Bioflavonoid-Induced Apoptosis and DNA Damage in Amastigotes and Promastigotes of Leishmania donovani: Deciphering the Mode of Action
Abstract
:1. Introduction
2. Results and Discussion
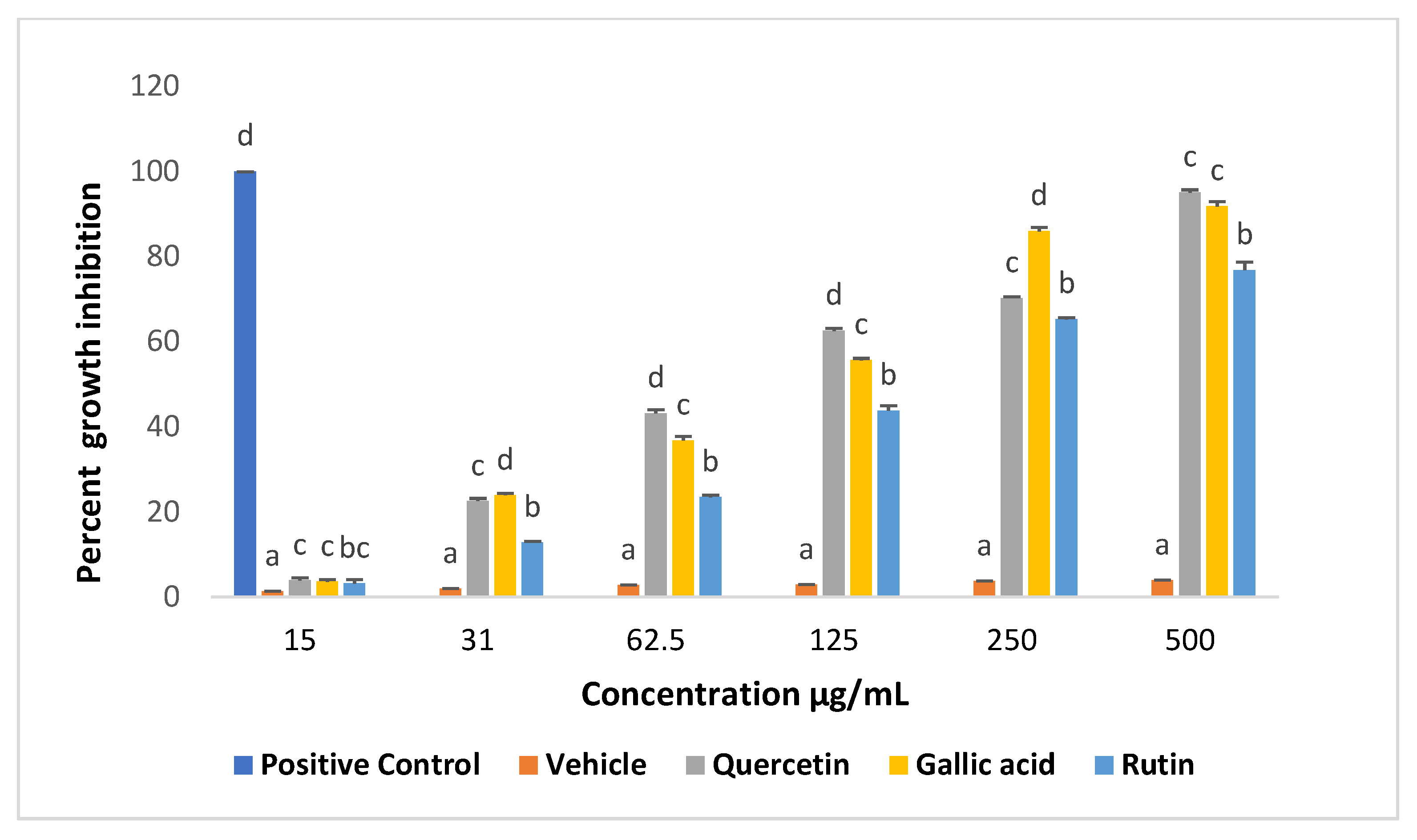
2.1. Effects of Compounds on Viability of Promastigotes
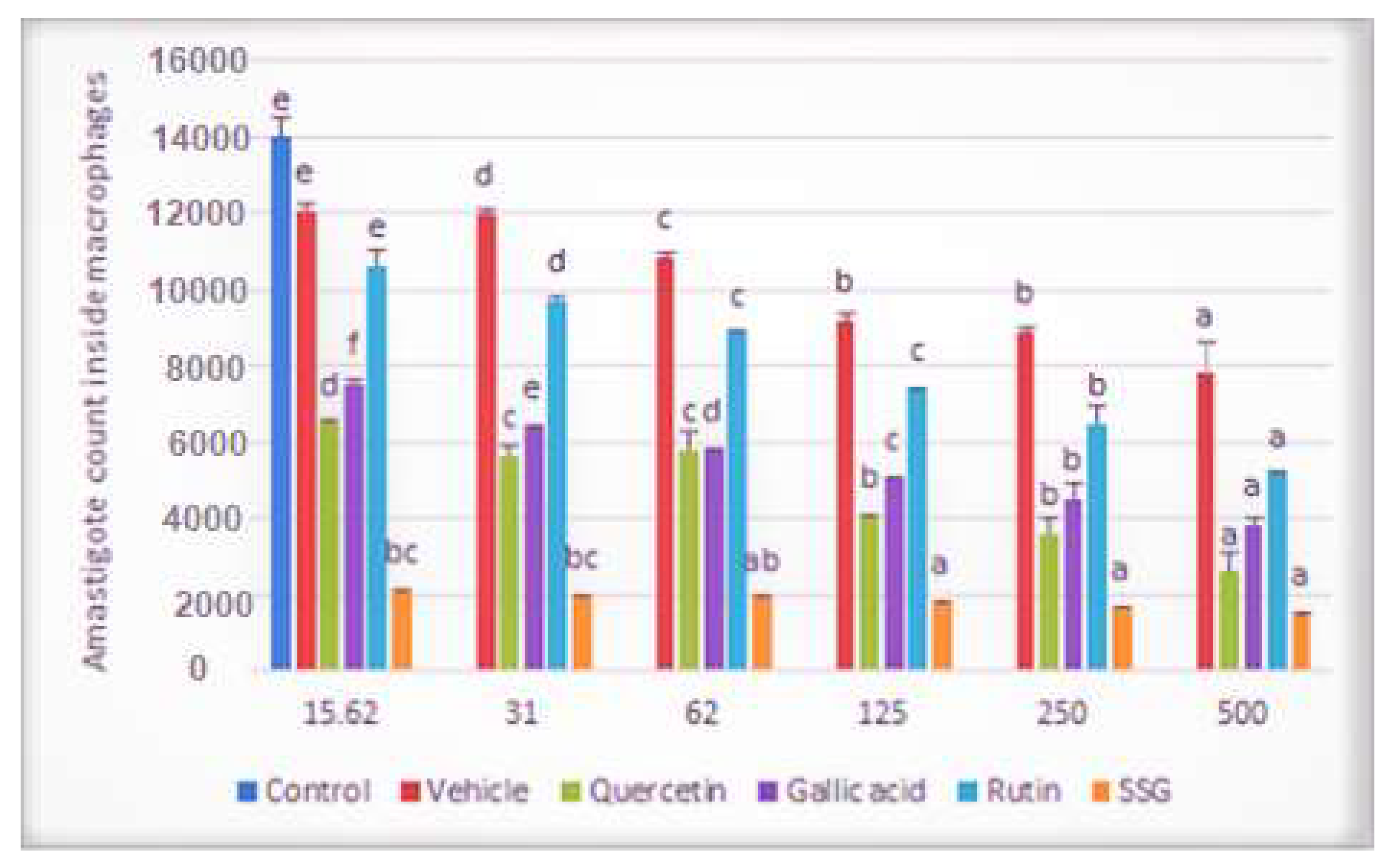
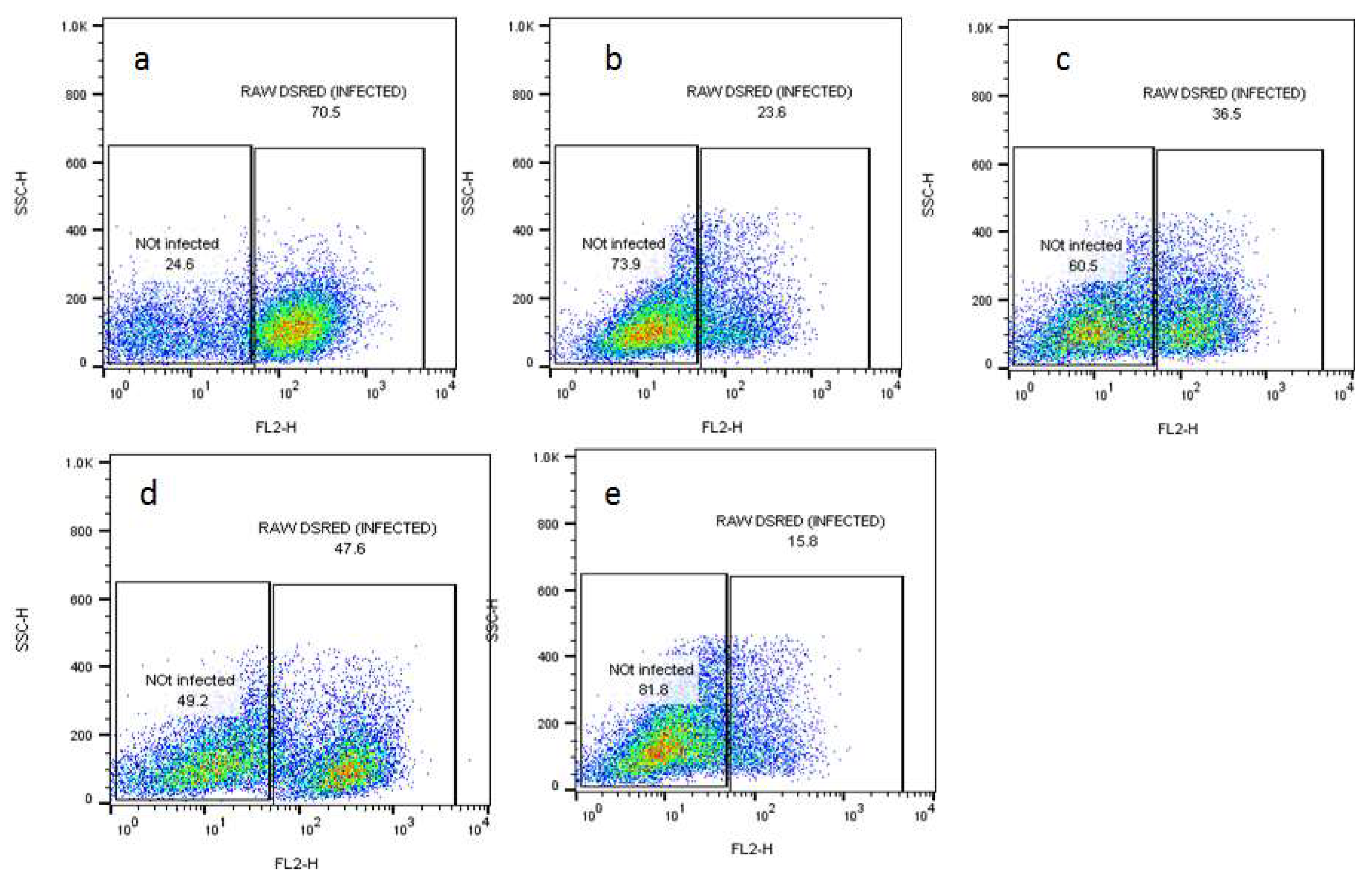
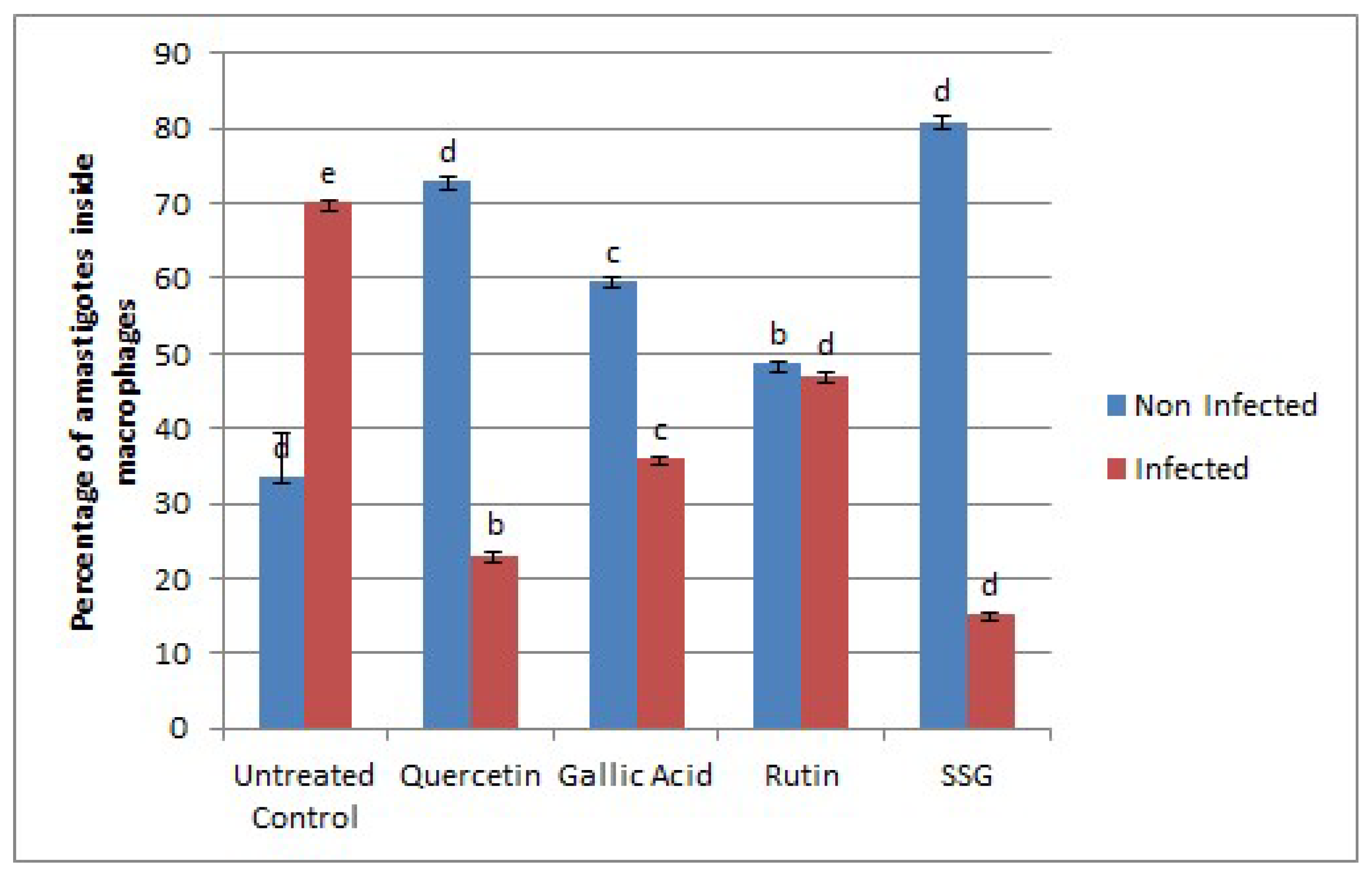
2.2. Effects of Compounds on the Viability of Intracellular Amastigotes
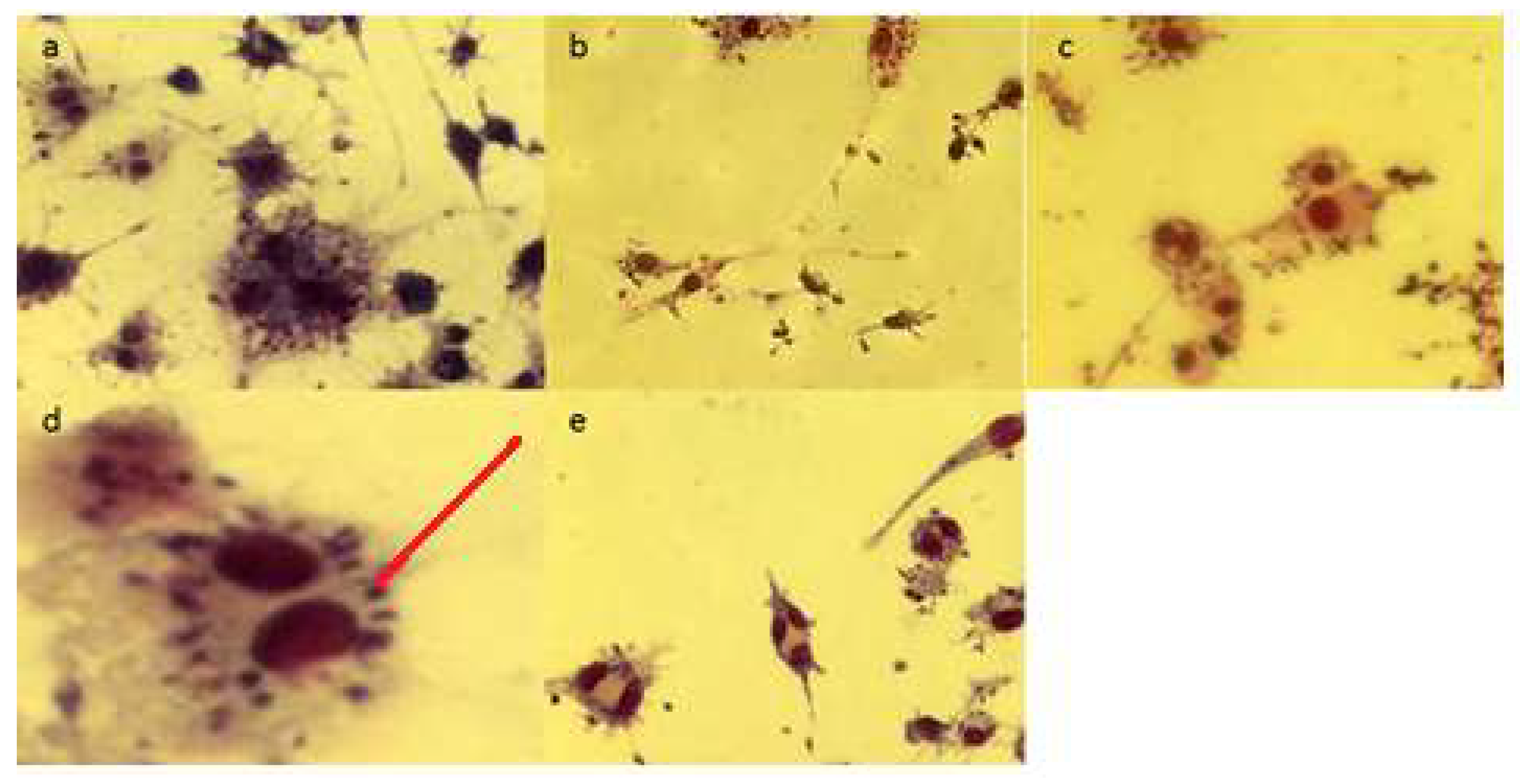
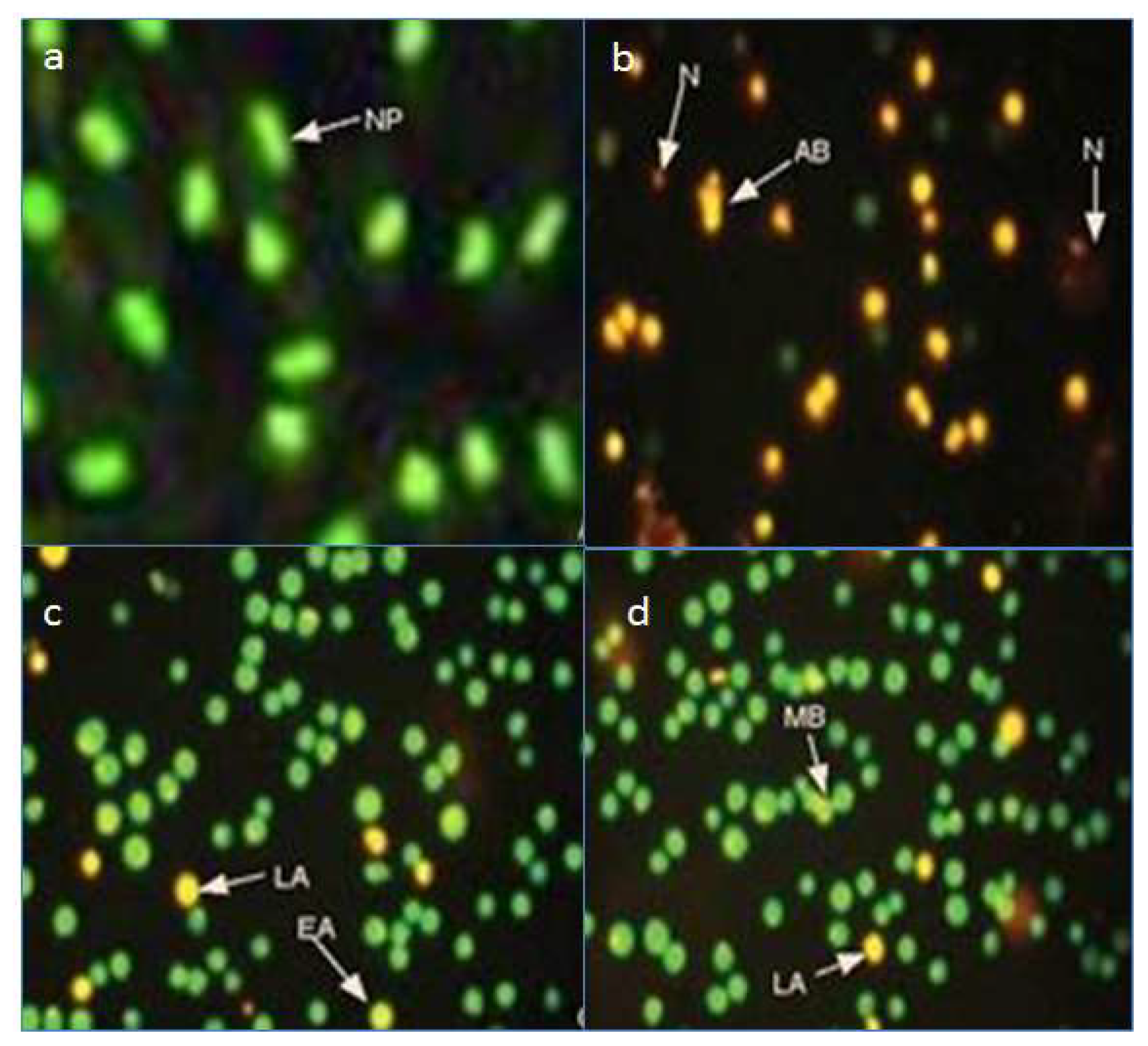
2.3. Fluorescence Microscopy Assay for Apoptosis and Necrosis
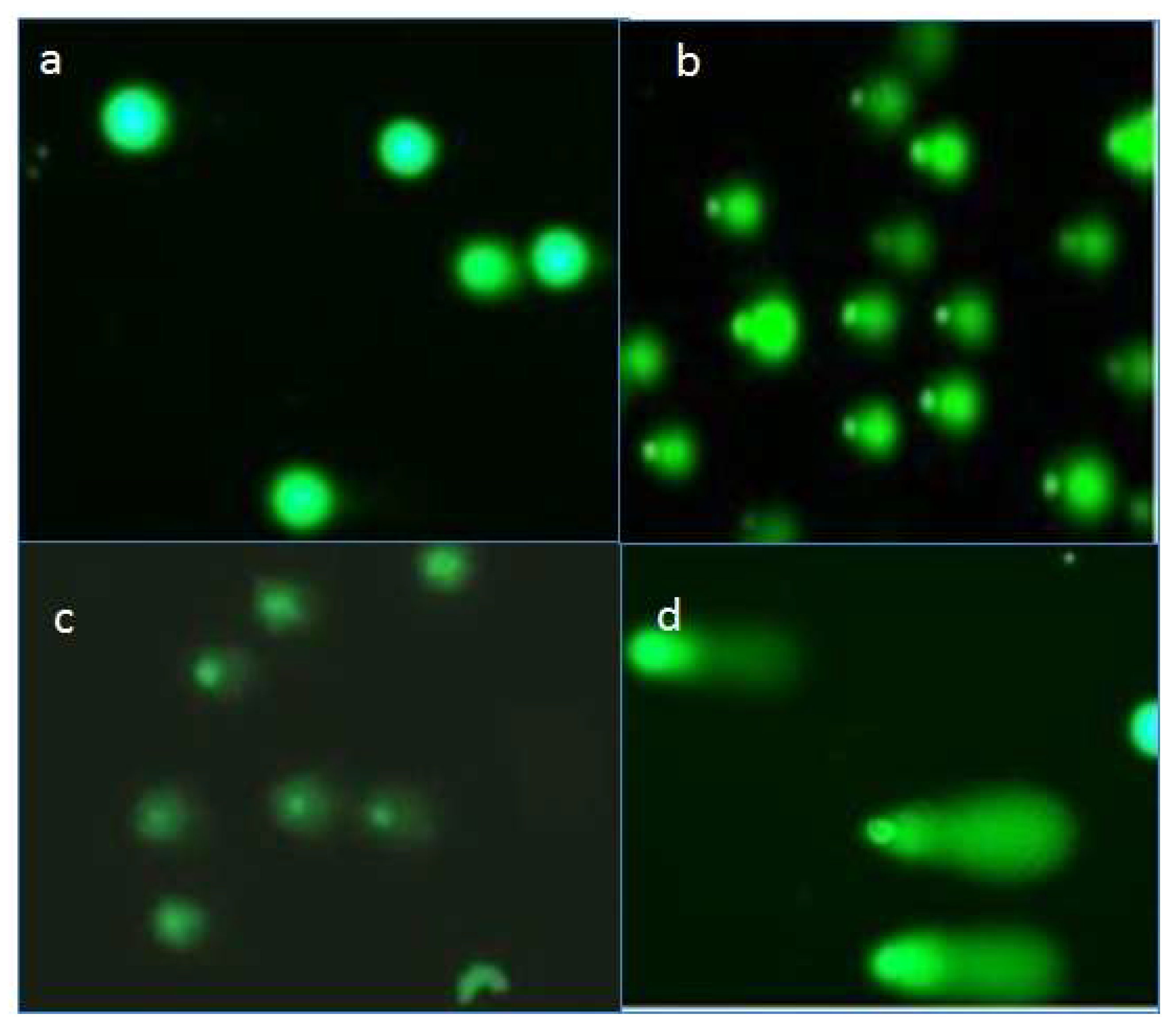
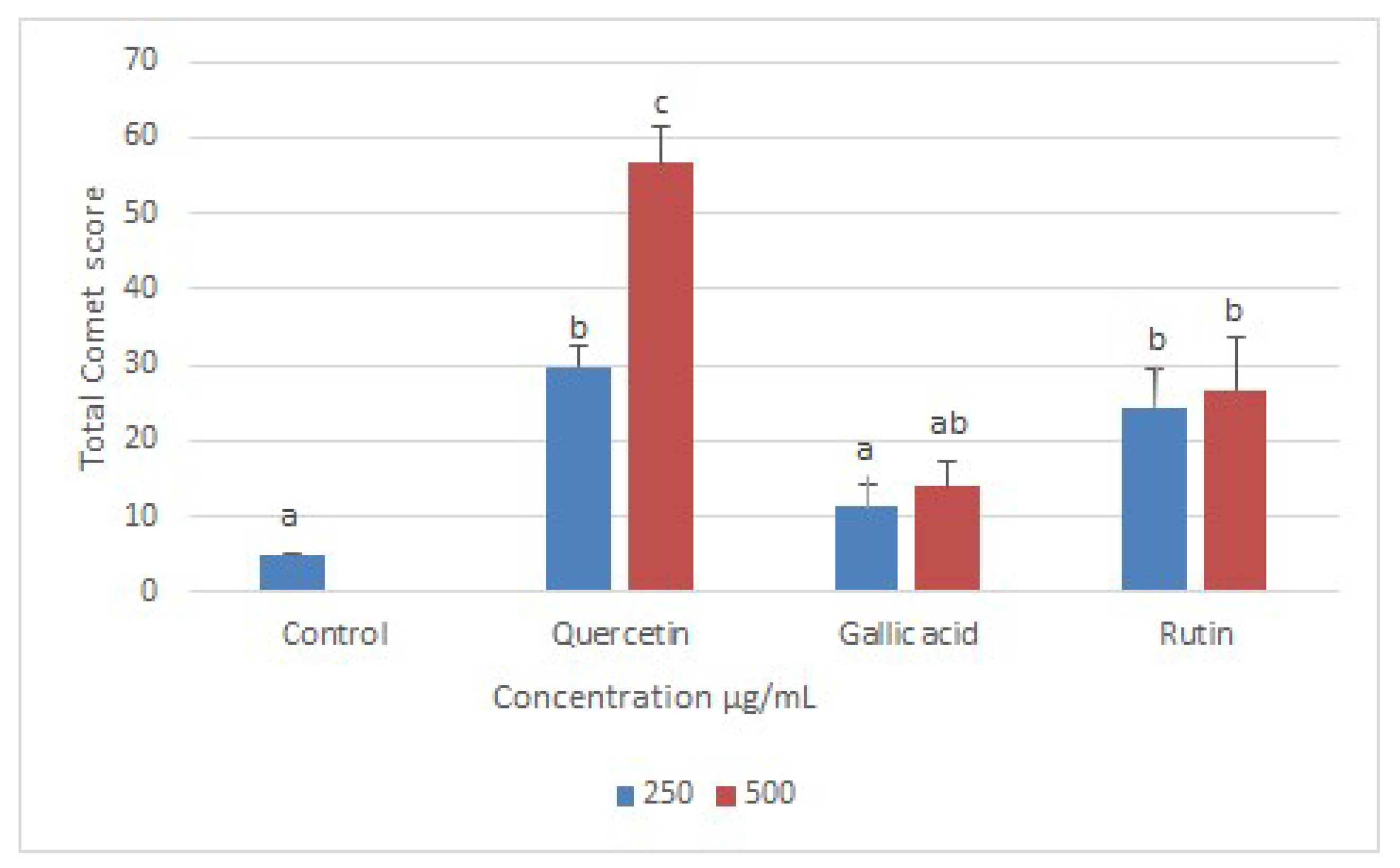
2.4. Effects of Compounds on DNA of Treated Promastigotes
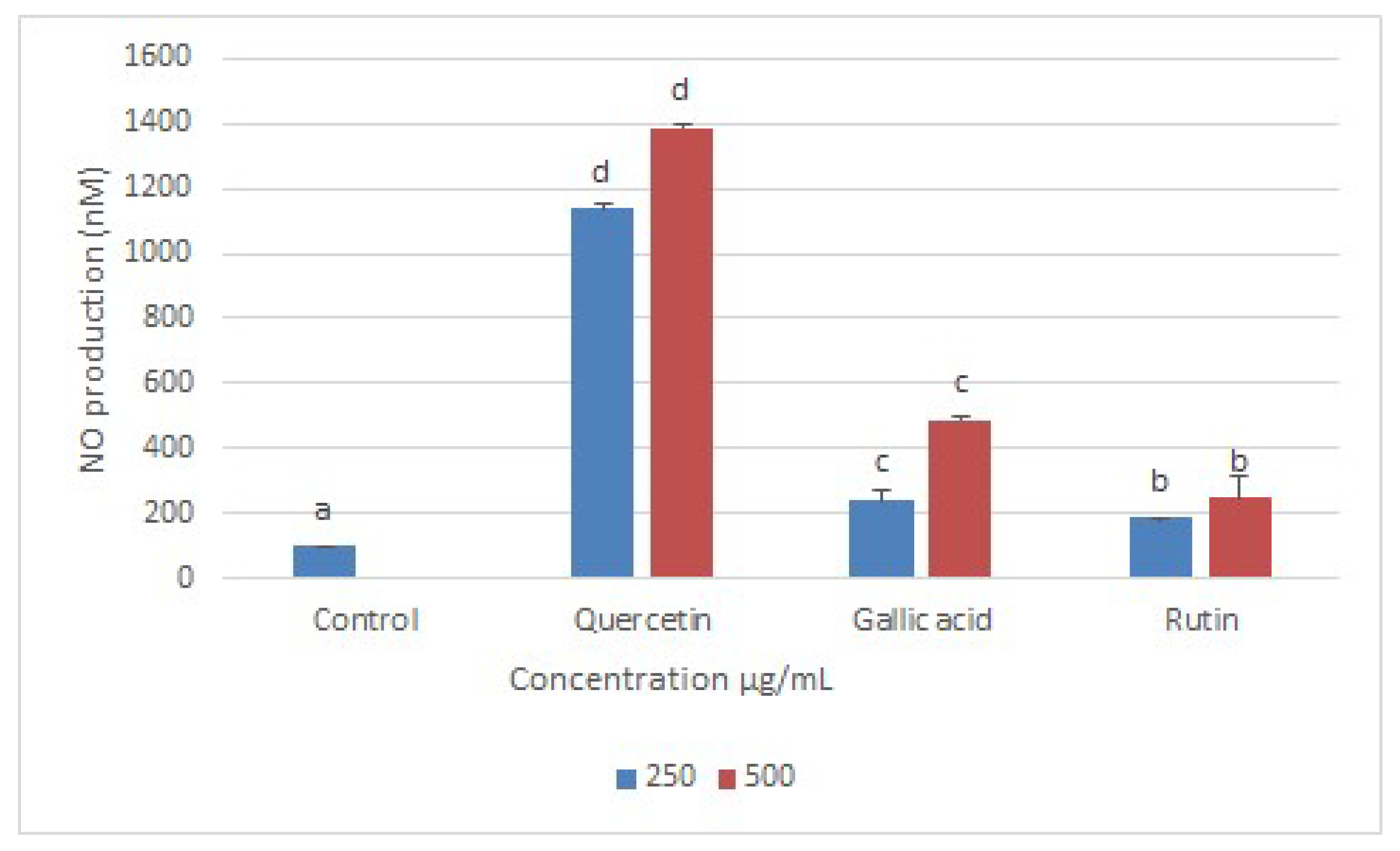
2.5. Nitric Oxide (NO) Production
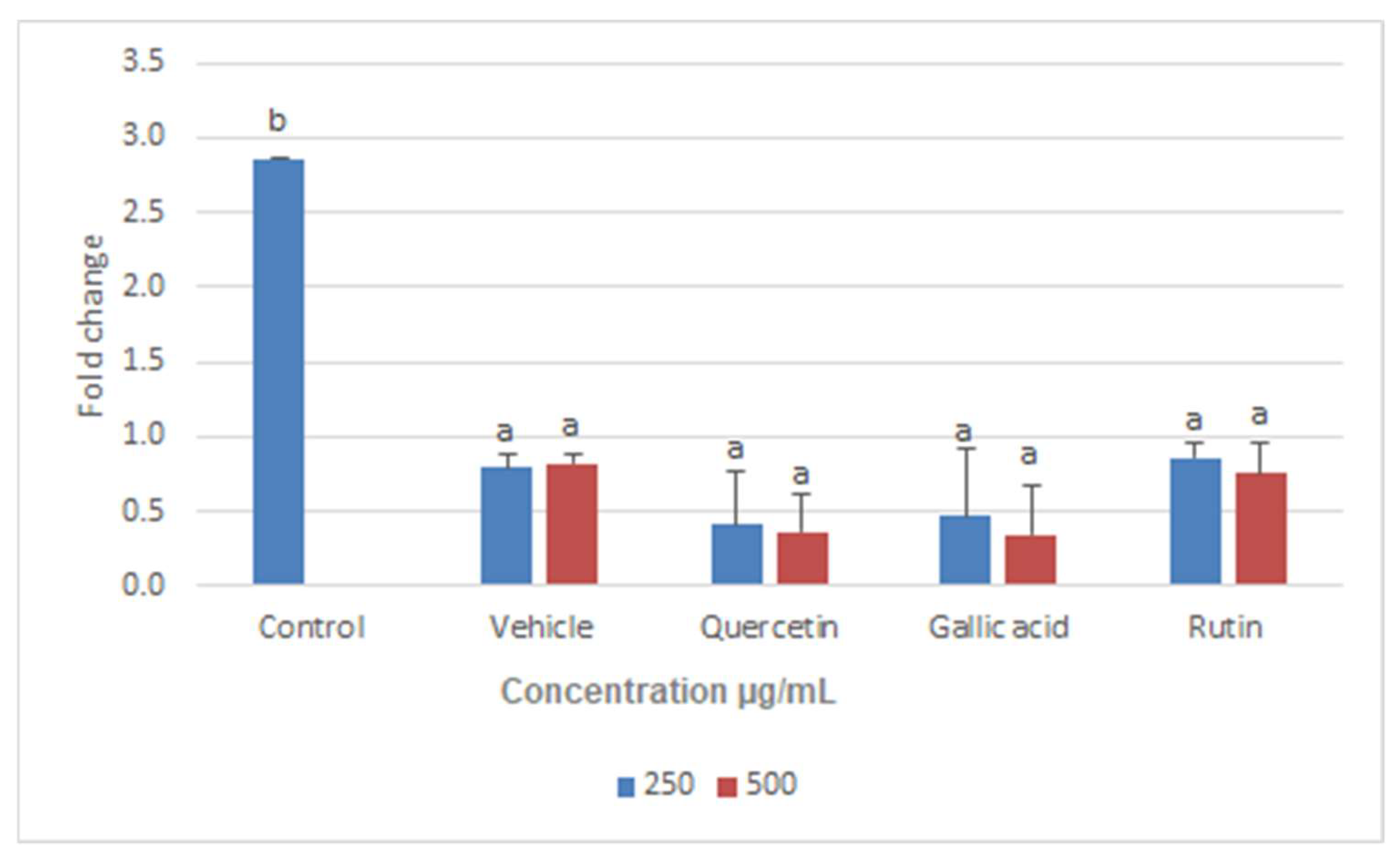
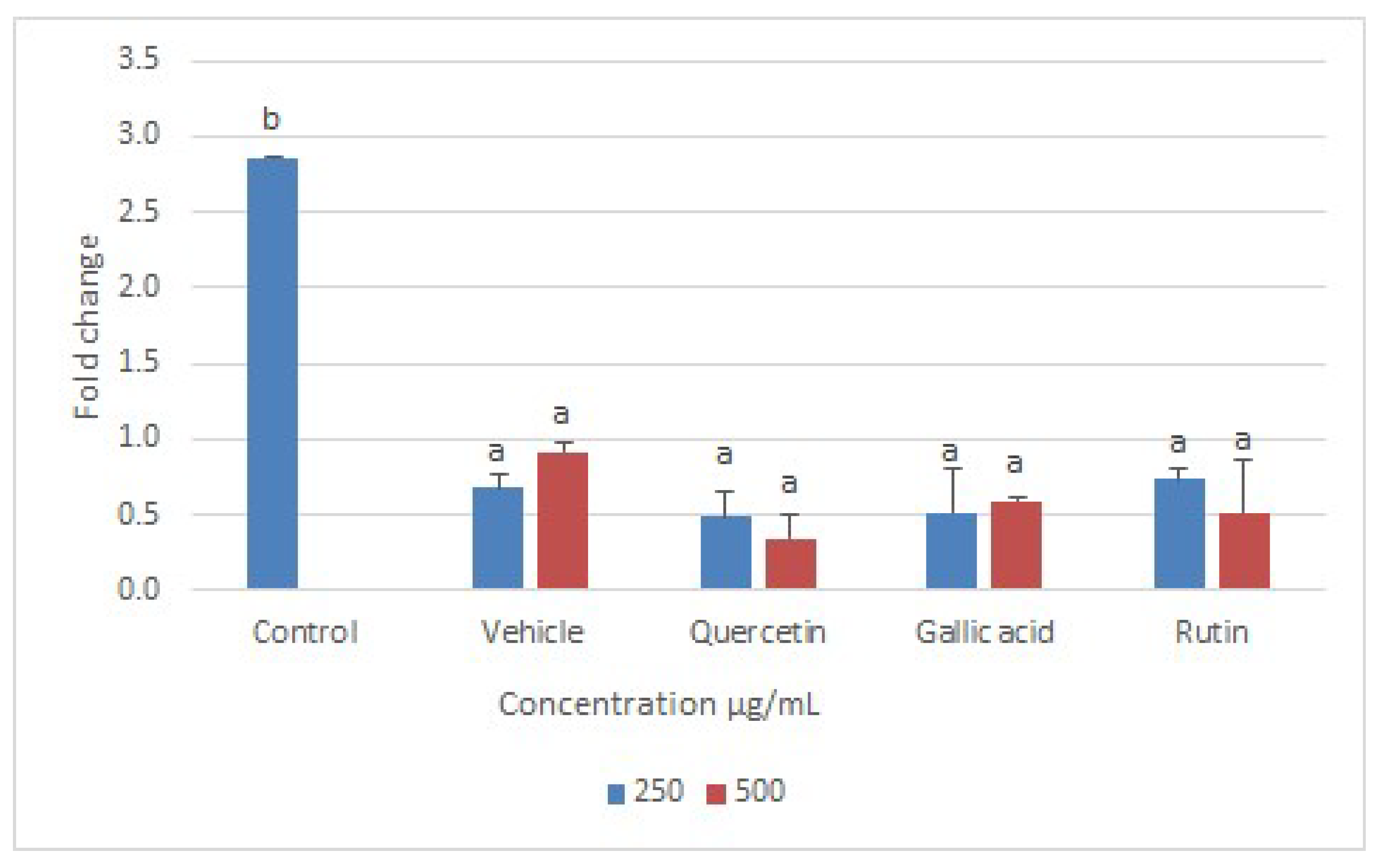
2.6. Real-Time PCR Assay
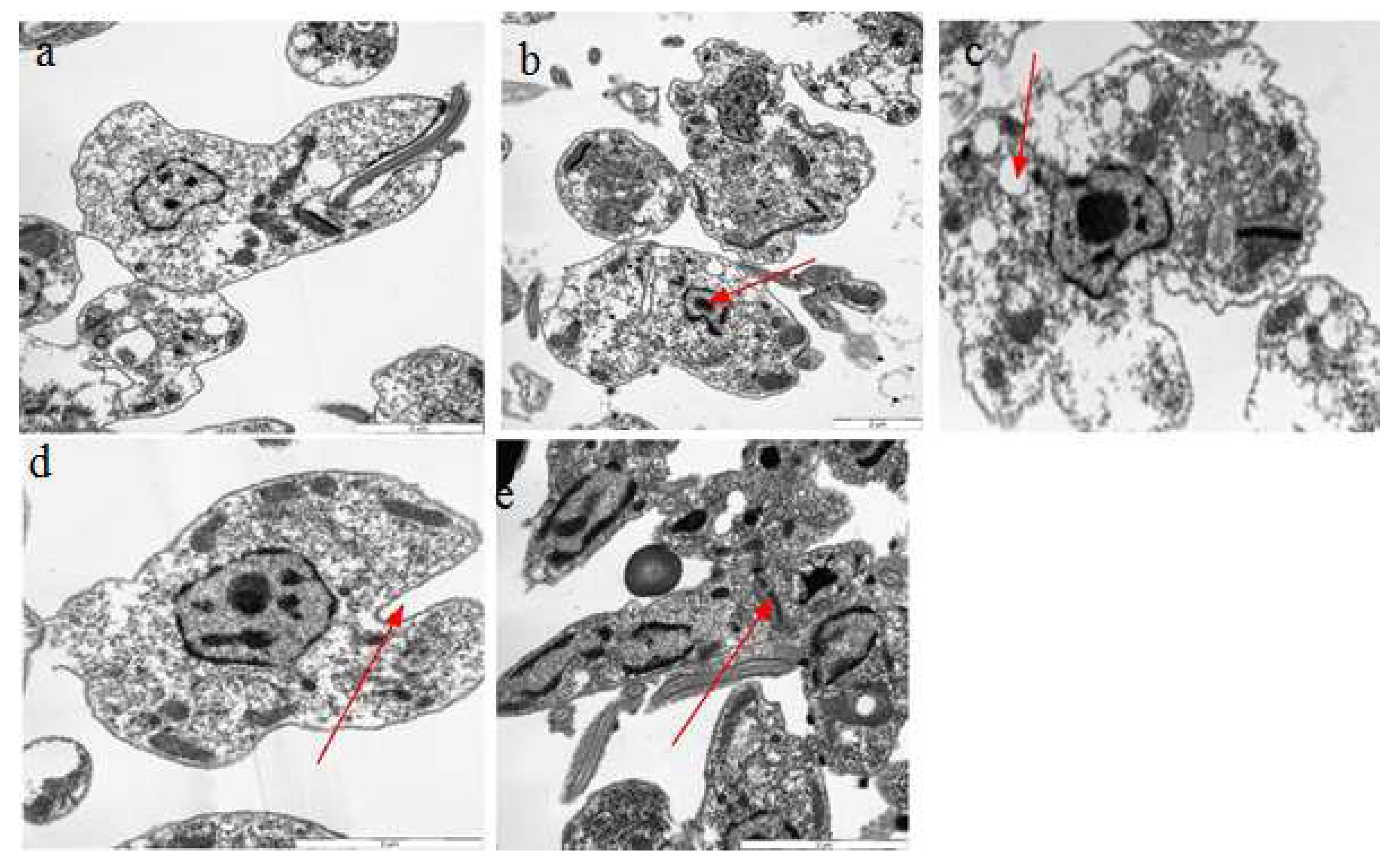
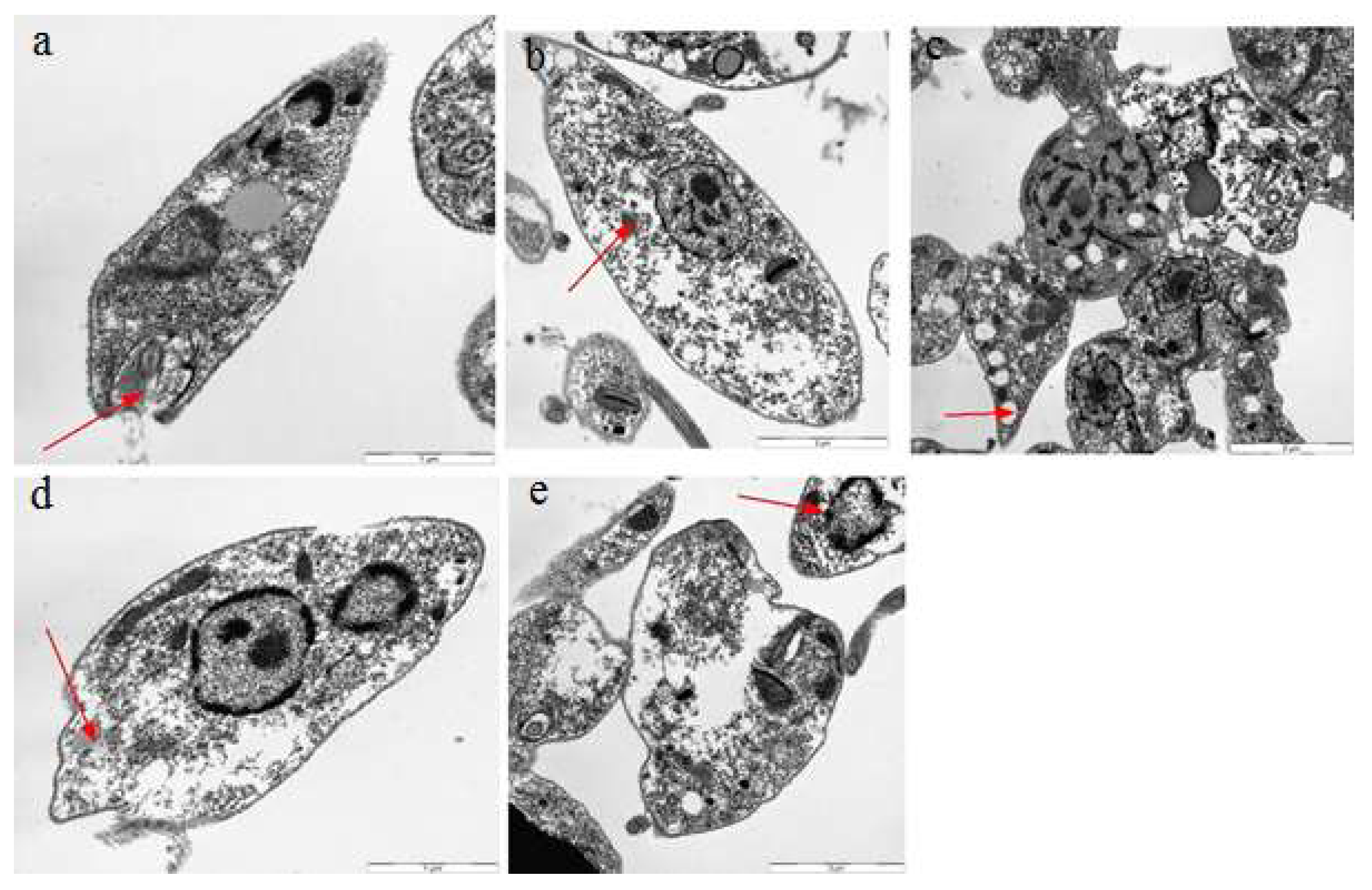
2.7. Transmission Electron Microscopy
3. Materials and Methods
3.1. Parasites and Cell Cultures
3.2. Effect of Compounds on Viability of Promastigotes
3.3. Effects of Compounds on Viability of Intracellular Amastigotes (via Giemsa Stain)
3.4. Flow Cytometry for Assessing the Effects of Compounds on Viability of Intracellular Amastigotes
3.5. Fluorescence Microscopy Assay for Apoptosis and Necrosis
3.6. Assessment of the Effect of Compounds on DNA of Promastigotes
3.7. Transmission Electron Microscopy for Ultrastructure Analysis
3.8. Real-Time PCR Assay for Quantification of mRNA
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- McGwire, B.; Satoskar, A. Leishmaniasis: Clinical syndromes and treatment. QJM Int. J. Med. 2013, 107, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimozako, H.J.; Wu, J.; Massad, E. Mathematical modelling for Zoonotic Visceral Leishmaniasis dynamics: A new analysis considering updated parameters and notified human Brazilian data. Infect. Dis. Model. 2017, 2, 143–160. [Google Scholar] [CrossRef]
- Ahamad, B.; Islam, A.; Khan, A.; Khan, M.A.; ul Haq, I.; Ahmad, M.; Mehwish, S.; Khan, A.; Ullah, N. Comprehensive investigations on anti-leishmanial potentials of Euphorbia wallichii root extract and its effects on membrane permeability and apoptosis. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 138–145. [Google Scholar] [CrossRef]
- Polonio, T.; Efferth, T. Leishmaniasis: Drug resistance and natural products. Int. J. Mol. Med. 2008, 22, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Ullah, N.; Nadhman, A.; Siddiq, S.; Mehwish, S.; Islam, A.; Jafri, L.; Hamayun, M. Plants as antileishmanial agents: Current scenario. Phytother. Res. 2016, 30, 1905–1925. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H. Special Issue “Flavonoids and Their Disease Prevention and Treatment Potential”: Recent Advances and Future Perspectives. Molecules 2020, 25, 4746. [Google Scholar] [CrossRef]
- Horáková, Ľ. Flavonoids in prevention of diseases with respect to modulation of Ca-pump function. Interdiscip. Toxicol. 2011, 4, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, K.; Kaur, G.; Kaur, S. Activity of rutin, a potent flavonoid against SSG-sensitive and-resistant Leishmania donovani parasites in experimental leishmaniasis. Int. Immunopharmacol. 2018, 64, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS ONE 2011, 6, e14666. [Google Scholar] [CrossRef]
- Fonseca-Silva, F.; Inacio, J.D.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive oxygen species production by quercetin causes the death of Leishmania amazonensis intracellular amastigotes. J. Nat. Prod. 2013, 76, 1505–1508. [Google Scholar] [CrossRef] [PubMed]
- Siqueira-Neto, J.L. An Image-Based High-Content Screening Assay for Compounds Targeting Intracellular Leishmania donovani Amastigotes in Human Macrophages. PLoS Negl. Trop. Dis. 2012, 6, e1671. [Google Scholar] [CrossRef] [Green Version]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Weiss, L.M.; Ma, Y.F.; Takvorian, P.M.; Tanowitz, H.B.; Wittner, M. Bradyzoite development in Toxoplasma gondii and the hsp70 stress response. Infect. Immun. 1998, 66, 3295–3302. [Google Scholar] [CrossRef] [Green Version]
- Khoo, C.; Falk, M. Polyphenols in the Prevention and Treatment of Vascular and Cardiac Disease, and Cancer. Polyphen. Hum. Health Dis. 2014, 2, 1049–1065. [Google Scholar]
- Varikuti, S.; Volpedo, G.; Saljoughian, N.; Hamza, O.M.; Halsey, G.; Ryan, N.M.; Sedmak, B.E.; Seidler, G.R.; Papenfuss, T.L.; Oghumu, S. The Potent ITK/BTK Inhibitor Ibrutinib Is Effective for the Treatment of Experimental Visceral Leishmaniasis Caused by Leishmania donovani. J. Infect. Dis. 2018, 219, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Balabhadrapathruni, S.; Thomas, T.; Yurkow, E.J.; Amenta, P.S.; Thomas, T. Effects of genistein and structurally related phytoestrogens on cell cycle kinetics and apoptosis in MDA-MB-468 human breast cancer cells. Oncol. Rep. 1999, 7, 3–15. [Google Scholar] [CrossRef]
- Duthie, S.J.; Johnson, W.; Dobson, V. The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 1997, 390, 141–151. [Google Scholar] [CrossRef]
- Murtaza, I.; Marra, G.; Schlapbach, R.; Patrignani, A.; Künzli, M.; Wagner, U.; Sabates, J.; Dutt, A. A preliminary investigation demonstrating the effect of quercetin on the expression of genes related to cell-cycle arrest, apoptosis and xenobiotic metabolism in human CO115 colon-adenocarcinoma cells using DNA microarray. Biotechnol. Appl. Biochem. 2006, 45, 29–36. [Google Scholar]
- Ramasamy, S.; Wahab, N.A.; Abidin, N.Z.; Manickam, S. Effect of extracts from Phyllanthus watsonii Airy Shaw on cell apoptosis in cultured human breast cancer MCF-7 cells. Exp. Toxicol. Pathol. 2013, 65, 341–349. [Google Scholar] [CrossRef]
- Filippi-Chiela, E.C.; Oliveira, M.M.; Jurkovski, B.; Callegari-Jacques, S.M.; Da Silva, V.D.; Lenz, G. Nuclear morphometric analysis (NMA): Screening of senescence, apoptosis and nuclear irregularities. PLoS ONE 2012, 7, e42522. [Google Scholar] [CrossRef] [Green Version]
- Ćurčić, M.G.; Stanković, M.S.; Mrkalić, E.M.; Matović, Z.D.; Banković, D.D.; Cvetković, D.M.; Đačić, D.S.; Marković, S.D. Antiproliferative and proapoptotic activities of methanolic extracts from Ligustrum vulgare L. as an individual treatment and in combination with palladium complex. Int. J. Mol. Sci. 2012, 13, 2521–2534. [Google Scholar] [CrossRef] [PubMed]
- Rondon, F.C.; Bevilaqua, C.M.; Accioly, M.P.; Morais, S.M.; Andrade-Junior, H.F.; Machado, L.K.; Cardoso, R.P.; Almeida, C.A.; Queiroz-Junior, E.M.; Rodrigues, A.C.M. In vitro effect of Aloe vera, Coriandrum sativum and Ricinus communis fractions on Leishmania infantum and on murine monocytic cells. Vet. Parasitol. 2011, 178, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Belkhelfa-Slimani, R.; Djerdjouri, B. Caffeic acid and quercetin exert caspases-independent apoptotic effects on Leishmania major promastigotes, and reactivate the death of infected phagocytes derived from BALB/c mice. Asian Pac. J. Trop. Biomed. 2017, 7, 321–331. [Google Scholar] [CrossRef]
- Antwi, C.A.; Amisigo, C.M.; Adjimani, J.P.; Gwira, T.M. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLoS Negl. Trop. Dis. 2019, 13, e0007206. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.; Nafisi, S.; Rajabi, M.; Shadaloi, A.; Tarantilis, P.; Polissiou, M.; Bariyanga, J.; Tajmir-Riahi, H. Structural analysis of DNA and RNA interactions with antioxidant flavonoids. Spectroscopy 2009, 23, 29–43. [Google Scholar] [CrossRef]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, D.; Long, H.; Liu, Y. Electrochemical behavior of gallic acid interaction with DNA and detection of damage to DNA. J. Electroanal. Chem. 2008, 624, 91–96. [Google Scholar] [CrossRef]
- Pinlaor, S.; Hiraku, Y.; Ma, N.; Yongvanit, P.; Semba, R.; Oikawa, S.; Murata, M.; Sripa, B.; Sithithaworn, P.; Kawanishi, S. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: A model of inflammation-mediated carcinogenesis. Nitric Oxide 2004, 11, 175–183. [Google Scholar] [CrossRef]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Rowe, L.A.; Degtyareva, N.; Doetsch, P.W. DNA damage-induced reactive oxygen species (ROS) stress response in Saccharomyces cerevisiae. Free Radic. Biol. Med. 2008, 45, 1167–1177. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Chen, C.; Hou, J.; Xin, W.; Mori, A. Nitric oxide induces oxidative stress and apoptosis in neuronal cells. Biochim. Biophys. Acta (Bba)-Mol. Cell Res. 2000, 1498, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, H.S.; Sohail, M.F.; Saljoughian, N.; Rehman, A.U.; Akhtar, S.; Nadhman, A.; Yasinzai, M.; Gendelman, H.E.; Satoskar, A.R.; Shahnaz, G. Design of mannosylated oral amphotericin B nanoformulation: Efficacy and safety in visceral leishmaniasis. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huk, I.; B Rovkovych, V.; Nanobash Vili, J.; Weigel, G.; Neumayer, C.; Partyka, L.; Patton, S.; Malinski, T. Bioflavonoid quercetin scavenges superoxide and increases nitric oxide concentration in ischaemia–reperfusion injury: An experimental study. Br. J. Surg. 1998, 85, 1080–1085. [Google Scholar] [CrossRef]
- Shoskes, D.A. Effect of bioflavonoids quercetin and curcumin on ischemic renal injury: A new class of renoprotective agents1. Transplantation 1998, 66, 147–152. [Google Scholar] [CrossRef]
- Shutenko, Z.; Henry, Y.; Pinard, E.; Seylaz, J.; Potier, P.; Berthet, F.; Girard, P.; Sercombe, R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1999, 57, 199–208. [Google Scholar] [CrossRef]
- Mehta, A.; Shaha, C. Mechanism of metalloid-induced death in Leishmania spp.: Role of iron, reactive oxygen species, Ca2+, and glutathione. Free Radic. Biol. Med. 2006, 40, 1857–1868. [Google Scholar] [CrossRef] [PubMed]
- Mehwish, S.; Khan, H.; Rehman, A.U.; Khan, A.U.; Khan, M.A.; Hayat, O.; Ahmad, M.; Wadood, A.; Ullah, N. Natural compounds from plants controlling leishmanial growth via DNA damage and inhibiting trypanothione reductase and trypanothione synthetase: An in vitro and in silico approach. 3 Biotech 2019, 9, 303. [Google Scholar] [CrossRef]
- Saito, K.; Jin, D.-H.; Ogawa, T.; Muramoto, K.; Hatakeyama, E.; Yasuhara, T.; Nokihara, K. Antioxidative properties of tripeptide libraries prepared by the combinatorial chemistry. J. Agric. Food Chem. 2003, 51, 3668–3674. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Souza, F.; de Oliveira, A.E.R.; Abreu-Silva, A.L.; da Silva Calabrese, K. In vitro activity of Morinda citrifolia Linn. fruit juice against the axenic amastigote form of Leishmania amazonensis and its hydrogen peroxide induction capacity in BALB/c peritoneal macrophages. BMC Res. Notes 2018, 11, 492. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.R.; Leitao, G.G.; Bizzo, H.R.; Alviano, D.S.; Alviano, C.S.; Leitão, S.G. Chemical and antimicrobial analyses of essential oil of Lippia origanoides HBK. Food Chem. 2007, 101, 236–240. [Google Scholar] [CrossRef]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Ueda-Nakamura, T.; Cortez, D.A.G.; Dias Filho, B.P.; Morgado-Díaz, J.A.; de Souza, W.; Nakamura, C.V. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium. Antimicrob. Agents Chemother. 2005, 49, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.T.; Dixo, M.; Pavan, D.; Verdade, V.K. A new species of Leposoma (Squamata, Gymnophthalmidae) from the remnant Atlantic forests of the state of Bahia, Brazil. Papéis Avulsos De Zool. 2002, 42, 335–350. [Google Scholar] [CrossRef]
- Ng, L.G.; Hsu, A.; Mandell, M.A.; Roediger, B.; Hoeller, C.; Mrass, P.; Iparraguirre, A.; Cavanagh, L.L.; Triccas, J.A.; Beverley, S.M. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008, 4, e1000222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weischenfeldt, J.; Porse, B. Bone marrow-derived macrophages (BMM): Isolation and applications. Cold Spring Harb. Protoc. 2008, 12, pdb.prot5080. [Google Scholar] [CrossRef] [Green Version]
- Terrazas, C.; Varikuti, S.; Kimble, J.; Moretti, E.; Boyaka, P.N.; Satoskar, A.R. IL-17A promotes susceptibility during experimental visceral leishmaniasis caused by Leishmania donovani. FASEB J. 2015, 30, 1135–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.; Khan, M.A.; Nadhman, A. Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl. Microbiol. Biotechnol. 2015, 99, 9923–9934. [Google Scholar]
- Collins, A.R. The comet assay for DNA damage and repair. Mol. Biotechnol. 2004, 26, 249. [Google Scholar] [CrossRef]
- Topuzogullari, M.; Koc, R.C.; Isoglu, S.D.; Bagirova, M.; Akdeste, Z.; Elcicek, S.; Oztel, O.N.; Baydar, S.Y.; Ates, S.C.; Allahverdiyev, A.M. Conjugation, characterization and toxicity of lipophosphoglycan-polyacrylic acid conjugate for vaccination against leishmaniasis. J. Biomed. Sci. 2013, 20, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Debnath, S.; Hazra, S.; Hartung, A.; Thomale, K.; Schultheis, M.; Kapkova, P.; Schurigt, U.; Moll, H.; Holzgrabe, U. Valeriana wallichii root extracts and fractions with activity against Leishmania spp. Parasitol. Res. 2011, 108, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Zakrajsek, B.A. Effect of experimental treatment on housekeeping gene expression: Validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 2000, 46, 69–81. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehwish, S.; Varikuti, S.; Ali Khan, M.; Khan, T.; Khan, I.U.; Satoskar, A.; Elsayed Elserehy, H.A.; Ullah, N. Bioflavonoid-Induced Apoptosis and DNA Damage in Amastigotes and Promastigotes of Leishmania donovani: Deciphering the Mode of Action. Molecules 2021, 26, 5843. https://doi.org/10.3390/molecules26195843
Mehwish S, Varikuti S, Ali Khan M, Khan T, Khan IU, Satoskar A, Elsayed Elserehy HA, Ullah N. Bioflavonoid-Induced Apoptosis and DNA Damage in Amastigotes and Promastigotes of Leishmania donovani: Deciphering the Mode of Action. Molecules. 2021; 26(19):5843. https://doi.org/10.3390/molecules26195843
Chicago/Turabian StyleMehwish, Shaila, Sanjay Varikuti, Mubarak Ali Khan, Tariq Khan, Imdad Ullah Khan, Abhay Satoskar, Hamed Abdelhamid Elsayed Elserehy, and Nazif Ullah. 2021. "Bioflavonoid-Induced Apoptosis and DNA Damage in Amastigotes and Promastigotes of Leishmania donovani: Deciphering the Mode of Action" Molecules 26, no. 19: 5843. https://doi.org/10.3390/molecules26195843
APA StyleMehwish, S., Varikuti, S., Ali Khan, M., Khan, T., Khan, I. U., Satoskar, A., Elsayed Elserehy, H. A., & Ullah, N. (2021). Bioflavonoid-Induced Apoptosis and DNA Damage in Amastigotes and Promastigotes of Leishmania donovani: Deciphering the Mode of Action. Molecules, 26(19), 5843. https://doi.org/10.3390/molecules26195843







