Hexaconazole-Micelle Nanodelivery System Prepared Using Different Surfactants for Ganoderma Antifungal Application
Abstract
:1. Introduction
2. Results and Discussion
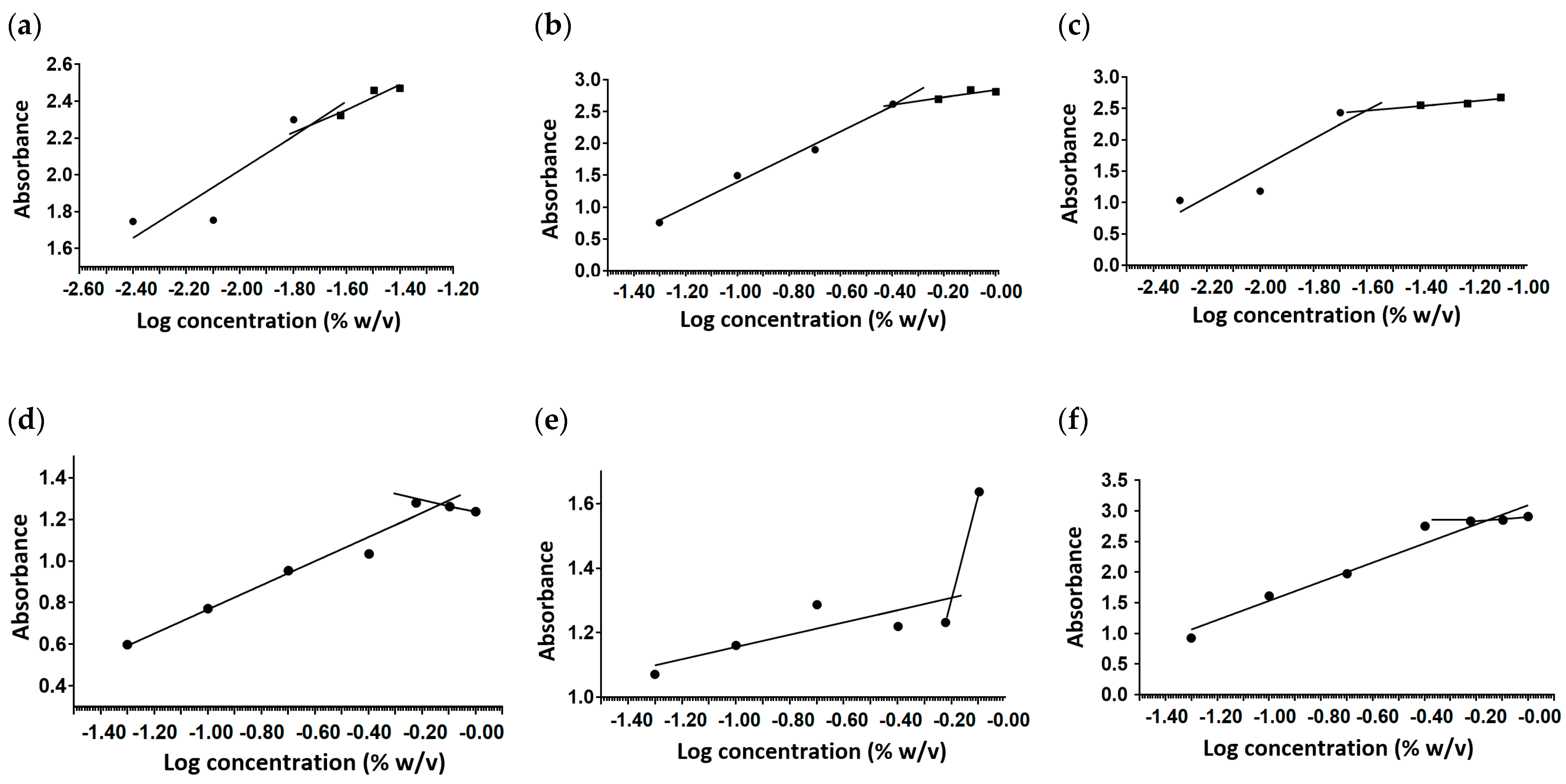
2.1. Critical Micelle Concentration
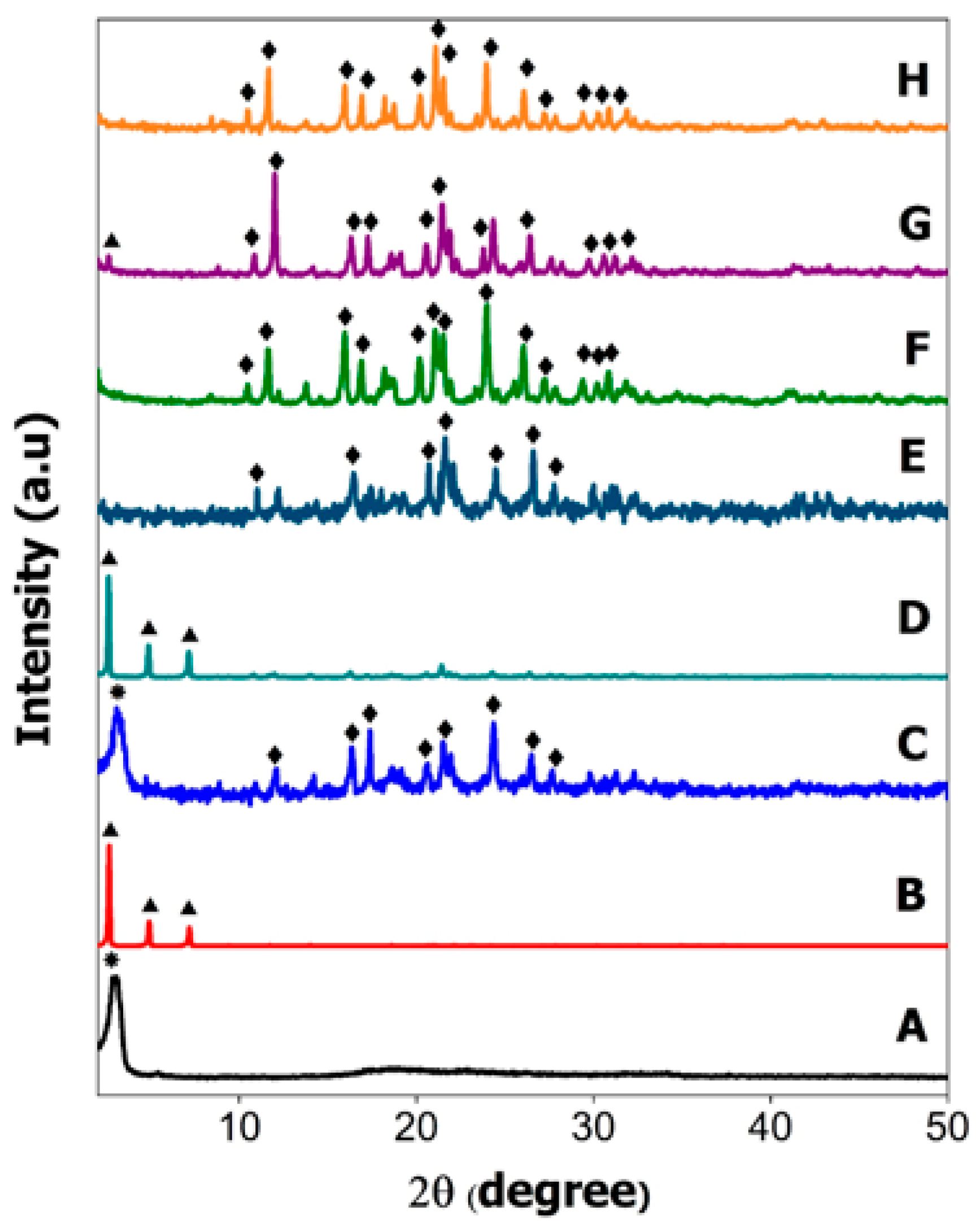
2.2. Powder X-ray Diffraction
 ) signifying that SDS surfactant also has crystalline property in the mixture. The high intensity of the XRD pattern of SDS in the HMDS sample has suppressed the XRD reflection peak of hexaconazole. Similar reflection peaks at 10.44, 11.64, 15.94, 16.9, 20.24, 21.08, 21.54, 23.94, 26.04, 27.18, 29.4, 30.34 and 30.82° (
) signifying that SDS surfactant also has crystalline property in the mixture. The high intensity of the XRD pattern of SDS in the HMDS sample has suppressed the XRD reflection peak of hexaconazole. Similar reflection peaks at 10.44, 11.64, 15.94, 16.9, 20.24, 21.08, 21.54, 23.94, 26.04, 27.18, 29.4, 30.34 and 30.82° (  ) can be seen in the XRD patterns, due to hexaconazole with only some changes in their intensity. Assuming that all the surfactants—SDBS, SDS and T80—used in this work have no reflection peak in their XRD patterns of the as-synthesized micelles (Figure 2F–H), it can be concluded that the surfactants formed the micelle system in an amorphous state [11].
) can be seen in the XRD patterns, due to hexaconazole with only some changes in their intensity. Assuming that all the surfactants—SDBS, SDS and T80—used in this work have no reflection peak in their XRD patterns of the as-synthesized micelles (Figure 2F–H), it can be concluded that the surfactants formed the micelle system in an amorphous state [11].2.3. Fourier-Transform Infrared Spectroscopy
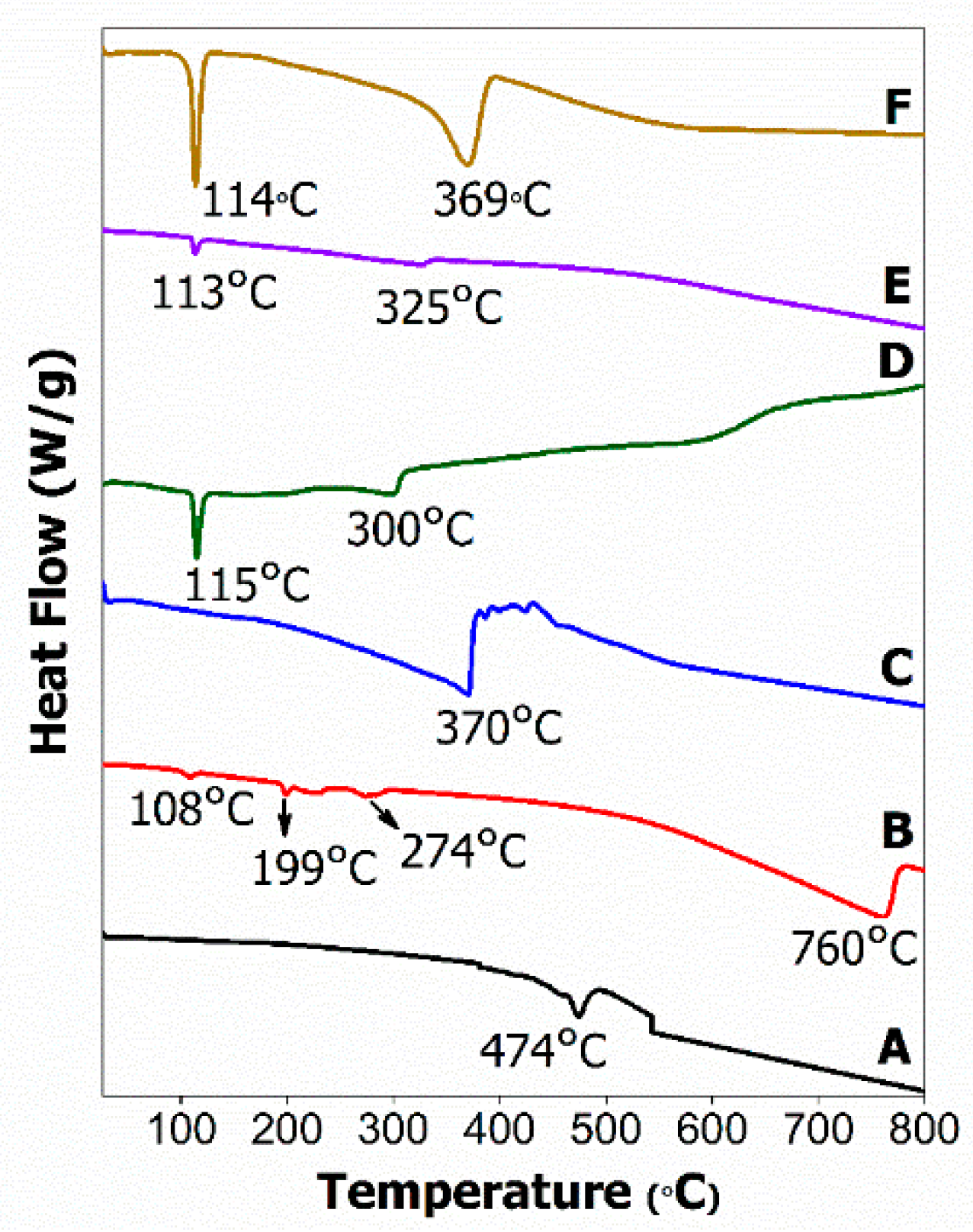
2.4. Thermal Analysis
2.5. Differential Scanning Calorimetry
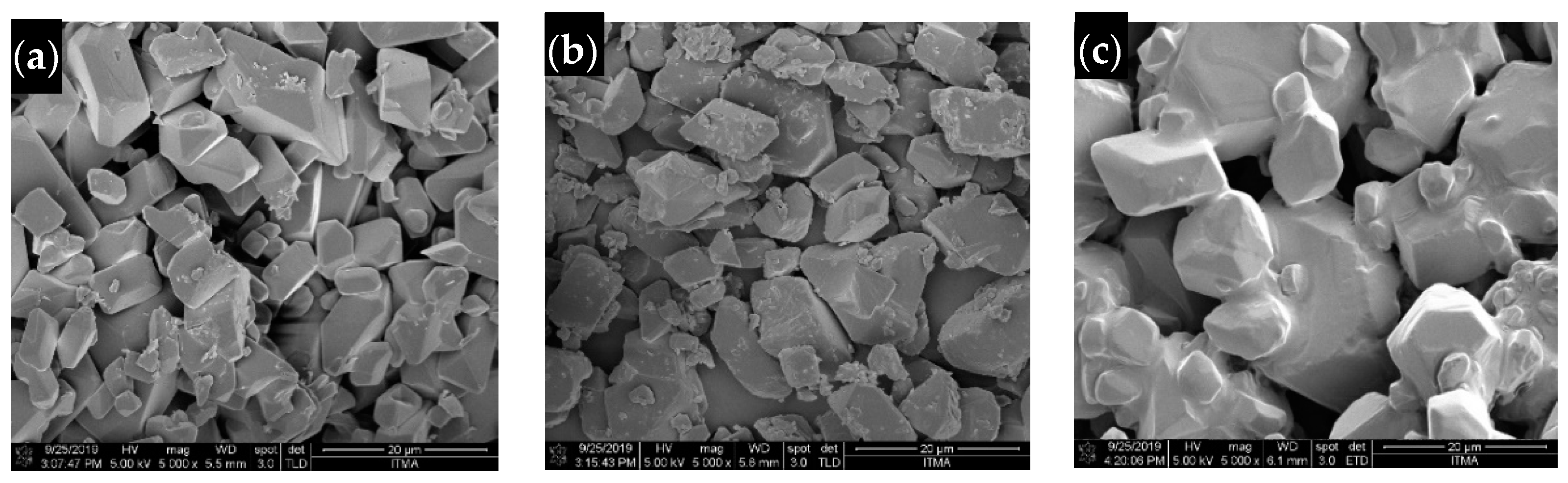
2.6. Surface Morphology
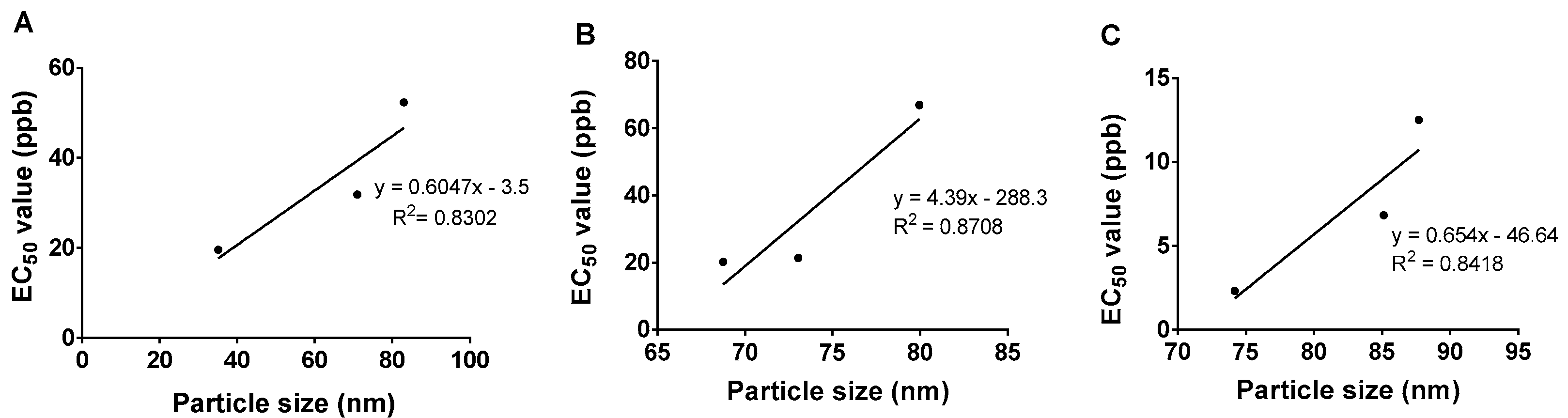
2.7. Particle Size and Its Stability
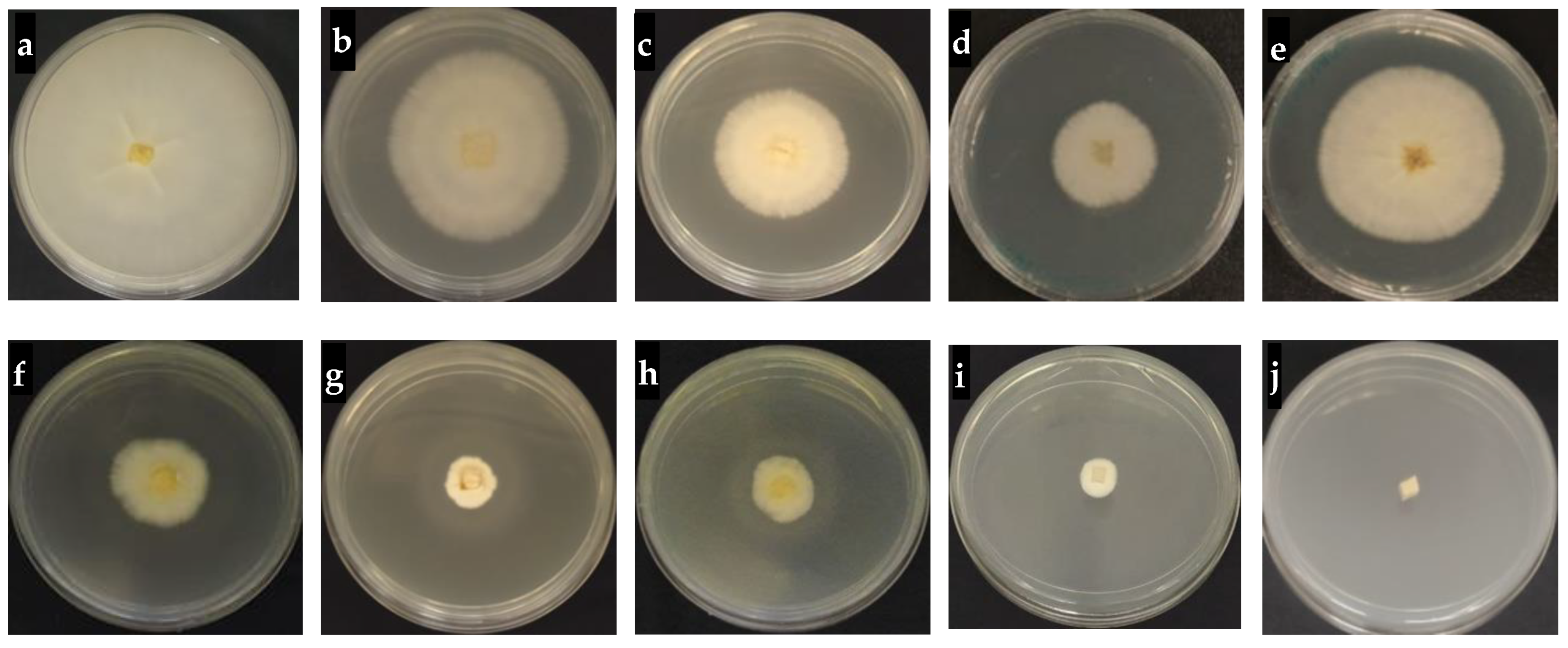
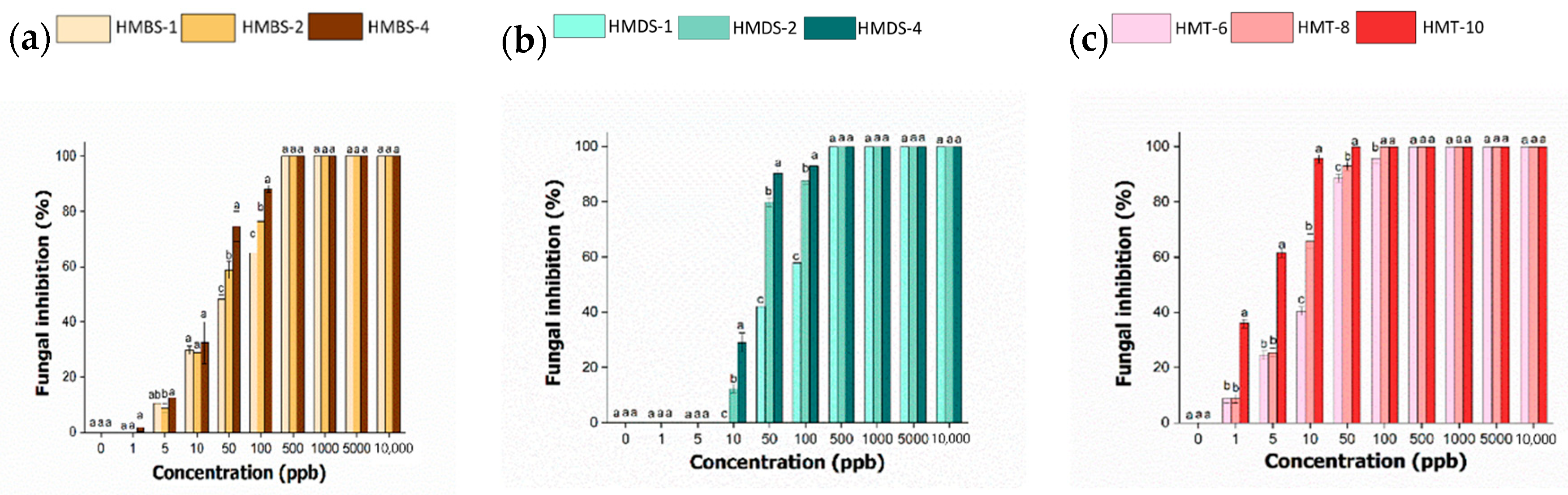
2.8. In Vitro Antifungal Activity of Hexaconazole-Micelles Nanodelivery System
3. Materials and Methods
3.1. Chemical Reagents
3.2. Micelle Preparation
3.3. Measurement of Critical Micelle Concentration
3.4. Characterization of Hexaconazole Micelles
3.5. Measurement of Fungicide Loading in Micelle System
3.6. Determination of Particle Size and Stability
3.7. Fungicidal Activity
3.7.1. In Vitro Antifungal Assay
3.7.2. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Idris, D.; Arifin, S.; Ahmad, H. Prolonging the productive life of Ganoderma-infected palms with hexaconazole. MPOB Inf. Ser. 2004, 214, 3–6. [Google Scholar]
- Maznah, Z.; Halimah, M.; Ismail, S.; Idris, A.S. Dissipation of the fungicide hexaconazole in oil palm plantation. Environ. Sci. Pollut. Res. 2015, 22, 19648–19657. [Google Scholar] [CrossRef] [PubMed]
- Baird, T.; DeLorenzo, M.E. Descriptive and mechanistic toxicity of conazole fungicides using the model test algaDunaliella tertiolecta(chlorophyceae). Environ. Toxicol. 2009, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, H.; Zainol, M.; Sahid, I.; Abu Seman, I. Determination of hexaconazole in field samples of an oil palm plantation. Drug Test. Anal. 2012, 4, 112–117. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z.; Saifullah, B.; Idris, A.S.; Hilmi, N.H.Z.; Fakurazi, S. Synthesis of (Hexaconazole-Zinc/Aluminum-Layered Double Hydroxide Nanocomposite) Fungicide Nanodelivery System for Controlling Ganoderma Disease in Oil Palm. J. Agric. Food Chem. 2018, 66, 806–813. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z.; Yusof, N.A.; Fakurazi, S.; Idris, A.S.; Hilmi, N.H.Z.; Daim, L.D.J. Preparation of Chitosan-Hexaconazole Nanoparticles as Fungicide Nanodelivery System for Combating Ganoderma Disease in Oil Palm. Molecules 2019, 24, 2498. [Google Scholar] [CrossRef] [Green Version]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef]
- Wu, D.; Lu, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Influence of nonionic and ionic surfactants on the antifungal and mycotoxin inhibitory efficacy of cinnamon oil nanoemulsions. Food Funct. 2019, 10, 2817–2827. [Google Scholar] [CrossRef]
- Jibrin, M.O.; Liu, Q.; Jones, J.B.; Zhang, S. Surfactants in plant disease management: A brief review and case studies. Plant Pathol. 2021, 70, 495–510. [Google Scholar] [CrossRef]
- Miyagishi, S. The Effect of Organic Additives on the Thermodynamic Parameters of Micellization. Bull. Chem. Soc. Jpn. 1976, 49, 34–37. [Google Scholar] [CrossRef]
- Liang, N.; Sun, S.; Gong, X.; Li, Q.; Yan, P.; Cui, F. Polymeric Micelles Based on Modified Glycol Chitosan for Paclitaxel Delivery: Preparation, Characterization and Evaluation. Int. J. Mol. Sci. 2018, 19, 1550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, P.; Liu, A.; Kang, L.; Zhu, M.; Dai, B. Heteropoly acid supported on sodium dodecyl benzene sulfonate modified layered double hydroxides as catalysts for oxidative desulfurization. New J. Chem. 2018, 42, 12830–12837. [Google Scholar] [CrossRef]
- Singh, M.K.; Agarwal, A.; Gopal, R.; Swarnkar, R.K.; Kotnala, R.K. Dumbbell shaped nickel nanocrystals synthesized by a laser induced fragmentation method. J. Mater. Chem. 2011, 21, 11074–11079. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Mandal, A.; Chakravorty, D.; Gopal, M.; Goswami, A. Evaluation of physicochemical properties, and antimicrobial efficacy of monoclinic sulfur-nanocolloid. J. Nanopart. Res. 2013, 15, 1491. [Google Scholar] [CrossRef]
- King, S.; McCafferty, L.; Stolojan, V.; Silva, S.R.P. Highly aligned arrays of super resilient carbon nanotubes by steam purification. Carbon 2015, 84, 130–137. [Google Scholar] [CrossRef]
- Ahmed, A.A.A.; Talib, Z.A.; Hussein, M.Z. Influence of sodium dodecyl sulfate concentration on the photocatalytic activity and dielectric properties of intercalated sodium dodecyl sulfate into Zn–Cd–Al layered double hydroxide. Mater. Res. Bull. 2015, 62, 122–131. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bin Hussein, M.Z.; Taufiq-Yap, Y.H. The Effect of Sodium Dodecyl Sulfate (SDS) and Cetyltrimethylammonium Bromide (CTAB) on the Properties of ZnO Synthesized by Hydrothermal Method. Int. J. Mol. Sci. 2012, 13, 13275–13293. [Google Scholar] [CrossRef] [Green Version]
- Larson, N.R.; Wei, Y.; Prajapati, I.; Chakraborty, A.; Peters, B.; Kalonia, C.; Hudak, S.; Choudhary, S.; Esfandiary, R.; Dhar, P.; et al. Comparison of Polysorbate 80 Hydrolysis and Oxidation on the Aggregation of a Monoclonal Antibody. J. Pharm. Sci. 2020, 109, 633–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukwada, L.T.; Mochane, M.J.; Motaung, T.E.; Motloung, S.V.; Koao, L.F. Effect of sodium dodecylbenzene sulphonate modifier and PP-g-MA on the morphology and thermal conductivity of PP/EG composites. Plast. Rubber Compos. 2017, 46, 469–475. [Google Scholar] [CrossRef]
- Parhizkar, M.; Edirisinghe, M.J.; Stride, E. The effect of surfactant type and concentration on the size and stability of microbubbles produced in a capillary embedded T-junction device. RSC Adv. 2014, 5, 10751–10762. [Google Scholar] [CrossRef]
- Landfester, K.; Bechthold, N.; Tiarks, A.F.; Antonietti, M. Formulation and Stability Mechanisms of Polymerizable Miniemulsions. Macromolecules 1999, 32, 5222–5228. [Google Scholar] [CrossRef]
- Hecht, L.L.; Wagner, C.; Landfester, K.; Schuchmann, H.P. Surfactant Concentration Regime in Miniemulsion Polymerization for the Formation of MMA Nanodroplets by High-Pressure Homogenization. Langmuir 2011, 27, 2279–2285. [Google Scholar] [CrossRef]
- Mustafa, I.F.; Hussein, M.Z. Synthesis and Technology of Nanoemulsion-Based Pesticide Formulation. Macromolecules 2020, 10, 1608. [Google Scholar] [CrossRef]
- Fu, J.; Cai, Z.; Gong, Y.; O’Reilly, S.; Hao, X.; Zhao, D. A new technique for determining critical micelle concentrations of surfactants and oil dispersants via UV absorbance of pyrene. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Danquah, M.; Mahato, R.I. Synthesis and Characterization of Amphiphilic Lipopolymers for Micellar Drug Delivery. Biomacromolecules 2010, 11, 2610–2620. [Google Scholar] [CrossRef]
- Ali, M.M.; Sadeghizadeh, M.; Najafi, F.; Ardestani, S.K.; Erfani-Moghadam, V.; Khaniki, M.; Rezaei, A.; Zamani, M.; Khodayari, S.; Khodayari, H.; et al. Encapsulation of Curcumin in Diblock Copolymer Micelles for Cancer Therapy. BioMed Res. Int. 2015, 2015, 824746. [Google Scholar] [CrossRef]
- Skidmore, A.; Dickinson, C. Colony interactions and hyphal interference between Septoria nodorum and phylloplane fungi. Trans. Br. Mycol. Soc. 1976, 66, 57–64. [Google Scholar] [CrossRef]




| Surfactant Used | Surfactant Concentration (% w/v) | Formulation Label | PSD * Intensity (nm) | |
|---|---|---|---|---|
| Initial | after 6 Months of Storage | |||
| Sodium dodecyl benzene sulfonate | 1 | HMBS-1 | 83 | 133 |
| 2 | HMBS-2 | 71 | 118 | |
| 4 | HMBS-4 | 35 | 90 | |
| Sodium dodecyl sulfate | 1 | HMDS-1 | 80 | 150 |
| 2 | HMDS-2 | 73 | 137 | |
| 4 | HMDS-4 | 69 | 103 | |
| Tween 80 | 6 | HMT-6 | 88 | 137 |
| 8 | HMT-8 | 87 | 122 | |
| 10 | HMT-10 | 74 | 91 | |
| Samples | EC50 (ppb) | EC50 95% Confidence Interval | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| HMBS-1 | 52 | 40 | 68 |
| HMBS-2 | 32 | 26 | 40 |
| HMBS-4 | 20 | 16 | 25 |
| HMDS-1 | 67 | 55 | 81 |
| HMDS-2 | 21 | 16 | 28 |
| HMDS-4 | 20 | 13 | 31 |
| HMT-6 | 13 | 10 | 15 |
| HMT-8 | 7 | 5 | 9 |
| HMT-10 | 2 | 1 | 4 |
| Growth | Micelle | Concentration | Surfactant | Concentration × Surfactant |
|---|---|---|---|---|
| p-value | HMBS | 0 | 0 | 0 |
| HMDS | 0 | 0 | 0 | |
| HMT | 0 | 0 | 0 | |
| F-value | HMBS | 3909 | 55 | 18 |
| HMDS | 25,348 | 1291 | 377 | |
| HMT | 12,690 | 1368 | 309 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, I.F.; Hussein, M.Z.; Idris, A.S.; Hilmi, N.H.Z.; Fakurazi, S. Hexaconazole-Micelle Nanodelivery System Prepared Using Different Surfactants for Ganoderma Antifungal Application. Molecules 2021, 26, 5837. https://doi.org/10.3390/molecules26195837
Mustafa IF, Hussein MZ, Idris AS, Hilmi NHZ, Fakurazi S. Hexaconazole-Micelle Nanodelivery System Prepared Using Different Surfactants for Ganoderma Antifungal Application. Molecules. 2021; 26(19):5837. https://doi.org/10.3390/molecules26195837
Chicago/Turabian StyleMustafa, Isshadiba Faikah, Mohd Zobir Hussein, Abu Seman Idris, Nur Hailini Zainol Hilmi, and Sharida Fakurazi. 2021. "Hexaconazole-Micelle Nanodelivery System Prepared Using Different Surfactants for Ganoderma Antifungal Application" Molecules 26, no. 19: 5837. https://doi.org/10.3390/molecules26195837
APA StyleMustafa, I. F., Hussein, M. Z., Idris, A. S., Hilmi, N. H. Z., & Fakurazi, S. (2021). Hexaconazole-Micelle Nanodelivery System Prepared Using Different Surfactants for Ganoderma Antifungal Application. Molecules, 26(19), 5837. https://doi.org/10.3390/molecules26195837






