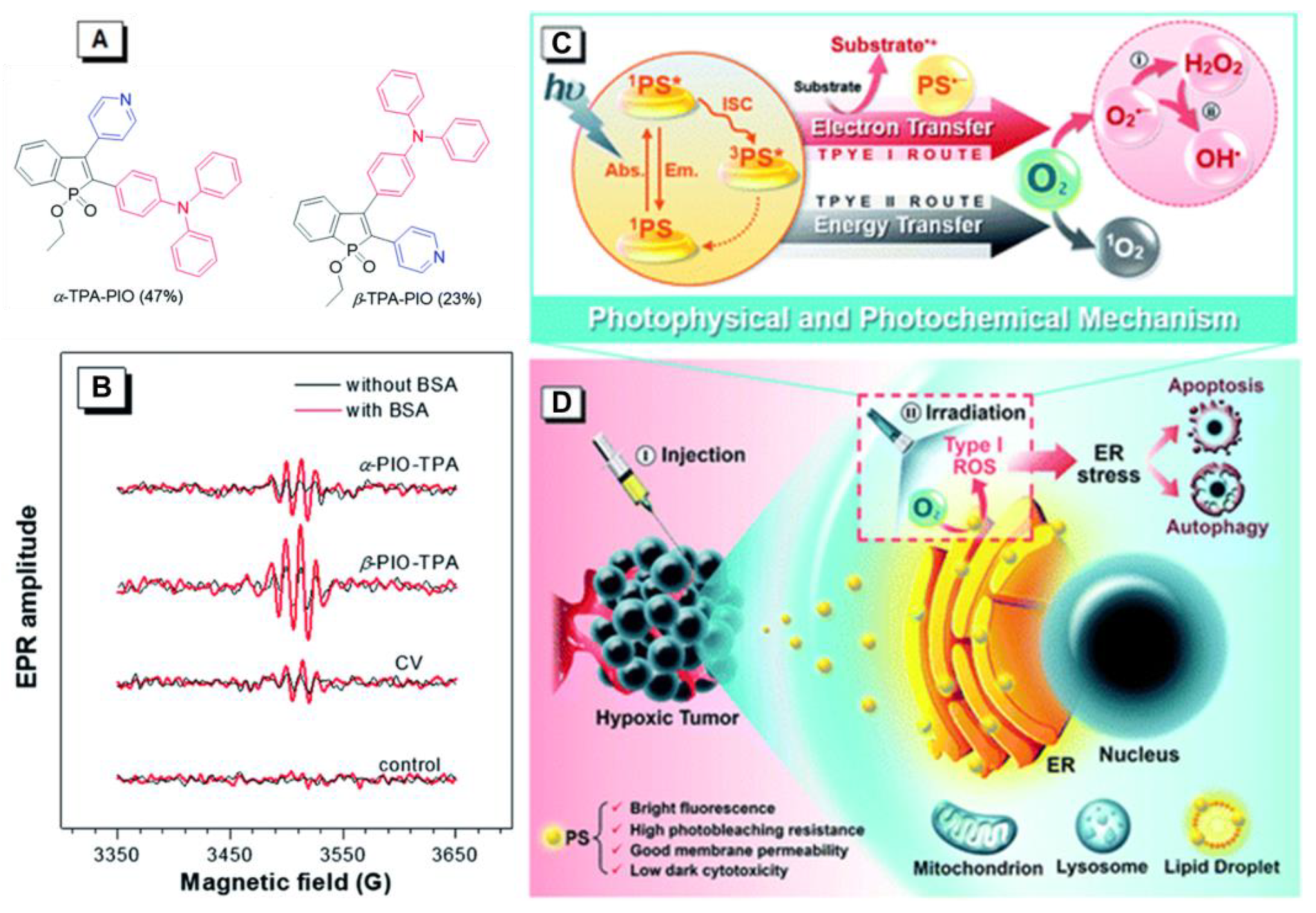
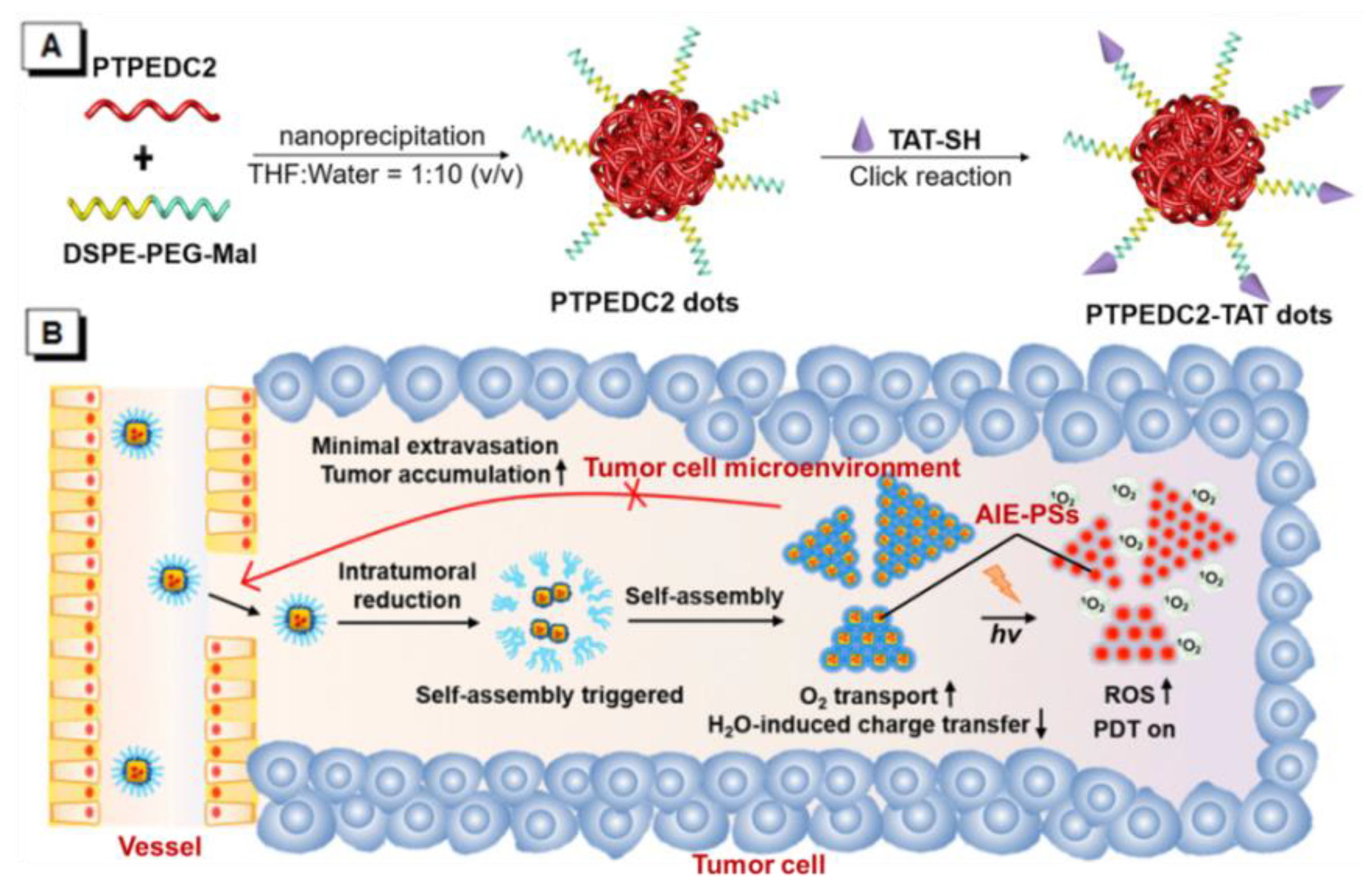
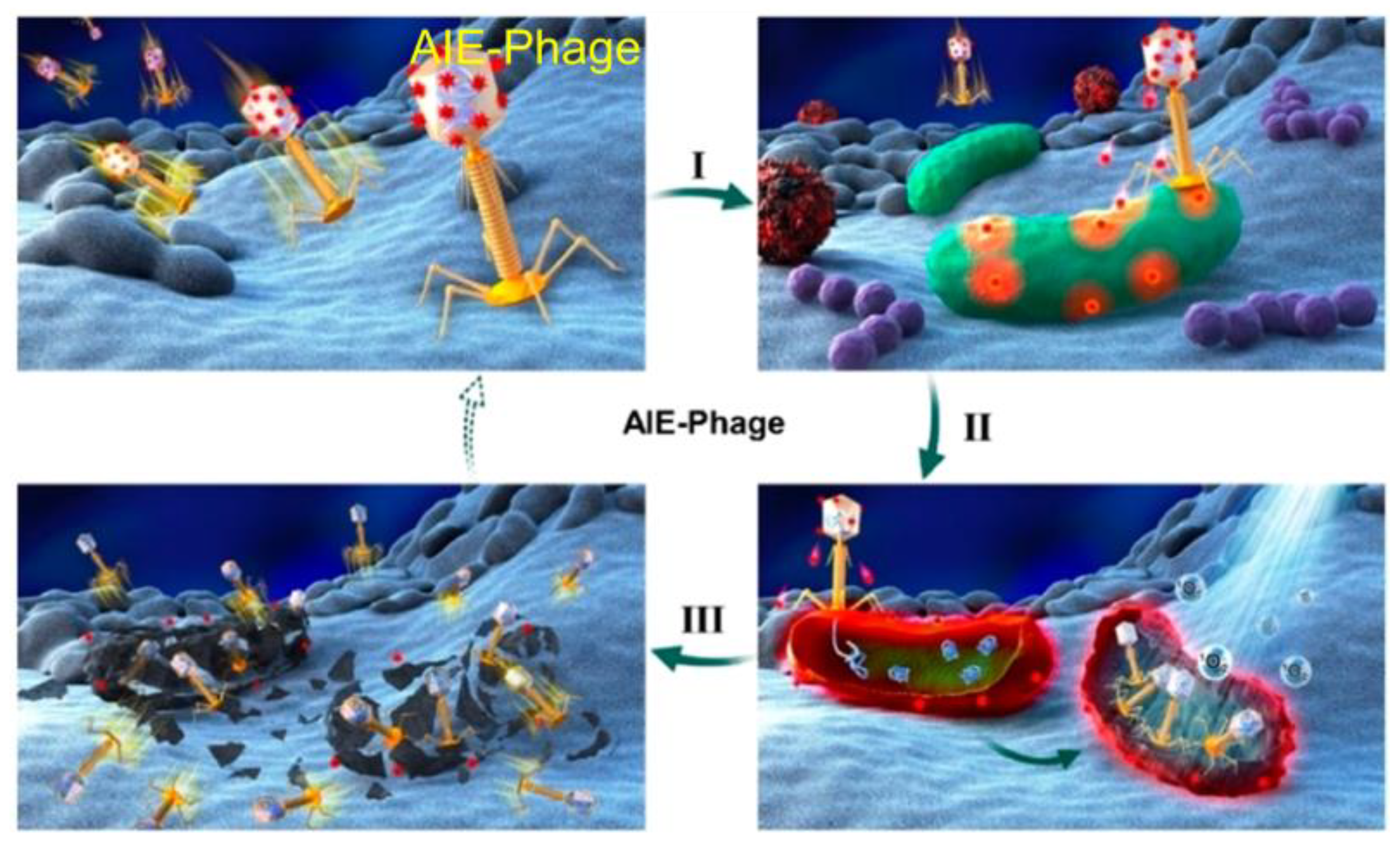
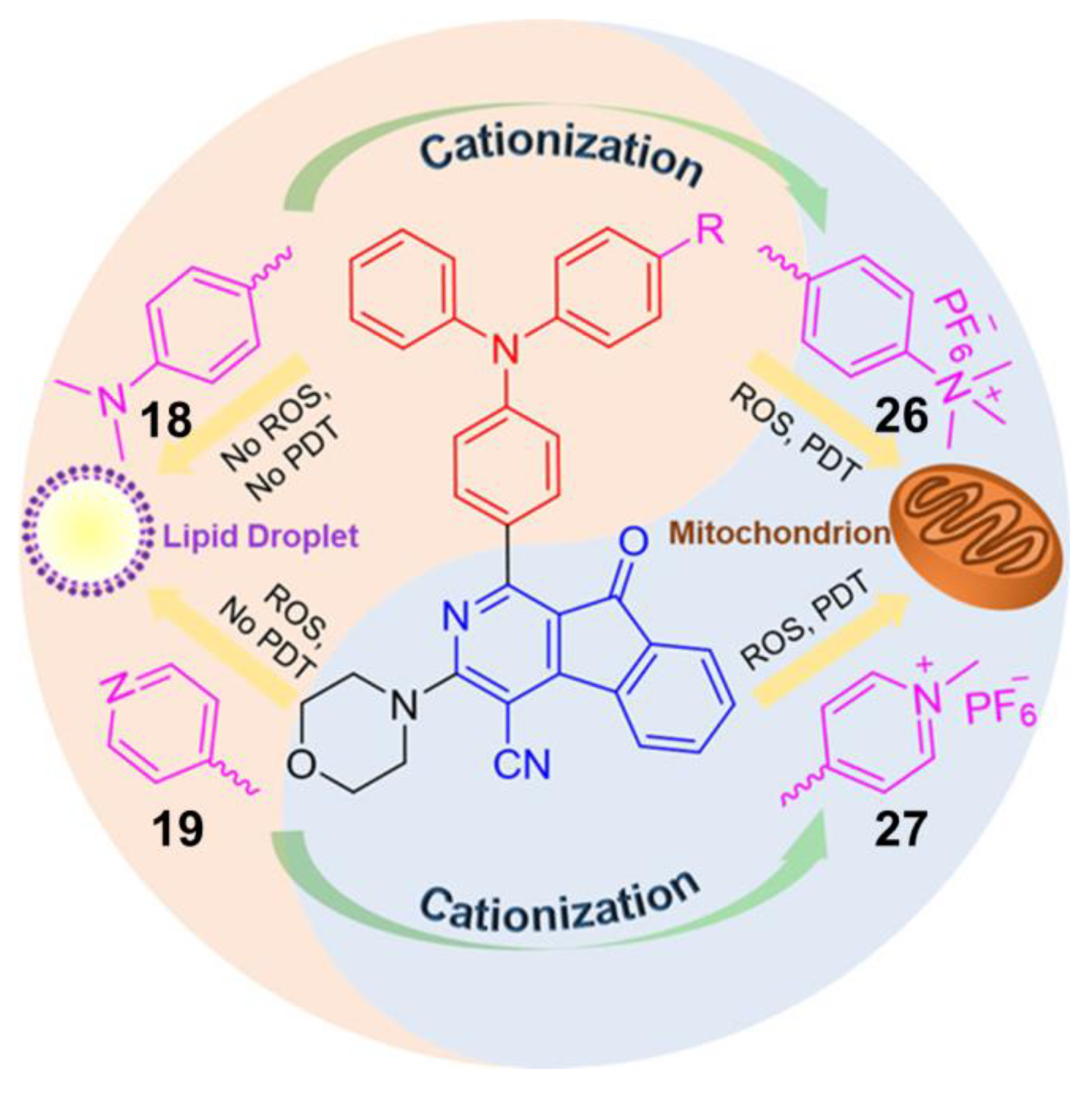
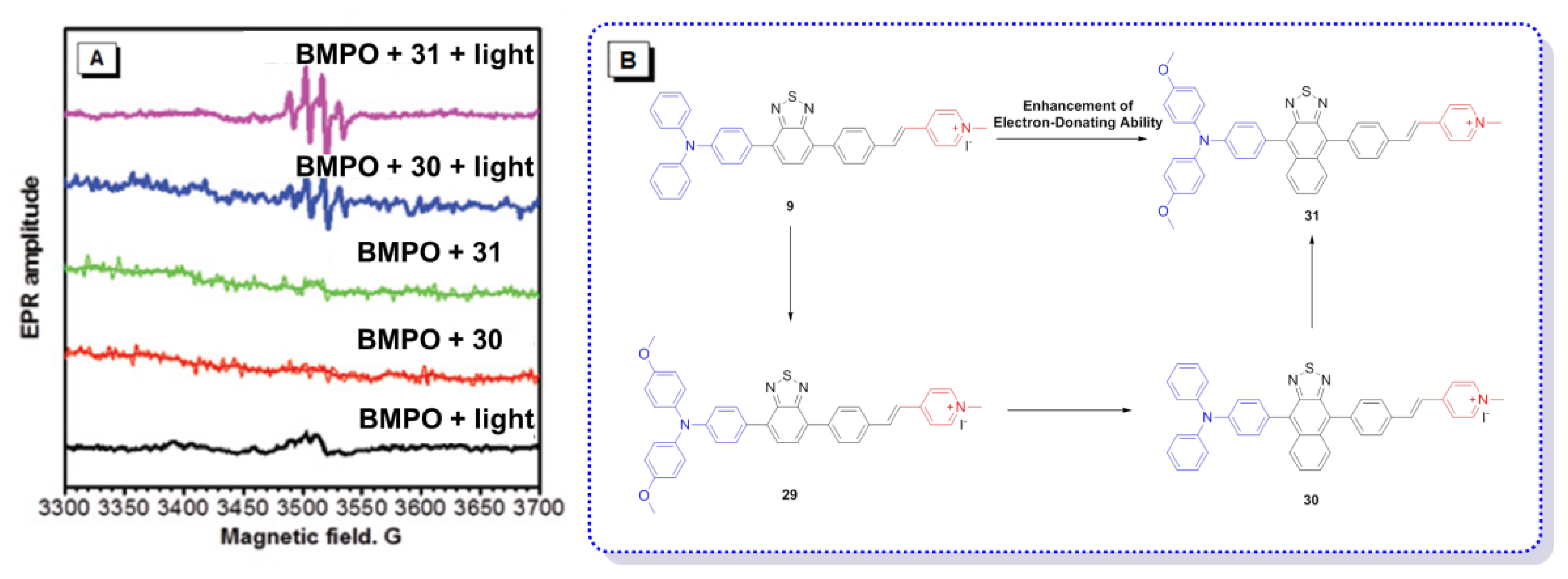
3. AIE–PSs Based on Electron Transfer (Type I) Mechanism
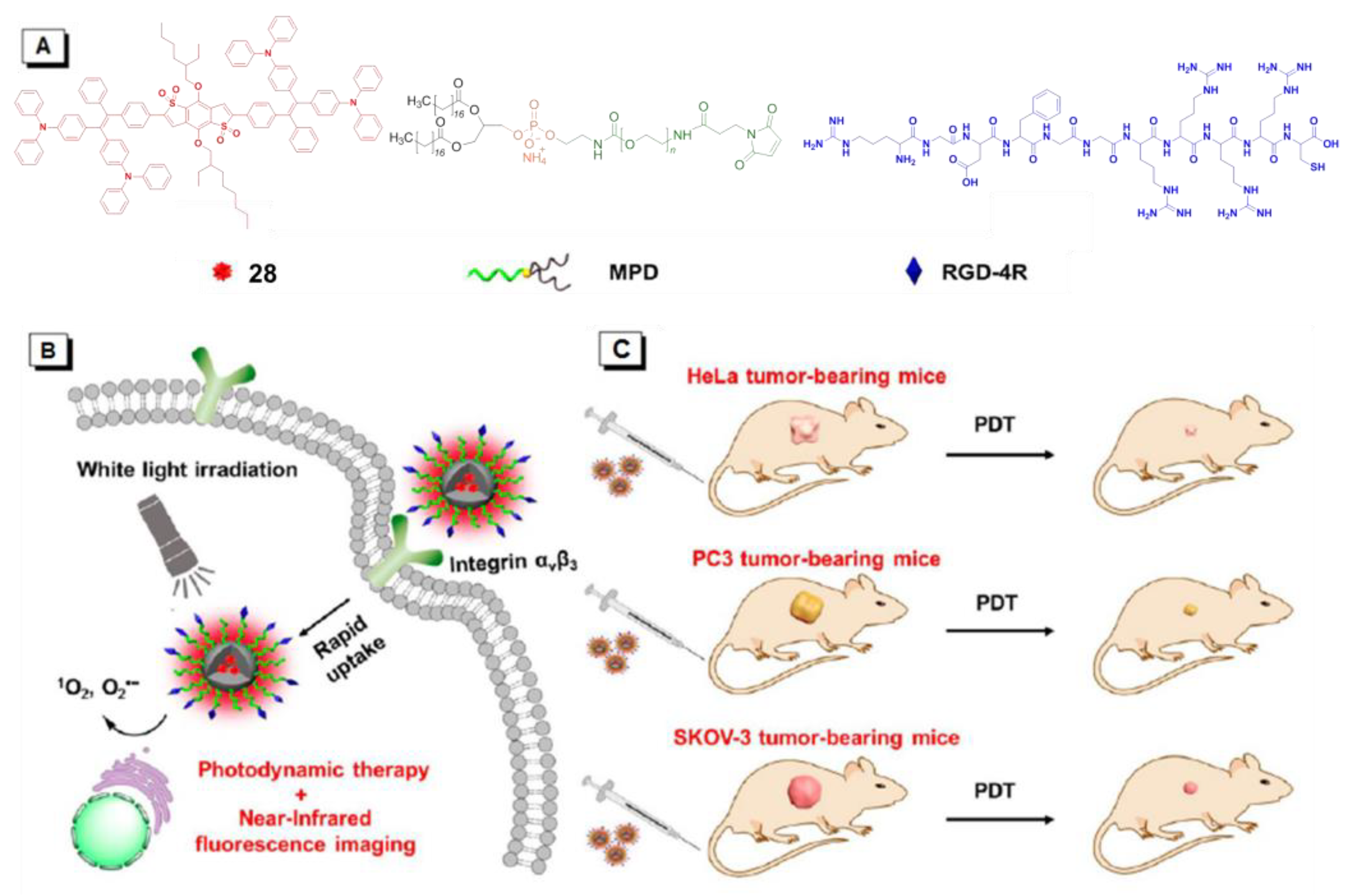
4. AIE-PSs Based on Energy Transfer (Type II) Mechanism
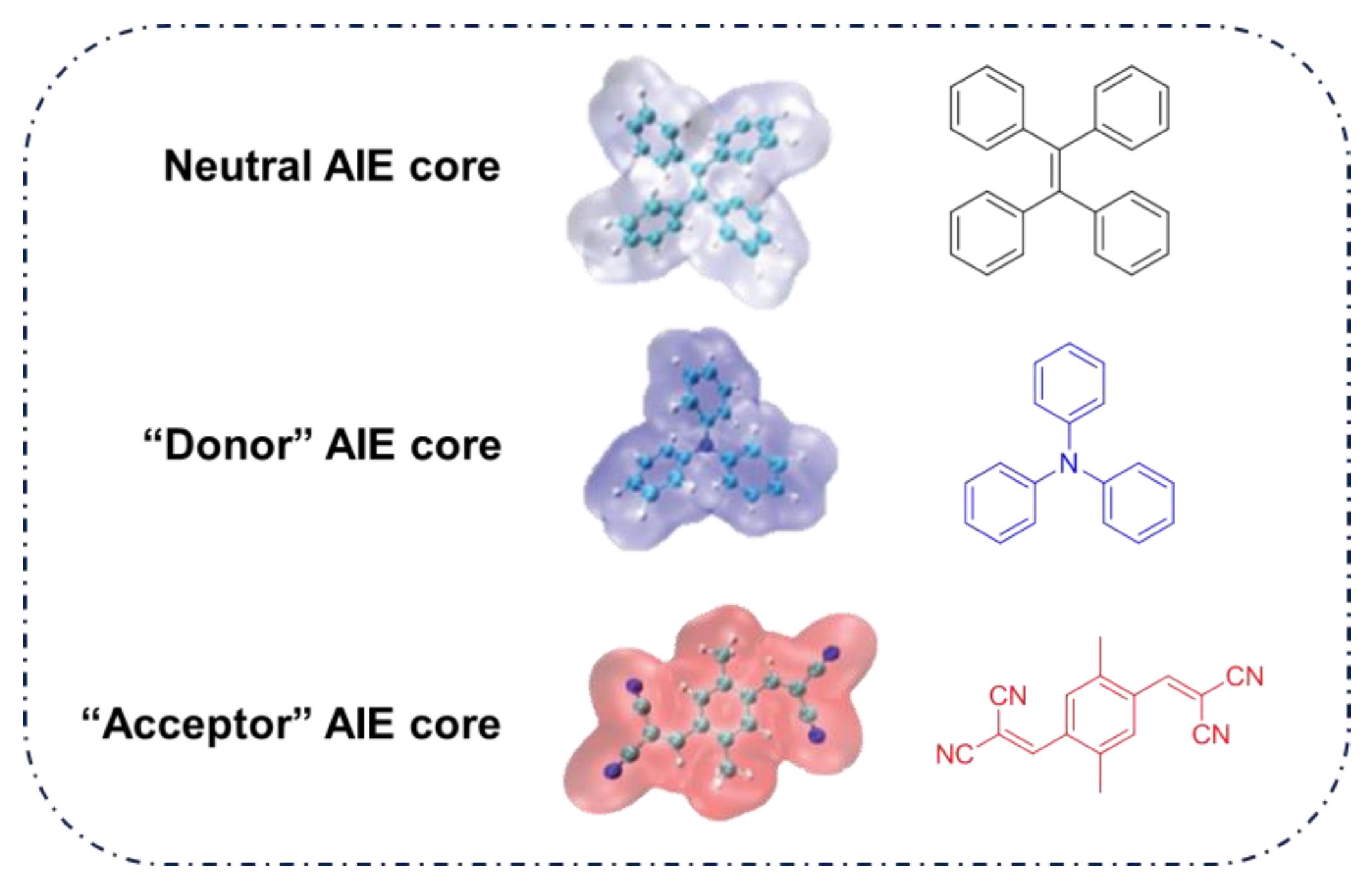
4.1. Donor-AIE (Neutral)-Acceptor PSs
4.2. AIE (Donor)-Acceptor PSs
5. Both Energy Transfer and Electron Transfer AIE–PSs
6. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, J.; Lu, H.; Zhao, X.; Li, W.; Guan, Y.; Zheng, Y.; Zhang, L.; Gao, H. A multi-functional fluorescent probe with aggregation-induced emission characteristics: Mitochondrial imaging, photodynamic therapy and visualizing therapeutic process in zebrafish model. Dye Pigment. 2018, 151, 45–53. [Google Scholar] [CrossRef]
- Umar, A.; Dunn, B.K.; Greenwald, P. Future directions in cancer prevention. Nat. Rev. Cancer 2012, 12, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-Induced Emission Photosensitizers: From Molecular Design to Photodynamic Therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–950. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. Comput. Chem. 2017, 70, 343–394. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Wong, R.C.H.; Lo, P.-C.; Ng, D.K. Stimuli responsive phthalocyanine-based fluorescent probes and photosensitizers. Coord. Chem. Rev. 2019, 379, 30–46. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Shan, G.; Leung, A.C.S.; Lee, M.M.S.; Sung, H.H.-Y.; Williams, I.D.; Lam, J.W.Y.; Tang, B.Z. Facile Synthesis of Red/NIR AIE Luminogens with Simple Structures, Bright Emissions, and High Photostabilities, and Their Applications for Specific Imaging of Lipid Droplets and Image-Guided Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1704039. [Google Scholar] [CrossRef]
- Tang, B.Z.; Huang, Y.; Chen, Y.; Zhao, E.; Hong, Y.; Chen, S.; Lam, J.W.Y.; Chen, Y.; Hou, J.; Tang, B.Z. Amphiphilic Tetraphenylethene-Based Pyridinium Salt for Selective Cell-Membrane Imaging and Room-Light-Induced Special Reactive Oxygen Species Generation. ACS Appl. Mater. Interfaces 2019, 11, 10567–10577. [Google Scholar] [CrossRef]
- He, X.; Situ, B.; Gao, M.; Guan, S.; He, B.; Ge, X.; Li, S.; Tao, M.; Zou, H.; Tang, B.Z.; et al. Stereotactic Photodynamic Therapy Using a Two-Photon AIE Photosensitizer. Small 2019, 15, e1905080. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Liu, Z.; Zou, H.; Zhao, Z.; Zhang, P.; Shan, G.-G.; Kwok, R.T.K.; Lam, J.W.Y.; Zheng, L.; Tang, B.Z. Tuning Organelle Specificity and Photodynamic Therapy Efficiency by Molecular Function Design. ACS Nano 2019, 13, 11283–11293. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, H.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: New Vistas at the Aggregate Level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907. [Google Scholar] [CrossRef]
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-active conjugated polymer with high ROS-generation ability and biocompatibility for efficient photodynamic therapy of bacterial infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 1, 4332–4353. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.-J.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, T.; Tanaka, K.; Kuwatani, Y.; Sung, J.; Nishinaga, T.; Kim, D.; Iyoda, M. Solvent-induced crystalline-state emission and multichromism of a bent π-surface system composed of dibenzocyclooctatetraene units. Chem. Eur. J. 2013, 19, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Saito, S.; Camacho, C.; Kowalczyk, T.; Irle, S.; Yamaguchi, S. Hybridization of a flexible cyclooctatetraene core and rigid aceneimide wings for multi-luminescent flapping π systems. Chem. Eur. J. 2014, 20, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.X.; Tao, X.T.; Ren, Y.; Li, Y.; Yang, J.X.; Yu, W.T.; Wang, L.; Jiang, M.H. Synthesis, structure, and aggregation-induced emission of a novel lambda (Λ)-shaped pyridinium salt based on Tröger’s base. J. Phys. Chem. C 2007, 111, 12811–12816. [Google Scholar] [CrossRef]
- Tang, B.Z.; Kwok, R.T.K.; Li, X.; Gui, C.; Lam, J.W.Y.; Qu, J.; Tang, B.Z. A simple mitochondrial targeting AIEgen for image-guided two-photon excited photodynamic therapy. J. Mater. Chem. B 2018, 6, 2557–2565. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.D.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, Y.; Liu, B. Recent advances of AIE dots in NIR imaging and phototherapy. Nanoscale 2019, 11, 19241–19250. [Google Scholar] [CrossRef]
- Foote, C.S. Definition of TYPE I and Type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coordin. Chem. Rev. 2002, 233, 351–371. [Google Scholar] [CrossRef]
- Boyer, R.F.; McCleary, C.J. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radic. Biol. Med. 1987, 3, 389–395. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Maidt, L.; Poyer, L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem. J. 1990, 269, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Oszajca, M.; Brindell, M.; Orzeł, Ł.; Dąbrowski, J.M.; Śpiewak, K.; Łabuz, P.; Pacia, M.; Stochel-Gaudyn, A.; Macyk, W.; Van Eldik, R.; et al. Mechanistic studies on versatile metal-assisted hydrogen peroxide activation processes for biomedical and environmental incentives. Coord. Chem. Rev. 2016, 327–328, 143–165. [Google Scholar] [CrossRef]
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simoes, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of Singlet-Oxygen and Superoxide-Ion Generation by Porphyrins and Bacteriochlorins and their Implications in Photodynamic Therapy. Chem. Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Arnaut, L.G.; Pereira, M.M.; Dąbrowski, J.M.; Silva, E.F.F.; Schaberle, F.A.; Abreu, A.R.; Rocha, L.B.; Barsan, M.M.; Urbańska, K.; Stochel, G.; et al. Photodynamic Therapy Efficacy Enhanced by Dynamics: The Role of Charge Transfer and Photostability in the Selection of Photosensitizers. Chem. A Eur. J. 2014, 20, 5346–5357. [Google Scholar] [CrossRef] [PubMed]
- Krzykawska-Serda, M.; Dąbrowski, J.M.; Arnaut, L.G.; Szczygieł, M.; Urbanska, K.; Stochel, G.; Elas, M. The role of strong hypoxia in tumors after treatment in the outcome of bacteriochlorin-based photodynamic therapy. Free. Radic. Biol. Med. 2014, 73, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, R.; Rocha, L.B.; Dąbrowski, J.M.; Arnaut, L.G. Modulation of Biodistribution, Pharmacokinetics, and Photosensitivity with the Delivery Vehicle of a Bacteriochlorin Photosensitizer for Photodynamic Therapy. ChemMedChem 2013, 9, 390–398. [Google Scholar] [CrossRef]
- Wilkinson, F.; Abdel-Shafi, A.A. Mechanism of Quenching of Triplet States by Oxygen: Biphenyl Derivatives in Acetonitrile. J. Phys. Chem. A 1997, 101, 5509–5516. [Google Scholar] [CrossRef]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef]
- Guan, Y.; Lu, H.; Li, W.; Zheng, Y.; Jiang, Z.; Zou, J.; Gao, H. Near-Infrared Triggered Upconversion Polymeric Nanoparticles Based on Aggregation-Induced Emission and Mitochondria Targeting for Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 26731–26739. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Xu, W.; Chu, Y.; Yang, J.; Yan, Y.; Hu, Y.; Wang, Y.; Hua, J. Fine tuning of pyridinium-functionalized dibenzo[a,c]phenazine near-infrared AIE fluorescent biosensors for the detection of lipopolysaccharide, bacterial imaging and photodynamic antibacterial therapy. J. Mater. Chem. C 2019, 7, 12509–12517. [Google Scholar] [CrossRef]
- Gao, F.; Wu, J.; Gao, H.; Hu, X.; Liu, L.; Midgley, A.C.; Liu, Q.; Sun, Z.; Liu, Y.; Ding, D.; et al. Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics. Biomaterials 2020, 230, 119635. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Dai, J.; Yu, M.; Li, J.; Shen, P.; Hu, R.; Lou, X.; Zhao, Z.; Tang, B.Z. Type I photosensitizers based on phosphindole oxide for photodynamic therapy: Apoptosis and autophagy induced by endoplasmic reticulum stress. Chem. Sci. 2020, 11, 3405–3417. [Google Scholar] [CrossRef]
- Hu, F.; Xu, S.; Liu, B. Photosensitizers with Aggregation-Induced Emission: Materials and Biomedical Applications. Adv. Mater. 2018, 30, e1801350. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Duan, Y.; Liu, B. Precise Molecular Design for High-Performance Luminogens with Aggregation-Induced Emission. Adv. Mater. 2020, 32, e1903530. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Nicol, A.; Qin, W.; Kwok, R.T.K.; Burkhartsmeyer, J.M.; Zhu, Z.; Su, H.; Luo, W.; Lam, J.W.Y.; Qian, J.; Wong, K.S.; et al. Functionalized AIE nanoparticles with efficient deep-red emission, mitochondrial specificity, cancer cell selectivity and multiphoton susceptibility. Chem. Sci. 2017, 8, 4634–4643. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Hu, F.; Xu, S.; Chen, C.; Zhang, C.-J.; Cheng, X.; Yuan, Y.; Ding, D.; Kong, D.; et al. A Highly Efficient and Photostable Photosensitizer with Near-Infrared Aggregation-Induced Emission for Image-Guided Photodynamic Anticancer Therapy. Adv. Mater. 2017, 29, 1700548. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Manghnani, P.; Xu, S.; Wang, Y.; Goh, C.C.; Ng, L.G.; Liu, B. Polymerization-Enhanced Two-Photon Photosensitization for Precise Photodynamic Therapy. ACS Nano 2019, 13, 3095–3105. [Google Scholar] [CrossRef]
- Kang, M.; Kwok, R.T.K.; Wang, J.; Zhang, H.; Lam, J.W.Y.; Li, Y.; Zhang, P.; Zou, H.; Gu, X.; Li, F.; et al. A multifunctional luminogen with aggregation-induced emission characteristics for selective imaging and photodynamic killing of both cancer cells and Gram-positive bacteria. J. Mater. Chem. B 2018, 6, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, W.; Guo, X.; Li, Y.; Ma, Y.; Chen, L.; Zhang, H.; Wang, T.; Zhang, X.; Wang, Z. Mitochondria-Targeted Polydopamine Nanocomposite with AIE Photosensitizer for Image-Guided Photodynamic and Photothermal Tumor Ablation. Small 2019, 15, e1902352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, L.; Ma, D.; Xu, S.; Wu, W.; Xu, L.; Panahandeh-Fard, M.; Zhu, X.; Wang, B.; Liu, B. Tumor-Activated and Metal–Organic Framework Assisted Self-Assembly of Organic Photosensitizers. ACS Nano 2020, 14, 13056–13068. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Zhang, R.; Zhuang, Z.; Li, Y.; Huang, Y.; Wang, Z.; Zhang, W.; Hou, J.; Tang, B.Z. Molecular Engineering to Boost AIE-Active Free Radical Photogenerators and Enable High-Performance Photodynamic Therapy under Hypoxia. Adv. Funct. Mater. 2020, 30, 2002057. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, L.; Ma, B.; Kong, Q.; Li, G.; Wang, Y.; Tang, B.Z. Multifunctional Two-Photon AIE Luminogens for Highly Mitochondria-Specific Bioimaging and Efficient Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20715–20724. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; He, B.; Zhang, Z.; Li, Y.; Kang, M.; Wang, Y.; Li, K.; Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens Married to 2D Black Phosphorus Nanosheets for Highly Efficient Multimodal Theranostics. Adv. Mater. 2020, 32, 2003382. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Guo, Y.; Lu, S.; Du, Y.; Li, J.-J.; Zhang, X.; Leung, N.L.C.; Zhao, Z.; Niu, G.; et al. Phage-Guided Targeting, Discriminative Imaging, and Synergistic Killing of Bacteria by AIE Bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969. [Google Scholar] [CrossRef]
- Shi, X.; Sung, S.H.P.; Chau, J.H.C.; Li, Y.; Liu, Z.; Kwok, R.T.K.; Liu, J.; Xiao, P.; Zhang, J.; Liu, B.; et al. Killing G(+) or G(−) Bacteria? The Important Role of Molecular Charge in AIE-Active Photosensitizers. Small Methods 2020, 4, 2000046. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Che, W.; Li, D.; Li, G.; Xie, Z.; Song, N.; Liu, S.; Tang, B.Z.; Liu, X.; et al. AIE Multinuclear Ir(III) Complexes for Biocompatible Organic Nanoparticles with Highly Enhanced Photodynamic Performance. Adv. Sci. 2019, 6, 1802050. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; He, X.; He, Z.; Yang, X.; Tian, S.; Meng, F.; Ding, D.; Luo, L.; Tang, B.Z. A Dual-Functional Photosensitizer for Ultraefficient Photodynamic Therapy and Synchronous Anticancer Efficacy Monitoring. Adv. Funct. Mater. 2019, 29, 1902673. [Google Scholar] [CrossRef]
- Li, Q.; Li, Y.; Min, T.; Gong, J.; Du, L.; Phillips, D.L.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; et al. Time-Dependent Photodynamic Therapy for Multiple Targets: A Highly Efficient AIE-Active Photosensitizer for Selective Bacterial Elimination and Cancer Cell Ablation. Angew. Chem. Int. Ed. 2020, 59, 9470–9477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kuang, H.; Xu, Y.; Shi, L.; Cao, W.; Zhu, K.; Xu, L.; Ma, J. Rational design of a high-performance quinoxalinone-based AIE photosensitizer for image-guided photodynamic therapy. ACS Appl. Mater. Interfaces 2020, 12, 42551–42557. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zheng, Q.C.; Xu, S.; Yuan, Y.; Cheng, X.; Jiang, S.; Kenry; Yu, Q.; Song, Z.; Liu, B.; et al. Theranostic Nanodots with Aggregation-Induced Emission Characteristic for Targeted and Image-Guided Photodynamic Therapy of Hepatocellular Carcinoma. Theranostics 2019, 9, 1264–1279. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhenyuan, M.; Han, G.; Liu, M.; Shan, B.; Dong, G.; Miao, Z.; Jianzhong, Y.; Tan, Z.; Li, B.; et al. Chlorin p6-Based Water-Soluble Amino Acid Derivatives as Potent Photosensitizers for Photodynamic Therapy. J. Med. Chem. 2016, 59, 4999–5010. [Google Scholar] [CrossRef]
- Xu, Y.; He, R.; Lin, D.; Ji, M.; Chen, J. Laser beam controlled drug release from Ce6–gold nanorod composites in living cells: A FLIM study. Nanoscale 2015, 7, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Y.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Structural and process controls of AIEgens for NIR-II theranostics. Chem. Sci. 2020. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Z.; Liu, S.; Zhang, H.; Wong, S.T.H.; Lam, J.W.Y.; Kwok, R.T.K.; Qian, J.; Tang, B.Z. Design of AIEgens for near-infrared IIb imaging through structural modulation at molecular and morphological levels. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Yu, C.Y.Y.; Xu, H.; Ji, S.; Kwok, R.T.K.; Lam, J.W.Y.; Li, X.; Krishnan, S.; Ding, D.; Tang, B.Z. Mitochondrion-anchoring photosensitizer with aggregation-induced emission characteristics synergistically boosts the radiosensitivity of cancer cells to ionizing radiation. Adv. Mater. 2017, 29, 1606167. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, T.; Liu, H.; Chen, Y.; Kwok, R.T.K.; Ma, C.; Zhang, P.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Bright Near-Infrared Aggregation-Induced Emission Luminogens with Strong Two-Photon Absorption, Excellent Organelle Specificity, and Efficient Photodynamic Therapy Potential. ACS Nano 2018, 12, 8145–8159. [Google Scholar] [CrossRef]
- Jin, G.; He, R.; Liu, Q.; Lin, M.; Dong, Y.; Li, K.; Tang, B.Z.; Liu, B.; Xu, F. Near-infrared light-regulated cancer theranostic nanoplatform based on aggregation-induced emission luminogen encapsulated upconversion nanoparticles. Theranostics 2019, 9, 246–264. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient Near-Infrared Photosensitizer with Aggregation-Induced Emission for Imaging-Guided Photodynamic Therapy in Multiple Xenograft Tumor Models. ACS Nano 2020, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]

| AIE-PSs | Chemical Structures | Absorption/Emission [nm] | ΔEST [eV] (a) | Consuming Rate of ABDA | Treatment Efficiency | 1O2 Quantum Yield (b) | Ref. |
|---|---|---|---|---|---|---|---|
| Donor-AIE (Neutral)-Acceptor AIE–PSs | |||||||
| 1 | 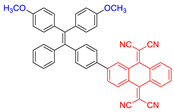 | 520/820 | 0.067 | 3.58 nmol min−1 | IC50 = 5.0 μg mL−1 (4T1 cell) White light: 60 mW cm−2 for 5 min | 0.08 | [49] |
| 2 | 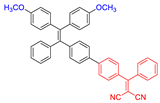 | 410/620 | 0.23 | 3.98 nmol min−1 | IC50 = 8.0 μg mL−1 (4T1 cell) White light: 60 mW cm−2 for 5 min | - | [50] |
| 3 | 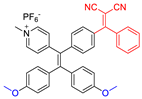 | 440/606 | - | - | IC50 = 1.5 × 10−6 M (HeLa cell) White light: 36 mW cm−2 for 40 min | - | [51] |
| 4 | 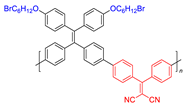 | 410/610 | - | 9.03 nmol min−1 | - | - | [50] |
| 5 | 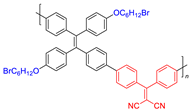 | 410/620 | - | 21.8 nmol min−1 | - | 0.55 | [50] |
| 6 | 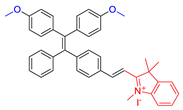 | 480/710 | 0.63 | - | IC50 = 70.72 μg mL−1 polymer (HeLa cell) White light: 100 mW cm−2 for 5 min | 0.89 | [52] |
| 7 | 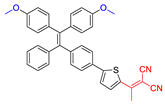 | 450/651 | 0.50 | 12.0 nmol min−1 | - | - | [45] |
| 8 |  | 450/650 | 0.33 | 27.0 nmol min−1 | IC50 = 1 μg mL−1 (MDA-MB-231) White light: 60 mW cm−2 for 5 min | Higher than 0.08 | [53] |
| AIE (Donor)-Acceptor AIE–PSs | |||||||
| 9 |  | 447/625 | 0.15 | - | - | - | [54] |
| 10 |  | 442/558 | - | 70.20 nmol min−1 | 55% killing effect of HeLa cell 10 × 10−6 M White light: 60 mW cm−2 for 30 min | 3.17 | [55] |
| 11 | 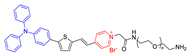 | 480/680 | - | - | IC50 = 10.0 μg mL−1 (4T1 cell) White light: 24 mW cm−2 for 30 min | - | [56] |
| 12 |  | 448/592 | - | 121.74 nmol min−1 | 75% killing effect of HeLa cell 10 × 10−6 M White light: 60 mW cm−2 for 30 min | 3.71 | [55] |
| 13 |  | 508/720 | 0.093 | - | 83.2% killing effect of E. Coli 10 × 10−6 M White light: 60 mW cm−2 for 10 min | 0.71 | [42] |
| 14 | 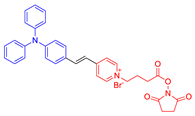 | 457/630 | - | - | 79% killing effect of P. aeruginosa 10 × 10−6 M White light: 4.2 mW cm−2 for 30 min | 0.72 | [57] |
| 15 | 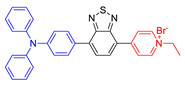 | 486/705 | 0.79 | 18.2 nmol min−1 | 98.4% killing effect of E. Coli 10 × 10−6 M White light: 4.2 mW cm−2 for 30 min | - | [58] |
| 16 | 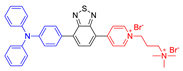 | 493/685 | - | 15.3 nmol min−1 | 87.3% killing effect of E. Coli 10 × 10−6 M White light: 4.2 mW cm−2 for 30 min | - | [58] |
| 17 | 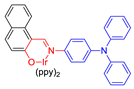 | 469/652 | - | - | IC50 = 1.1 × 10−5 M (HeLa cell) White light: 20 mW cm−2 for 30 min | - | [59] |
| 18 |  | 417/601 | 0.09 | - | - | - | [13] |
| 19 |  | 409/593 | 0.16 | 0.7 nmol min−1 | - | - | [13] |
| 20 | 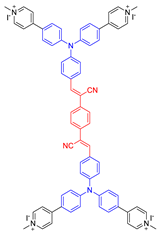 | 405/580 | - | - | IC50 = 3.0 × 10−7 M (HeLa cell) White light: 4 mW cm−2 for 10 min | 0.98 | [60] |
| 21 | 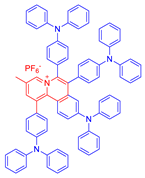 | 440/590 | 0.55 | - | More than 70% killing effect of E. Coli 5 × 10−6 M White light: 10 mW cm−2 for 15 min | 0.98 | [61] |
| 22 |  | 530/800 | 0.12 | - | IC50 = 3.26 μg mL−1 (Hela cell) White light: 100 mW cm−2 for 5 min | 0.63 | [62] |
| 23 | 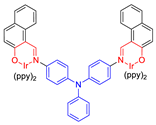 | 469/671 | - | - | IC50 = 6.3 × 10−6 M (HeLa cell) White light: 20 mW cm−2 for 30 min | - | [59] |
| 24 | 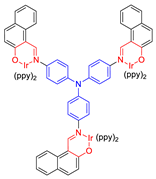 | 469/690 | - | - | IC50 = 1.1 × 10−6 M (HeLa cell) White light: 20 mW cm−2 for 30 min | - | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Wang, Y.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction. Molecules 2021, 26, 268. https://doi.org/10.3390/molecules26020268
Ni J, Wang Y, Zhang H, Sun JZ, Tang BZ. Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction. Molecules. 2021; 26(2):268. https://doi.org/10.3390/molecules26020268
Chicago/Turabian StyleNi, Juechen, Yijia Wang, Haoke Zhang, Jing Zhi Sun, and Ben Zhong Tang. 2021. "Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction" Molecules 26, no. 2: 268. https://doi.org/10.3390/molecules26020268
APA StyleNi, J., Wang, Y., Zhang, H., Sun, J. Z., & Tang, B. Z. (2021). Aggregation-Induced Generation of Reactive Oxygen Species: Mechanism and Photosensitizer Construction. Molecules, 26(2), 268. https://doi.org/10.3390/molecules26020268








