Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking
Abstract
:1. Introduction
2. Results and Discussion
2.1. Nutritional Composition
2.2. Antioxidant Profile
2.3. Obtention of Phenolic-Enriched Extracts of G. tinctoria Leaves
2.3.1. Total Phenolic Content
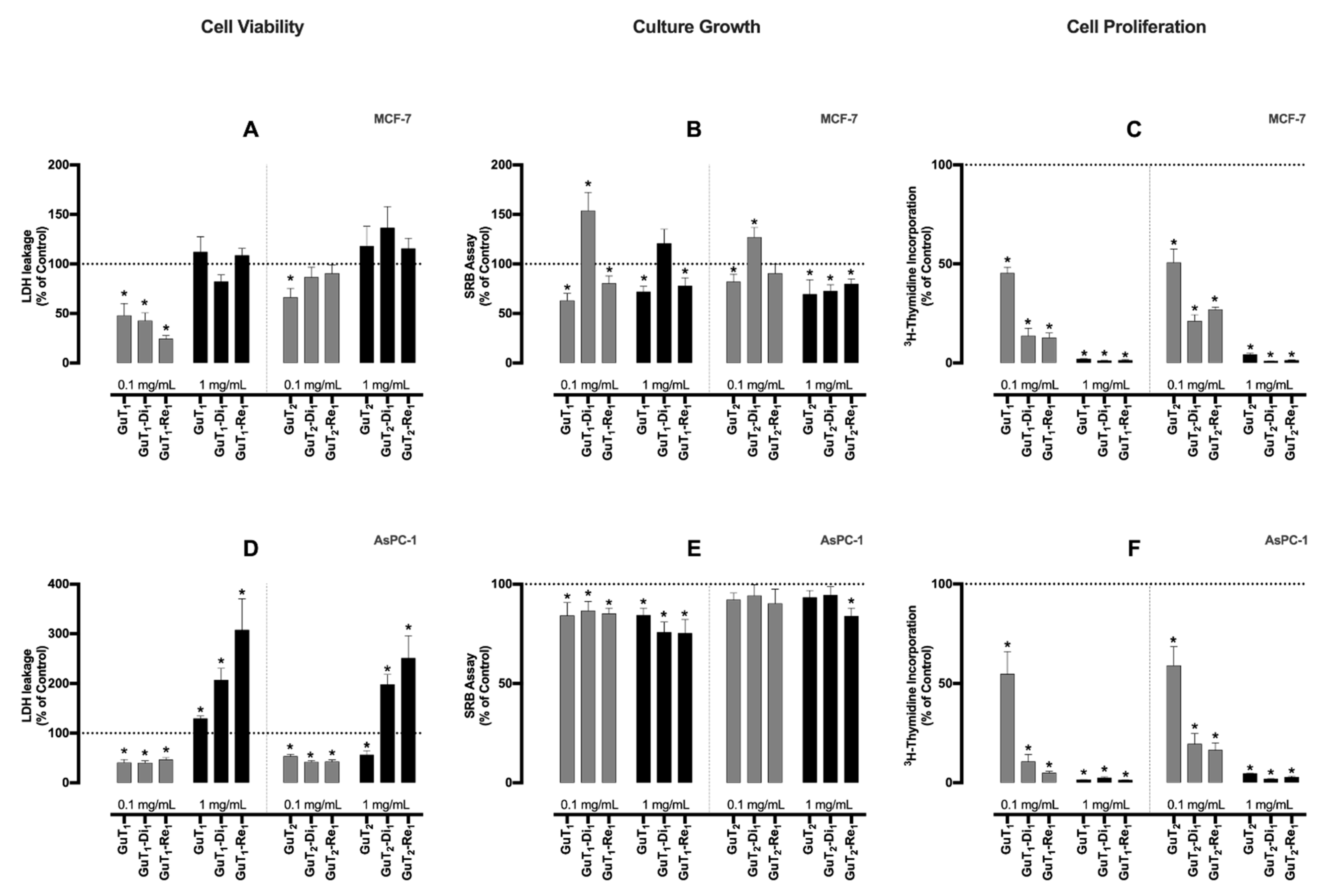
2.4. Bioassays
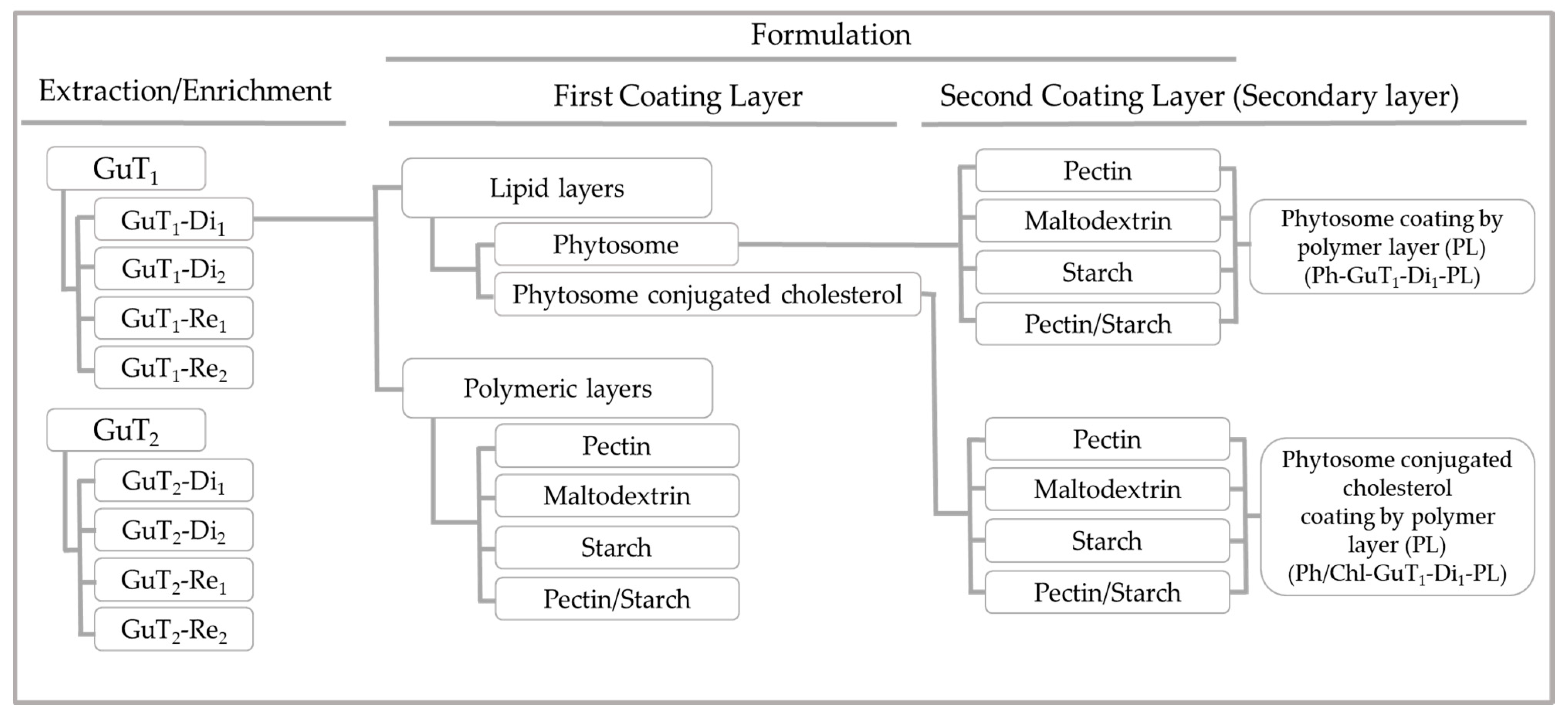
2.5. Physicochemical Properties of Nano/Micro-Particles
2.5.1. Formulation Yield
2.5.2. Fourier-Transform Infrared Spectroscopy Analyses (FTIR)
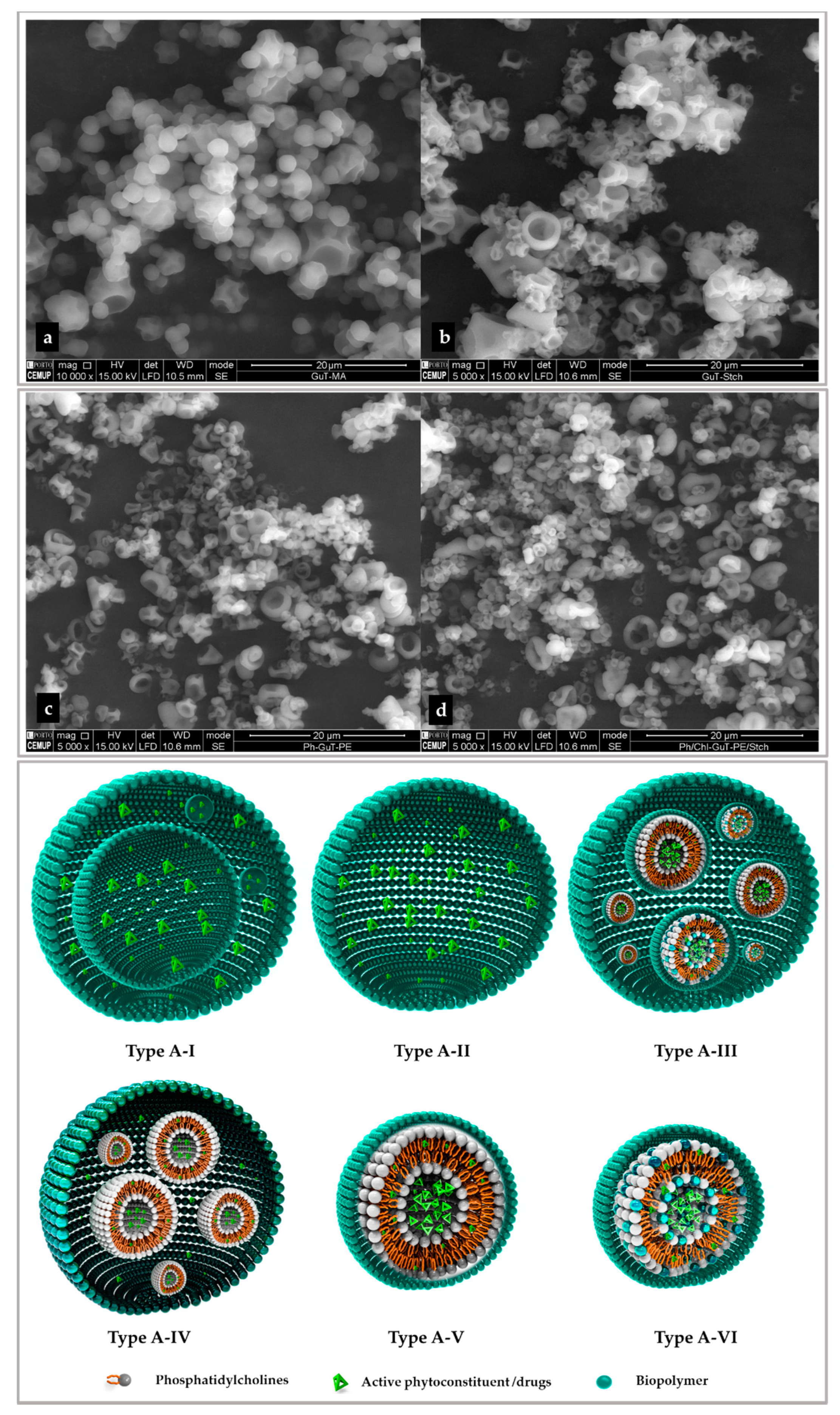
2.5.3. Particle Size Distribution and Morphology
2.5.3.1. Lipid-Based Layers
2.5.3.2. Polymeric Layers
2.5.3.3. Secondary Layers
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Zeta Potential (Surface Charge)
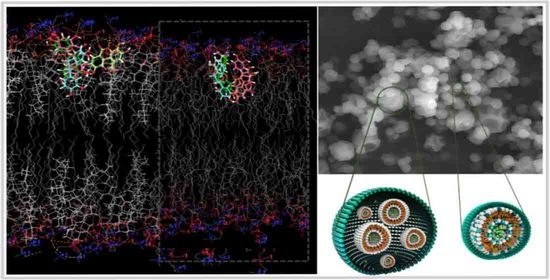
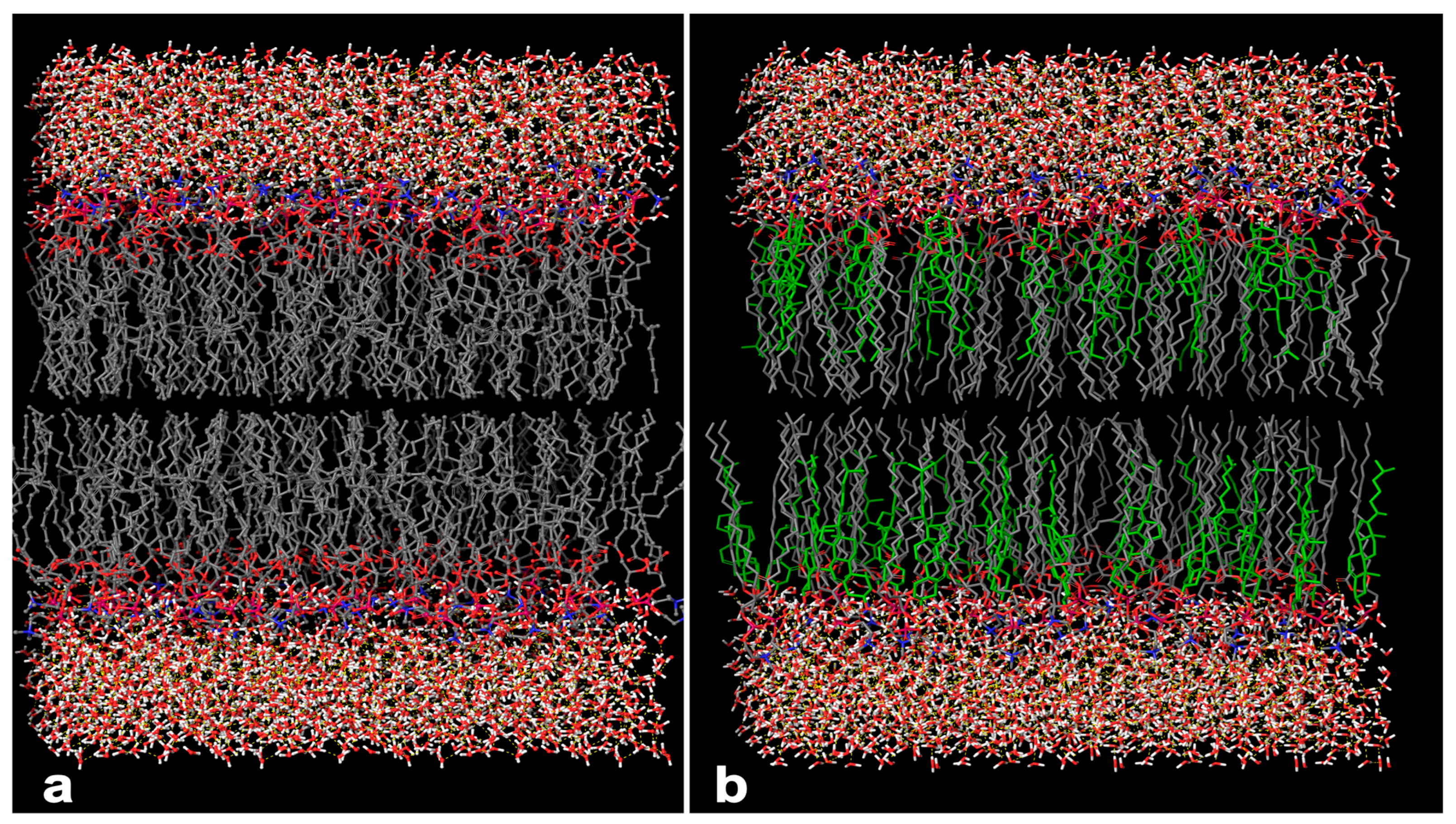
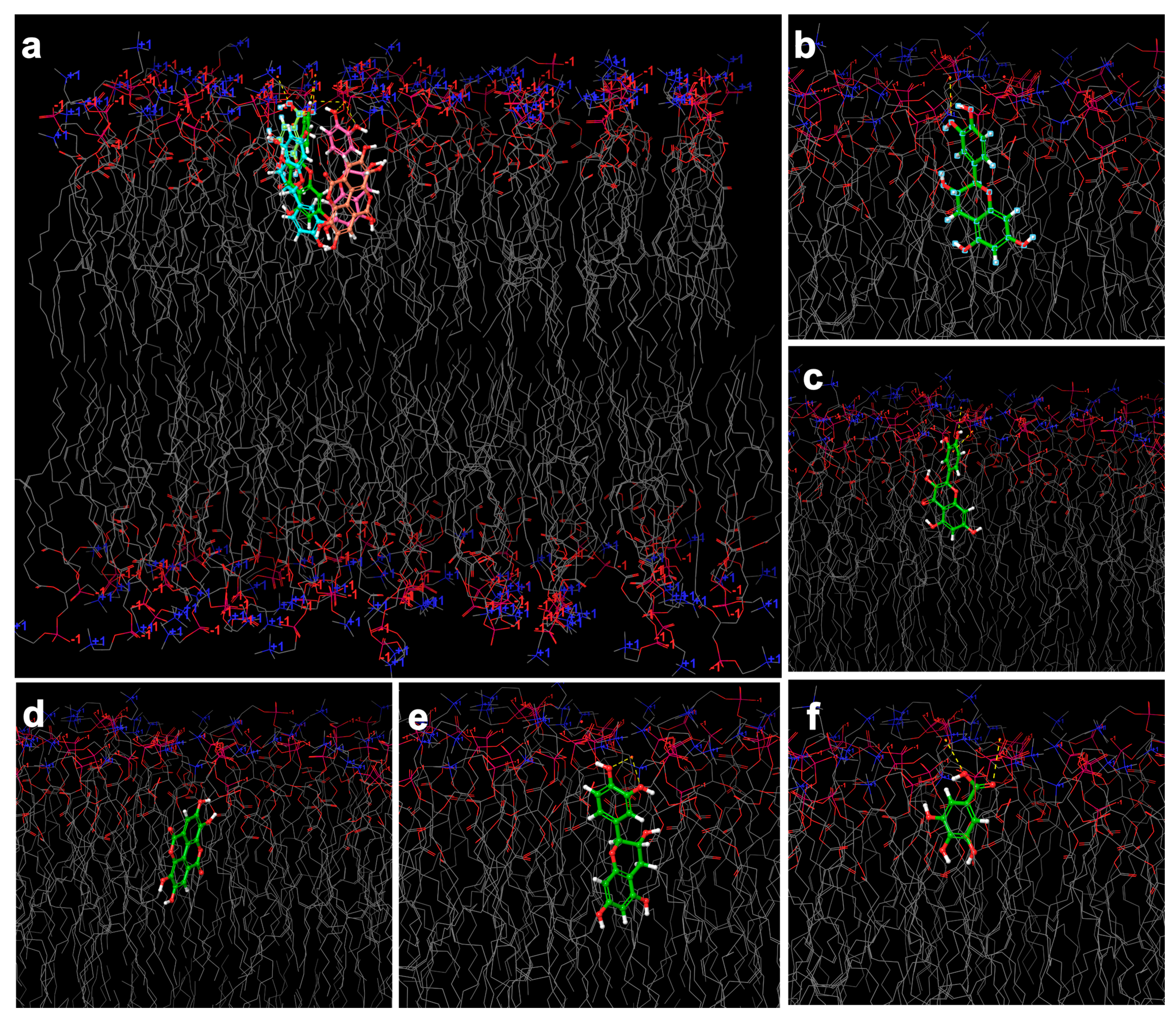
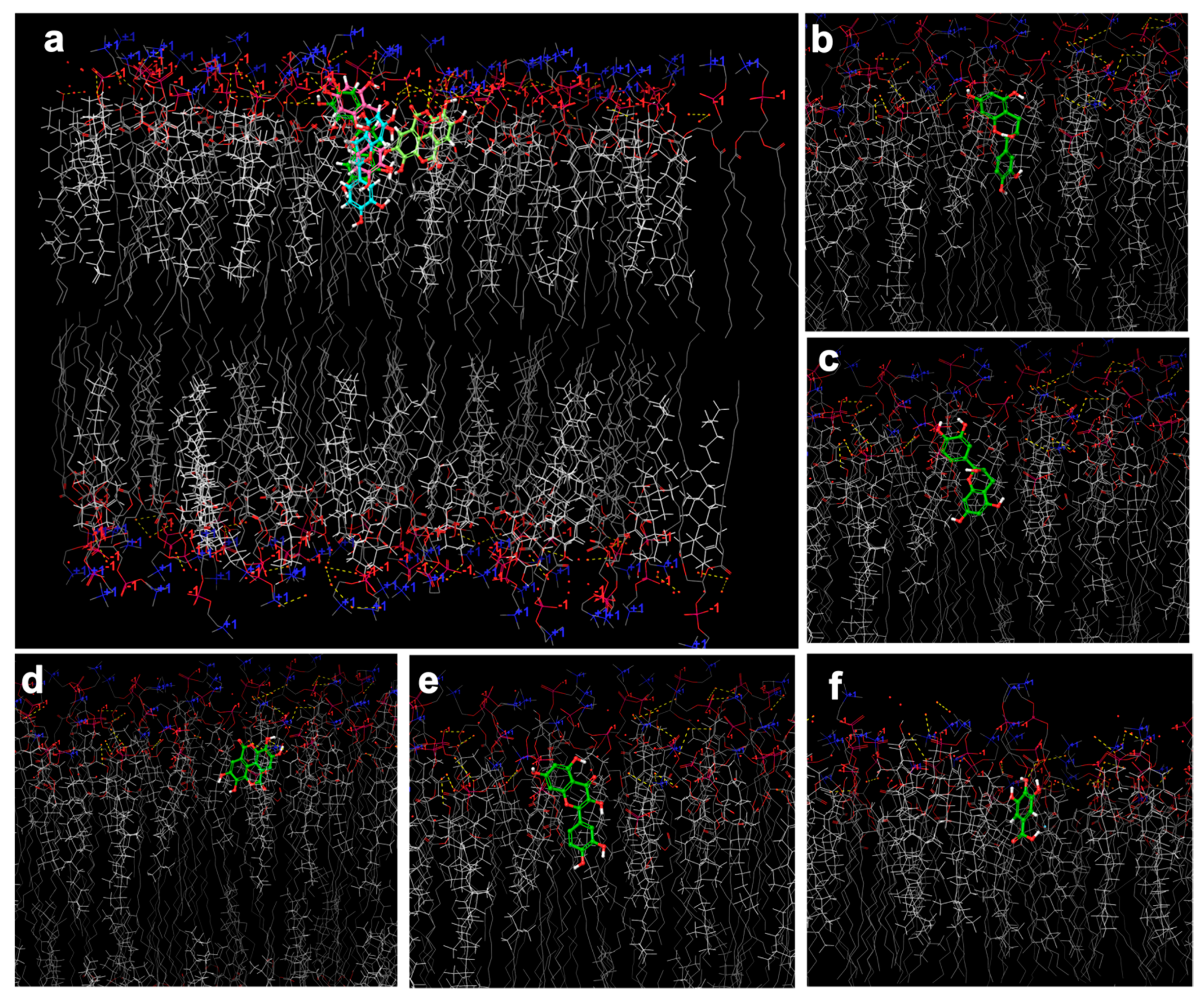
2.6. In Silico Investigation Based on Molecular Arrangement
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
3.3. Nutritional Composition
3.3.1. Moisture Content
3.3.2. Ash Content
3.3.3. Protein Content
3.3.4. Fat Content
3.3.5. Total, Soluble, and Insoluble Fiber Content
3.3.6. Available Carbohydrates
3.4. Antioxidant Profile
3.4.1. Total Phenolics Content
3.4.2. Total Flavonoids Content
3.4.3. Ferric Reducing Antioxidant Power
3.4.4. DPPH● Scavenging Ability
3.5. Obtention of Phenolic-Enriched Extracts of G. tinctoria Leaves
Total Phenolic Content
3.6. Bioassays
3.6.1. Cells and Cell Cultures
3.6.2. Cell Viability
3.6.3. Cell Growth
3.6.4. Evaluation of Cell Proliferation
3.7. Particles Production
3.7.1. Preparation of Nano-Phytosome and Cholesterol-Conjugated Phytosome
3.7.2. Polymeric Layer
3.7.3. Secondary Layer
- GuT1-Di1, lecithin, biopolymer (individual) (1:1:0.5:10);
- GuT1-Di1, lecithin, biopolymer (complex) (1:1:0.5:5:5);
- GuT1-Di1, lecithin, cholesterol, biopolymer (individual) (1:1:0.5:15);
- GuT1-Di1, lecithin, cholesterol, biopolymer (complex) (1:1:0.5:7.5:7.5).
3.7.4. Spray-Drying Conditions
3.8. Physicochemical Properties of Nano/Micro-Particles
3.8.1. Fourier-Transform Infrared Spectroscopy Analyses (FTIR)
3.8.2. Particle Size Distribution
3.8.3. Scanning Electron Microscopy (SEM)
3.8.4. Zeta Potential (Surface Charge)
3.8.5. Molecular Docking Arrangement
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bernardini, S.; Tiezzi, A.; Laghezza, M.V.; Ovidi, E. Natural products for human health: An historical overview of the drug discovery approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Ogle, C.C.; Timmins, S.M.; La, C.G.D.; Clarkson, J. Chilean Rhubarb (Gunnera Tinctoria): Biology, Ecology and Conservation Impacts in New Zealand; Department of Conservation: Wellington, New Zealand, 2005; pp. 1–27. [Google Scholar]
- Fuller, D.Q.; Hickey, L.J. Systematics and leaf architecture of the Gunneraceae. Bot. Rev. 2005, 71, 295–353. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Biological flora of the british Isles: Gunnera tinctoria. Ecology 2013, 101, 243–264. [Google Scholar] [CrossRef]
- Silva, L.; Tavares, J.; Pena, A. Ecological basis for the control of Gunnera tinctoria in São Miguel Island. In Proceedings of the Second International Weed Control Congress Copenhagen, Brown, Copenhagen, Denmark, 25–28 June 1996. [Google Scholar]
- Petzold, G.; Catril, G.; Duarte, C. Caracterización fisicoquímica de pecíolos del pangue (Gunnera tinctoria). Rev. Chil. Nutr. 2006, 33, 539–543. [Google Scholar] [CrossRef]
- Estomba, D.; Ladio, A.; Lozada, M. Medicinal wild plant knowledge and gathering patterns in a Mapuche community from North-western Patagonia. J. Ethnopharmacol. 2006, 103, 109–119. [Google Scholar] [CrossRef]
- Sabando, C.; Rodríguez-Díaz, M.; Ide, W.; Pastene, E.; Avello, M.; Simirgiotis, M.; Rojas, S.; Villarroel, E.; Silva-Grecchi, T.; Gutiérrez, C. Improvement of endothelial function by Gunnera tinctoria extract with antioxidant properties. J. Biol. Res. 2020, 53, 1–14. [Google Scholar]
- Silva, C.; Correia-Branco, A.; Andrade, N.; Ferreira, A.C.; Soares, M.L.; Sonveaux, P.; Stephenne, J.; Martel, F. Selective pro-apoptotic and antimigratory effects of polyphenol complex catechin: Lysine 1: 2 in breast, pancreatic and colorectal cancer cell lines. Eur. J. Pharmacol. 2019, 859, 172533. [Google Scholar] [CrossRef]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Bayati, M.; Mehrbod, P.; Ebrahimi, S.N. Natural products as inhibitors of COVID-19 main protease–A virtual screening by molecular docking. Res. Square 2020, 1–13. [Google Scholar] [CrossRef]
- Ballester, P.J.; Mitchell, J.B. A machine learning approach to predicting protein–ligand binding affinity with applications to molecular docking. J. Bioinform. 2010, 26, 1169–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Commission, E. Commission Directive 2006/141/EC of 22 December 2006 on infant formulae and follow-on formulae and amending Directive 1999/21/EC. Off. J. Eur. Union 2006, 49, 1–33. [Google Scholar]
- Zamorano, P.; Rojano, B.I.; Morales, M.; Magariños, H.; Godoy, P.; Muñoz, O. Biological and antioxidant activity of Gunnera tinctoria (Nalca). J. Med. Plants Res. 2017, 11, 318–330. [Google Scholar]
- Davoodi, M.; Rustaiyan, A.; Ebrahimi, S.N. Monoterpene flavonoid from aerial parts of Satureja khuzistanica. Rec. Nat. Prod. 2018, 12, 175–178. [Google Scholar] [CrossRef]
- Kokabi, M.; Ebrahimi, S.N. Polyphenol rnriched extract of pomegranate peel; A novel precursor for the biosynthesis of zinc oxide nanoparticles and application in sunscreens. Pharm. Sci. 2020, 27, 102–110. [Google Scholar] [CrossRef]
- Scordino, M.; Di Mauro, A.; Passerini, A.; Maccarone, E. Selective recovery of anthocyanins and hydroxycinnamates from a byproduct of citrus processing. J. Agric. Food Chem. 2005, 53, 651–658. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Bridi, R.; Giordano, A.; Peñailillo, M.F.; Montenegro, G. Antioxidant effect of extracts from native Chilean plants on the lipoperoxidation and protein oxidation of bovine muscle. Molecules 2019, 24, 3264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, Y.; Toledo, R.T. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef]
- Oyenihi, A.; Smith, C. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 2019, 229, 54–72. [Google Scholar] [CrossRef]
- Teleki, A.; Hitzfeld, A.; Eggersdorfer, M. 100 years of vitamins: The science of formulation is the key to functionality. Kona 2013, 30, 144–163. [Google Scholar] [CrossRef] [Green Version]
- Tontul, I.; Topuz, A. Spray-drying of fruit and vegetable juices: Effect of drying conditions on the product yield and physical properties. Trends Food Sci. Technol. 2017, 63, 91–102. [Google Scholar] [CrossRef]
- Cano-Higuita, D.M.; Vélez, H.A.V.; Telis, V.R.N. Microencapsulation of turmeric oleoresin in binary and ternary blends of gum Arabic, maltodextrin and modified starch. Cienc. Agrotecnol. 2015, 39, 173–182. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Lević, S.M.; Pavlovic, V.B.; Markovic, S.B.; Pjanovic, R.V.; Đorđević, V.B.; Nedović, V.; Bugarski, B.M. Freeze versus spray drying for dry wild thyme (Thymus serpyllum L.) extract formulations: The impact of gelatin as a coating material. Molecules 2021, 26, 3933. [Google Scholar] [CrossRef] [PubMed]
- Keshani, S.; Daud, W.R.W.; Nourouzi, M.; Namvar, F.; Ghasemi, M. Spray drying: An overview on wall deposition, process and modeling. J. Food Eng. 2015, 146, 152–162. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Xu, D.; Lin, X.; Feng, Y.; Hong, Y. Hydroxypropyl methylcellulose reduces particle adhesion and improves recovery of herbal extracts during spray drying of Chinese herbal medicines. Dry. Technol. 2014, 32, 557–566. [Google Scholar] [CrossRef]
- Hou, Z.; Li, Y.; Huang, Y.; Zhou, C.; Lin, J.; Wang, Y.; Cui, F.; Zhou, S.; Jia, M.; Ye, S. Phytosomes loaded with mitomycin C–soybean phosphatidylcholine complex developed for drug delivery. Mol. Pharm. 2013, 10, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, Q.; Xu, X.; Li, N. Development and evaluation of a novel phytosome-loaded chitosan microsphere system for curcumin delivery. Int. J. Pharm. 2013, 448, 168–174. [Google Scholar] [CrossRef]
- Nazari, M.; Ghanbarzadeh, B.; Kafil, H.S.; Zeinali, M.; Hamishehkar, H. Garlic essential oil nanophytosomes as a natural food preservative: Its application in yogurt as food model. Colloids Interface Sci. Commun. 2019, 30, 100176. [Google Scholar] [CrossRef]
- Souza, J.E.D.; Casanova, L.M.; Costa, S.S. Bioavailability of phenolic compounds: A major challenge for drug development? Rev. Fitos (Rio J.) 2015, 9, 55–67. [Google Scholar]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Rasaee, S.; Ghanbarzadeh, S.; Mohammadi, M.; Hamishehkar, H. Nano phytosomes of quercetin: A promising formulation for fortification of food products with antioxidants. J. Pharm. Sci. 2014, 20, 96–101. [Google Scholar]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int. J. Biol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Ansari, F.; Sobhani, A.; Salavati-Niasari, M. Simple sol-gel synthesis and characterization of new CoTiO3/CoFe2O4 nanocomposite by using liquid glucose, maltose and starch as fuel, capping and reducing agents. J. Colloid Interface Sci. 2018, 514, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Golding, J.B.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, U.A.B.; Gutiérrez, M.C.; Mendoza, D.G.; Salitre, L.G.; Vargas, A.S.; Catzim, C.E.A.; Durán, C.C.; Valenzuela, B.E.L. Microencapsulation and antimicrobial activity of extract acetone-methanol of Hibiscus sabdariffa L. using a blend modified starch and pectin as a wall material. Ind. Crops Prod. 2021, 170, 113725. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery, 1st ed.; McNeil, S., Ed.; Springer: Gewerbesrasse, Switzerland, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Campos, J.C.; Ferreira, D.C.; Lima, S.; Reis, S.; Costa, P.J. Swellable polymeric particles for the local delivery of budesonide in oral mucositis. Int. J. Pharm. 2019, 566, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Gangadharappa, H.; Mruthunjaya, K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2018, 122, 214–229. [Google Scholar] [CrossRef]
- Hindarto, C.K.; Surini, S.; Saputri, F.C.; Irawan, C. In vivo evaluation of luteolin-loaded phytosome. J. Pharm. Innov. 2017, 6, 347–349. [Google Scholar]
- Nagaraju, P.G.; Sindhu, P.; Dubey, T.; Chinnathambi, S.; Priyadarshini, P.C.G.; Rao, P.J. Influence of sodium caseinate, maltodextrin, pectin and their Maillard conjugate on the stability, in vitro release, anti-oxidant property and cell viability of eugenol-olive oil nanoemulsions. Int. J. Biol. Macromol. 2021, 183, 158–170. [Google Scholar] [CrossRef]
- Jeong, O.; Shin, M. Preparation and stability of resistant starch nanoparticles, using acid hydrolysis and cross-linking of waxy rice starch. Food Chem. 2018, 256, 77–84. [Google Scholar] [CrossRef]
- Li, X.; Kong, X.; Shi, S.; Zheng, X.; Guo, G.; Wei, Y.; Qian, Z. Preparation of alginate coated chitosan microparticles for vaccine delivery. BMC Biotechnol. 2008, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, M.I.; Shaikh, M.; tul-Wahab, A.; ur-Rahman, A. In silico identification of potential inhibitors of key SARS-CoV-2 3CL hydrolase (Mpro) via molecular docking, MMGBSA predictive binding energy calculations, and molecular dynamics simulation. PLoS ONE 2020, 15, 1–15. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-phytosome as a potential food-grade delivery system. Food Biosci. 2016, 15, 126–135. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Associationo Of Analytical Chemistry, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Ayadi, M.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Food energy—Methods of analysis and conversion factors. Report of a technical workshop. In Food and Agriculture Organization of the United Nations Technical Workshop Report 77; Food and Nutrition Paper; FAO: Rome, Italy, 2003; ISSN 0254-4725. [Google Scholar]
- Costa, A.S.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P. Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Puga, H.; Alves, R.C.; Costa, A.S.; Vinha, A.F.; Oliveira, M.B.P. Multi-frequency multimode modulated technology as a clean, fast, and sustainable process to recover antioxidants from a coffee by-product. J. Clean. Prod. 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Shehzad, O.; Jin-Ha, I.; Park, Y.; Wan-Ha, Y.; Shik-Kim, Y. Development of a rapid and convenient method to separate eight ginsenosides from Panax ginseng by high-speed counter-current chromatography coupled with evaporative light scattering detection. J. Sep. Sci. 2011, 34, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Nunes, C.; Correia-Branco, A.; Araújo, J.R.; Martel, F. Insulin exhibits an antiproliferative and hypertrophic effect in first trimester human extravillous trophoblasts. Reprod. Sci. 2017, 24, 582–594. [Google Scholar] [CrossRef]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimaraes, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fathi, F.; Ebrahimi, S.N. Investigation of physiochemical properties nanophytosome obtained from of polyphenolic enrich fraction of Satureja khuzistanica by freeze-drying. Nashrieh Shimi va Mohandesi Shimi Iran 2021, in press. Available online: https://www.nsmsi.ir/article_242399.html?lang=en (accessed on 20 August 2021).
- Gandhi, A.; Dutta, A.; Pal, A.; Bakshi, P. Recent trends of phytosomes for delivering herbal extract with improved bioavailability. J. Pharmacogn. Phytochem. 2012, 1, 6–14. [Google Scholar]
- Anwar, E.; Farhana, N. Formulation and evaluation of phytosome-loaded maltodextrin-gum Arabic microsphere system for delivery of Camellia sinensis extract. J. Young Pharm. 2018, 10, 56–62. [Google Scholar] [CrossRef] [Green Version]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The effect of spray-drying and freeze-drying on encapsulation efficiency, in vitro bioaccessibility and oxidative stability of krill oil nanoemulsion system. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Tuyen, C.K.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. Microencapsulation of gac oil: Optimisation of spray drying conditions using response surface methodology. J. Powder Technol. 2014, 264, 298–309. [Google Scholar]
- Santana, A.A.; de Oliveira, R.A.; Kurozawa, L.E.; Park, K.J. Microencapsulation of pequi pulp by spray drying: Use of modified starches as encapsulating agent. Eng. Agric. 2014, 34, 980–991. [Google Scholar] [CrossRef] [Green Version]
- Knight, C.J.; Hub, J.S. MemGen: A general web server for the setup of lipid membrane simulation systems. J. Bioinform. 2015, 31, 2897–2899. [Google Scholar] [CrossRef]


| Roots | Inflorescences | Baby Leaves | Adult Leaves | |
|---|---|---|---|---|
| Moisture | 61.20 ± 0.11 d | 91.35 ± 0.06 a | 90.55 ± 0.10 b | 85.95 ± 0.08 c |
| Ash | 5.13 ± 0.05 d | 11.37 ± 0.12 a | 7.13 ± 0.06 c | 9.96 ± 0.12 b |
| Protein | 2.53 ± 0.05 d | 8.79 ± 0.31 c | 10.55 ± 0.23 b | 13.62 ± 0.85 a |
| Fat | 0.03 ± 0.00 d | 7.96 ± 0.13 a | 1.14 ± 0.16 c | 2.10 ± 0.23 b |
| Total Fiber | 12.62 ± 0.53 d | 41.31 ± 0.55 b | 36.54 ± 0.43 c | 44.19 ± 0.32 a |
| Insoluble Fiber | 10.42 ± 0.25 c | 39.24 ± 1.21 a | 31.55 ± 1.49 b | 39.94 ± 0.93 a |
| Soluble Fiber | 2.21 ± 0.78 b | 2.07 ± 0.66 b | 4.98 ± 1.06 a | 4.25 ± 1.26 a |
| Available Carbohydrates | 79.75 ± 0.59 a | 30.76 ± 0.78 c | 44.78 ± 0.46 b | 30.24 ± 1.60 c |
| Roots | Inflorescences | Baby Leaves | Adult Leaves | |
|---|---|---|---|---|
| TPC (mg GAE/g) | 115.4 ± 3.0 d | 176.2 ± 3.8 b | 308.0 ± 2.7 a | 151.4 ± 13.8 c |
| TFC (mg CE/g) | 30.1 ± 1.06 a | 13.76 ± 0.59 c | 11.86 ± 0.28 d | 18.28 ± 0.66 b |
| FRAP (mmol FSE/g) | 5677 ± 165 c | 6427 ± 205 b | 13153 ± 214 a | 6437 ± 276 b |
| DPPH● SA(mg TE/g) | 220.9 ± 13.3 c | 542.3 ± 86.1 b | 705.0 ± 26.1 a | 515.2 ± 65.9 b |
| Sample | Code | Yield | TFC (mg CE/L) | ER | TPC (mg GAE/L) | ER |
|---|---|---|---|---|---|---|
| Hydroethanolic Extract | GuT1 | 58.00 | 24.74 ± 0.43 c | - | 34.93 ± 1.53 e | - |
| E-GuT of Di | GuT1-Di1 | 38.55 | 43.14 ± 0.25 a | 1.74 | 82.82 ± 4.02 b | 2.37 |
| DF-GuT of Di | GuT1-Di2 | DC * | 01.26 ± 0.43 g | 0.05 | 3.05 ± 0.06 f | 0.09 |
| E-GuT of Re | GuT1-Re1 | 36.11 | 21.26 ± 0.87 cd | 0.86 | 77.84 ± 0.76 c | 2.23 |
| DF-GuT of Re | GuT1-Re2 | DC * | 02.42 ± 0.25 f | 0.10 | 3.01 ± 0.18 f | 0.10 |
| Ultrasonication Extract | GuT2 | 15.29 | 09.96 ± 0.00 e | - | 41.84 ± 2.47 d | - |
| E-GuT of Di | GuT2-Di1 | 21.00 | 18.51 ± 0.25 d | 1.86 | 80.38 ± 2.07 bc | 1.92 |
| DF-GuT of Di | GuT2-Di2 | DC * | 02.57 ± 0.43 f | 0.26 | 3.29 ± 0.70 f | 0.10 |
| E-GuT of Re | GuT2-Re1 | 22.00 | 37.78 ± 0.43 b | 3.79 | 87.40 ± 2.22 a | 2.09 |
| DF-GuT of Re | GuT2-Re2 | DC * | 01.70 ± 0.43 g | 0.17 | 0.93 ± 0.34 g | 0.04 |
| Sample Name | Yield | ED 1 In Volume | ED 1 In Number | ZP 1 Inner Layer | ZP 1 External Layer | |
|---|---|---|---|---|---|---|
| Lipid layer | Ph-GuT1-Di1 | - | 120.0 | 33.5 | −67.50 ± 1.43 | |
| Ph/Chl-GuT1-Di1 | - | 354.4 | 140.2 | −62.57 ± 2.95 | ||
| Polymeric layer | GuT1-Di1-PE | 34.55 | 1196.5 | 916.5 | −29.72 ± 1.32 | −16.21 ± 8.44 |
| GuT1-Di1-Malto | 46.00 | 397.9 | 327.1 | −33.82 ± 0.76 | −31.40 ± 10.29 | |
| GuT1-Di1-Stch | 68.55 | 509.1 | 355.0 | −55.01 ± 3.24 | −53.47 ± 1.94 | |
| GuT1-Di1-PE/Stch | 38.00 | 824.6 | 737.2 | −43.07 ± 2.17 | −28.24 ± 3.06 | |
| Secondary layer | Ph-GuT1-Di1-PE | 43.54 | 1364.6 | 1016.1 | −21.26 ± 7.19 | −17.21 ± 8.34 |
| Ph-GuT1-Di1-Malto | 37.23 | 411.5 | 226.3 | −33.82 ± 0.76 | −39.14 ± 5.92 | |
| Ph-GuT1-Di1-Stch | 47.38 | 523.1 | 446.7 | −31.38 ± 3.95 | −25.37 ± 1.75 | |
| Ph-GuT1-Di1-PE/Stch | 41.67 | 951.2 | 752.7 | −44.76 ± 3.38 | −18.87 ± 9.20 | |
| Secondary layer | Ph/Chl-GuT1-Di1-PE | 68.74 | 1207.9 | 1073.0 | −31.67 ± 1.69 | −14.99 ± 2.79 |
| Ph/Chl-GuT1-Di1-Malto | 66.22 | 392.7 | 212.7 | −34.18 ± 3.41 | −29.31 ± 3.38 | |
| Ph/Chl-GuT1-Di1-Stch | 70.22 | 680.5 | 555.1 | −43.07 ± 2.17 | −38.46 ± 1.94 | |
| Ph/Chl-GuT1-Di1-PE/Stch | 52.97 | 707.6 | 528.1 | −34.40 ± 2.84 | −30.09 ± 1.99 | |
| Phenolic Structure | Docking Score | Grid Energy | |||||
|---|---|---|---|---|---|---|---|
| Min | Max | Average | Min | Max | Average | ||
| Phytosome designed with lecithin | Gallic acid | −4.47 | −4.86 | −4.73 ± 0.23 | −29.98 | −31.37 | −30.83 ± 0.75 |
| Ellagic acid | −4.32 | −4.47 | −4.31 ± 0.15 | −31.33 | −4.47 | −32.88 ± 1.31 | |
| Quercetin | −5.31 | −5.41 | −5.05 ± 0.50 | −29.90 | −34.23 | −33.04 ± 2.57 | |
| Catechin | −3.50 | −5.36 | −4.49 ± 0.66 | −19.71 | −38.93 | −29.14 ± 9.18 | |
| Epicatechin | −3.38 | −5.23 | −4.36 ± 0.66 | −22.43 | −39.96 | −33.05 ± 6.71 | |
| Phytosome designed with lecithin conjugated with cholesterol | Gallic acid | −4.06 | −4.13 | −4.09 ± 0.04 | −12.66 | −15.75 | −14.62 ±1.70 |
| Ellagic acid | −3.54 | −3.75 | −3.62 ± 0.10 | −18.79 | −19.24 | −19.05 ± 0.21 | |
| Quercetin | −5.12 | −6.79 | −5.69 ± 0.65 | −23.47 | −26.29 | −25.01 ± 1.15 | |
| Catechin | −5.93 | −6.95 | −6.42 ± 0.44 | −21.62 | −25.47 | −23.51 ± 1.41 | |
| Epicatechin | −4.89 | −7.15 | −6.50 ± 0.57 | −20.84 | −25.09 | −24.09 ± 1.49 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathi, F.; Ebrahimi, S.N.; Valadão, A.I.G.; Andrade, N.; Costa, A.S.G.; Silva, C.; Fathi, A.; Salehi, P.; Martel, F.; Alves, R.C.; et al. Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking. Molecules 2021, 26, 5935. https://doi.org/10.3390/molecules26195935
Fathi F, Ebrahimi SN, Valadão AIG, Andrade N, Costa ASG, Silva C, Fathi A, Salehi P, Martel F, Alves RC, et al. Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking. Molecules. 2021; 26(19):5935. https://doi.org/10.3390/molecules26195935
Chicago/Turabian StyleFathi, Faezeh, Samad N. Ebrahimi, Ana I. G. Valadão, Nelson Andrade, Anabela S. G. Costa, Cláudia Silva, Alireza Fathi, Peyman Salehi, Fátima Martel, Rita C. Alves, and et al. 2021. "Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking" Molecules 26, no. 19: 5935. https://doi.org/10.3390/molecules26195935
APA StyleFathi, F., Ebrahimi, S. N., Valadão, A. I. G., Andrade, N., Costa, A. S. G., Silva, C., Fathi, A., Salehi, P., Martel, F., Alves, R. C., & Oliveira, M. B. P. P. (2021). Exploring Gunnera tinctoria: From Nutritional and Anti-Tumoral Properties to Phytosome Development Following Structural Arrangement Based on Molecular Docking. Molecules, 26(19), 5935. https://doi.org/10.3390/molecules26195935