Supercritical Carbon Dioxide Isolation of Cellulose Nanofibre and Enhancement Properties in Biopolymer Composites
Abstract
:1. Introduction
2. Results
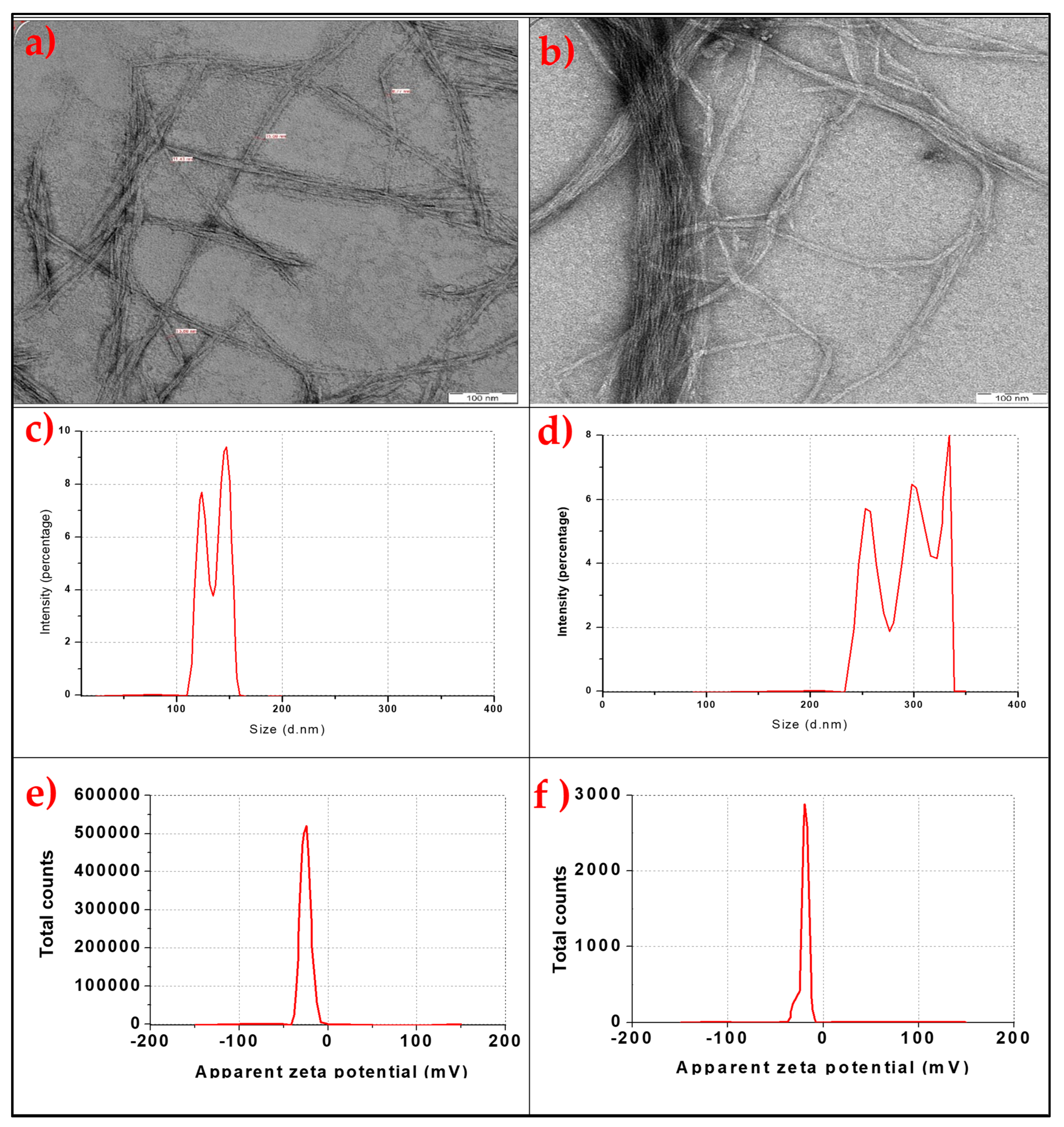
2.1. The Properties of Bamboo and Commercial Cellulose Nanofibres
2.2. The Physical Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
2.3. The Mechanical Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
2.4. The Morphological Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
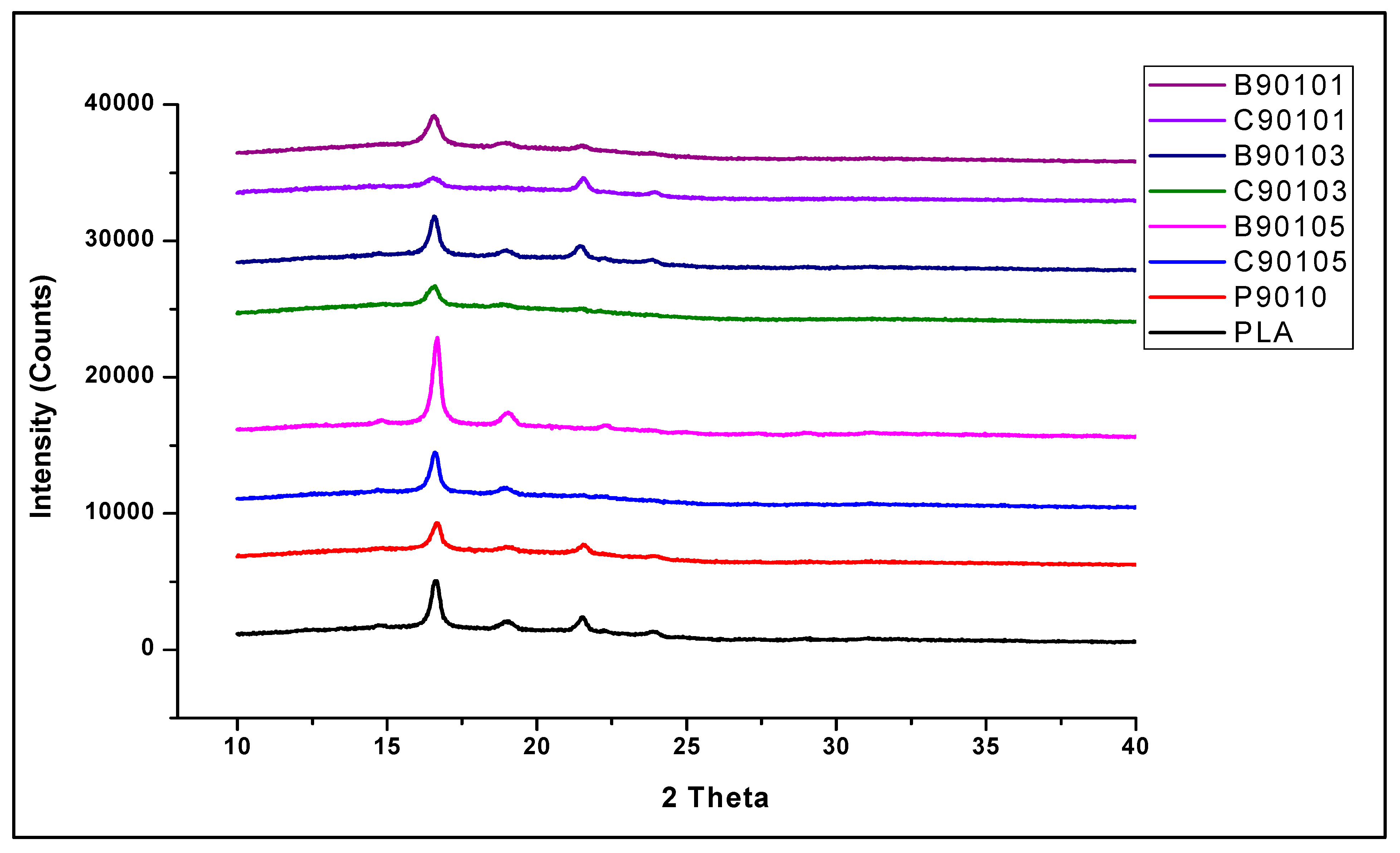
2.5. The Structural Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
2.6. The Thermal Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
2.7. The Wettability Properties of Bamboo and Commercial CNF-Reinforced PLA/Chitin Bionanocomposites
3. Materials and Methods
3.1. Materials
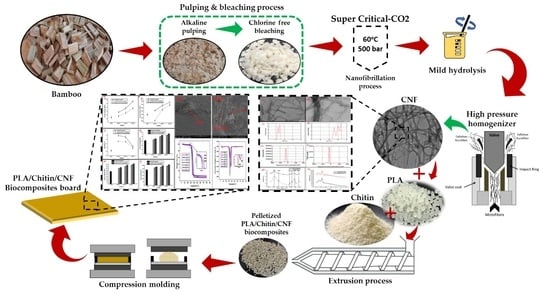
3.2. The Isolation and Characterisation of Cellulose Nanofibrillated Fibre from Bamboo
3.3. The Preparation and Characterisation of CNF-Reinforced Bionanocomposite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brebu, M. Environmental Degradation of Plastic Composites with Natural Fillers—A Review. Polymers 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Aldana, D.S.; Villa, E.D.; De Dios Hernández, M.; Sánchez, G.G.; Cruz, Q.R.; Gallardo, S.F.; Castillo, H.P.; Casarrubias, L.B. Barrier properties of polylactic acid in cellulose based packages using montmorillonite as filler. Polymers 2014, 6, 2386–2403. [Google Scholar] [CrossRef] [Green Version]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Henton, D.E.; Gruber, P.; Lunt, J.; Randall, J. Polylactic acid technology. Nat. Fibers Biopolym. Biocompos. 2005, 16, 527–577. [Google Scholar]
- Cinelli, P.; Coltelli, M.; Mallegni, N.; Morganti, P.; Lazzeri, A. Degradability and sustainability of nanocomposites based on polylactic acid and chitin nano fibrils. Chem. Eng. Trans. 2017, 60, 115–120. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.; Abdat, M.; Nam, N.; Halim, A.S.; Kumar, U.; Bairwan, R. A review on micro-to nanocellulose biopolymer scaffold forming for tissue engineering applications. Polymers 2020, 12, 2043. [Google Scholar] [CrossRef]
- Huang, L.-B.; Xu, W.; Tian, W.; Han, J.-C.; Zhao, C.-H.; Wu, H.-L.; Hao, J. Ultrasonic-assisted ultrafast fabrication of polymer nanowires for high performance triboelectric nanogenerators. Nano Energy 2020, 71, 104593. [Google Scholar] [CrossRef]
- Huang, L.-B.; Xu, W.; Zhao, C.; Zhang, Y.-L.; Yung, K.-L.; Diao, D.; Fung, K.H.; Hao, J. Multifunctional water drop energy harvesting and human motion sensor based on flexible dual-mode nanogenerator incorporated with polymer nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 24030–24038. [Google Scholar] [CrossRef]
- Bahari, S.A.; Krause, A. Utilizing Malaysian bamboo for use in thermoplastic composites. J. Clean. Prod. 2016, 110, 16–24. [Google Scholar] [CrossRef]
- Bahari, S.A.; Krause, A. Analysis on malaysian bamboo particle for thermoplastic composites production. In Proceedings of the First International Conference on Resource Efficiency in Interorganizational Networks (ResEff 2013), Göttingen, Germany, 13–14 November 2013; Geldermann, J., Schumann, M., Eds.; Universitätsverlag Göttingen: Göttingen, Germany, 2013; pp. 17–27. [Google Scholar]
- Benton, A. Priority species of bamboo. In Bamboo; Springer: Cham, Switzerland, 2015; pp. 31–41. [Google Scholar]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Irvin, C.W.; Satam, C.C.; Meredith, J.C.; Shofner, M.L. Mechanical reinforcement and thermal properties of PVA tricomponent nanocomposites with chitin nanofibers and cellulose nanocrystals. Compos. Part A Appl. Sci. Manuf. 2019, 116, 147–157. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G. Properties and skin compatibility of films based on poly (lactic acid) (PLA) bionanocomposites incorporating chitin nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, J.; Feng, D.; Zhao, J.; Sun, J.; Li, D. Comparative Study on Properties of Polylactic Acid Nanocomposites with Cellulose and Chitin Nanofibers Extracted from Different Raw Materials. J. Nanomater. 2017, 2017, 7193263. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, mechanical and thermal properties of poly (lactic acid) (PLA)/cellulose nanofibrils (CNF) composites nanofiber for tissue engineering. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.; Jinap, S.; Karim, A.; Abbas, K.; Norulaini, N.; Omar, A. Application of supercritical CO2 in lipid extraction–A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Jiang, H.; Song, Y.; Zhou, Z.; Zhao, H. Fabrication and characterization of nano-cellulose aerogels via supercritical CO2 drying technology. Mater. Lett. 2016, 183, 179–182. [Google Scholar] [CrossRef]
- Matsuyama, K.; Morotomi, K.; Inoue, S.; Nakashima, M.; Nakashima, H.; Okuyama, T.; Kato, T.; Muto, H.; Sugiyama, H. Antibacterial and antifungal properties of Ag nanoparticle-loaded cellulose nanofiber aerogels prepared by supercritical CO2 drying. J. Supercrit. Fluids 2019, 143, 1–7. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Porosity, density and mechanical properties of the paper of steam exploded bamboo microfibers controlled by nanofibrillated cellulose. J. Mater. Res. Technol. 2019, 8, 3612–3622. [Google Scholar] [CrossRef]
- Pachuau, L.; Vanlalfakawma, D.C.; Tripathi, S.K.; Lalhlenmawia, H. Muli bamboo (Melocanna baccifera) as a new source of microcrystalline cellulose. J. Appl. Pharm. Sci. 2014, 4, 87–94. [Google Scholar]
- Soni, B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr. Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Piorkowska, E.; Kulpinski, P.; Pracella, M. Mechanical and thermal properties of PLA composites with cellulose nanofibers and standard size fibers. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1509–1514. [Google Scholar] [CrossRef]
- Ohwoavworhua, F.; Adelakun, T. Some physical characteristics of microcrystalline cellulose obtained from raw cotton of Cochlospermum planchonii. Trop. J. Pharm. Res. 2005, 4, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.; Capadona, J.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S. Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Velazquez, G.; Vázquez, M. Novel composite films from regenerated cellulose-glycerol-polyvinyl alcohol: Mechanical and barrier properties. Food Hydrocoll. 2019, 89, 481–491. [Google Scholar] [CrossRef]
- Muhamad, M.; Hornsby, P.; Carmichael, E.; Zakaria, M.; Seok, Y.B.; Mohamed, S.; Sharma, S. Characterisation of cellulose nanofibres derived from chemical and mechanical treatments. In Proceedings of the MATEC Web of Conferences, Nantes, France, 10–14 June 2019; EDP Sciences: Paris, France, 2019. [Google Scholar]
- Abral, H.; Chairani, M.K.; Rizki, M.D.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.; Ilyas, R. Characterization of compressed bacterial cellulose nanopaper film after exposure to dry and humid conditions. J. Mater. Res. Technol. 2021, 11, 896–904. [Google Scholar] [CrossRef]
- Al-Dulaimi, A.A.; Wanrosli, W. Isolation and characterization of nanocrystalline cellulose from totally chlorine free oil palm empty fruit bunch pulp. J. Polym. Environ. 2017, 25, 192–202. [Google Scholar] [CrossRef]
- Atiqah, M.; Gopakumar, D.A.; FAT, O.; Pottathara, Y.B.; Rizal, S.; Aprilia, N.; Hermawan, D.; Paridah, M.; Thomas, S.; HPS, A.K. Extraction of Cellulose Nanofibers via Eco-friendly Supercritical Carbon Dioxide Treatment Followed by Mild Acid Hydrolysis and the Fabrication of Cellulose Nanopapers. Polymers 2019, 11, 1813. [Google Scholar] [CrossRef] [Green Version]
- Qua, E.; Hornsby, P.; Sharma, H.; Lyons, G. Preparation and characterisation of cellulose nanofibres. J. Mater. Sci. 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Colburn, A.; Vogler, R.J.; Patel, A.; Bezold, M.; Craven, J.; Liu, C.; Bhattacharyya, D. Composite membranes derived from cellulose and lignin sulfonate for selective separations and antifouling aspects. Nanomaterials 2019, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Wang, S. Preparation and characterization of acrylonitrile-butadiene-styrene nanocomposites reinforced with cellulose nanocrystal via solution casting method. Polym. Compos. 2017, 38, E167–E173. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Hou, Q.; Liu, H.; Lei, H.; Jian, B.; Li, X. Preparation of cellulose nanofibrils from okara by high pressure homogenization method using deep eutectic solvents. Cellulose 2020, 27, 2511–2520. [Google Scholar] [CrossRef]
- Wang, H.; Farooq, A.; Memon, H. Influence of cotton fiber properties on the microstructural characteristics of mercerized fibers by regression analysis. Wood Fiber Sci. 2020, 52, 13–27. [Google Scholar] [CrossRef]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Koyama, K. Thermomechanical and viscoelastic properties of green composites of PLA using chitin micro-particles as fillers. J. Polym. Res. 2020, 27, 1–11. [Google Scholar] [CrossRef]
- Rizvi, R.; Cochrane, B.; Naguib, H.; Lee, P.C. Fabrication and characterization of melt-blended polylactide-chitin composites and their foams. J. Cell. Plast. 2011, 47, 283–300. [Google Scholar] [CrossRef]
- Luddee, M.; Pivsa-Art, S.; Sirisansaneeyakul, S.; Pechyen, C. Particle size of ground bacterial cellulose affecting mechanical, thermal, and moisture barrier properties of PLA/BC biocomposites. Energy Procedia 2014, 56, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Mu, B.; Wang, H.; Hao, X.; Wang, Q. Morphology, mechanical properties and dimensional stability of biomass particles/high density polyethylene composites: Effect of species and composition. Polymers 2018, 10, 308. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Gopakumar, D.A.; Olaiya, N.G.; Zarlaida, F.; Alfian, A.; Aprinasari, C.; Alfatah, T.; Rizal, S.; Abdul Khalil, H.P.S. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int. J. Biol. Macromol. 2020, 156, 896–905. [Google Scholar] [CrossRef]
- Wan, Y.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Compos. Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.; Abdullah, C.; N. G., O.; Mohamad Haafiz, M.; Yahya, E.B.; Sabaruddin, F.; Abdul Khalil, H.P.S. Isolation of Textile Waste Cellulose Nanofibrillated Fibre Reinforced in Polylactic Acid-Chitin Biodegradable Composite for Green Packaging Application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef]
- Rizal, S.; Saharudin, N.; Olaiya, N.G.; Abdul Khalil, H.P.S.; Haafiz, M.; Ikramullah, I.; Muksin, U.; Olaiya, F.G.; Abdullah, C.; Yahya, E.B. Functional Properties and Molecular Degradation of Schizostachyum Brachycladum Bamboo Cellulose Nanofibre in PLA-Chitosan Bionanocomposites. Molecules 2021, 26, 2008. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.; Kenny, J.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef]
- Yang, W.; Qi, G.; Kenny, J.M.; Puglia, D.; Ma, P. Effect of cellulose nanocrystals and lignin nanoparticles on mechanical, antioxidant and water vapour barrier properties of glutaraldehyde crosslinked PVA films. Polymers 2020, 12, 1364. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.; Korchagina, E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Nasution, H.; Olaiya, N.G.; Haafiz, M.M.; Abdullah, C.; Bakar, S.A.; Olaiya, F.G.; Mohamed, A.; HPS, A.K. The role of amphiphilic chitosan in hybrid nanocellulose–reinforced polylactic acid biocomposite. Polym. Adv. Technol. 2021. [Google Scholar] [CrossRef]
- Zhong, T.; Wolcott, M.P.; Liu, H.; Wang, J. Developing chitin nanocrystals for flexible packaging coatings. Carbohydr. Polym. 2019, 226, 115276. [Google Scholar] [CrossRef] [PubMed]
- Rizal, S.; Lai, T.K.; Muksin, U.; Olaiya, N.G.; Abdullah, C.; Yahya, E.B.; Chong, E.; Abdul Khalil, H.P.S. Properties of Macroalgae Biopolymer Films Reinforcement with Polysaccharide Microfibre. Polymers 2020, 12, 2554. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Zhang, H.; Song, M.-L.; Zhou, Y.; Yao, J.; Ni, Q.-Q. From cellulose nanospheres, nanorods to nanofibers: Various aspect ratio induced nucleation/reinforcing effects on polylactic acid for robust-barrier food packaging. ACS Appl. Mater. Interfaces 2017, 9, 43920–43938. [Google Scholar] [CrossRef] [PubMed]
- Ni’mah, H.; Ningrum, E.O.; Sumarno; Rizkiyah, D.N.; Divta, I.G.C.; Meiliefiana; Subaghio, M.A. Effect of particle size and crystallinity of cellulose filler on the properties of poly (L-lactic acid): Mechanical property and thermal stability. In Proceedings of the AIP Conference Proceedings, Santiago, Chile, 26–29 September 2017; AIP Publishing LLC: New York, NY, USA, 2017. [Google Scholar]
- Clarkson, C.M.; El Awad Azrak, S.M.; Chowdhury, R.; Shuvo, S.N.; Snyder, J.; Schueneman, G.; Ortalan, V.; Youngblood, J.P. Melt spinning of cellulose nanofibril/polylactic acid (CNF/PLA) composite fibers for high stiffness. ACS Appl. Polym. Mater. 2018, 1, 160–168. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Torres-Hernández, Y.G.; Ortega-Díaz, G.M.; Téllez-Jurado, L.; Castrejón-Jiménez, N.S.; Altamirano-Torres, A.; García-Pérez, B.E.; Balmori-Ramírez, H. Biological compatibility of a polylactic acid composite reinforced with natural chitosan obtained from shrimp waste. Materials 2018, 11, 1465. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, F.A.; Iulianelli, G.C.; Tavares, M.I. Effect of microcrystalline and nanocrystals cellulose fillers in materials based on PLA matrix. Polym. Test. 2017, 61, 280–288. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Zarina, S.; Ahmad, I. Biodegradable composite films based on κ-carrageenan reinforced by cellulose nanocrystal from kenaf fibers. BioResources 2015, 10, 256–271. [Google Scholar] [CrossRef] [Green Version]
- Trifol, J.; Plackett, D.; Sillard, C.; Hassager, O.; Daugaard, A.E.; Bras, J.; Szabo, P. A comparison of partially acetylated nanocellulose, nanocrystalline cellulose, and nanoclay as fillers for high-performance polylactide nanocomposites. J. Appl. Polym. Sci. 2016, 133, 43257. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, H.; Cai, H.; Han, X.; Lin, X.; Qian, M.; Zhao, Y.; Huo, E.; Villota, E.M.; Mateo, W. Improvement on the properties of microcrystalline cellulose/polylactic acid composites by using activated biochar. J. Clean. Prod. 2020, 252, 119898. [Google Scholar] [CrossRef]
- Jonoobi, M.; Mathew, A.P.; Abdi, M.M.; Makinejad, M.D.; Oksman, K. A comparison of modified and unmodified cellulose nanofiber reinforced polylactic acid (PLA) prepared by twin screw extrusion. J. Polym. Environ. 2012, 20, 991–997. [Google Scholar] [CrossRef]







| Filler Contents (wt.%) | Droplet Image | Contact Angle for PLA/Chitin Embedded with B-CNF (θ) | Droplet Image | Contact Angle for PLA/Chitin Embedded with C-CNF (θ) |
|---|---|---|---|---|
| P90/10 |  | 78.80° ± 0.23 d | ||
| 90/10/1 |  | 74.24° ± 0.18 d |  | 73.70° ± 0.22 de |
| 90/10/3 |  | 70.80° ± 0.27 c |  | 69.80° ± 0.24 c |
| 90/10/5 |  | 66.80° ± 0.27 a |  | 65.80° ± 0.20 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N. G., O.; H. P. S., A.K.; El-Bahy, S.M.; Rafatullah, M.; Abdullah, C.K.; El-Bahy, Z.M.; Grace, O.F. Supercritical Carbon Dioxide Isolation of Cellulose Nanofibre and Enhancement Properties in Biopolymer Composites. Molecules 2021, 26, 5276. https://doi.org/10.3390/molecules26175276
N. G. O, H. P. S. AK, El-Bahy SM, Rafatullah M, Abdullah CK, El-Bahy ZM, Grace OF. Supercritical Carbon Dioxide Isolation of Cellulose Nanofibre and Enhancement Properties in Biopolymer Composites. Molecules. 2021; 26(17):5276. https://doi.org/10.3390/molecules26175276
Chicago/Turabian StyleN. G., Olaiya, Abdul Khalil H. P. S., Salah M. El-Bahy, Mohd Rafatullah, Che K. Abdullah, Zeinhom M. El-Bahy, and Olaiya F. Grace. 2021. "Supercritical Carbon Dioxide Isolation of Cellulose Nanofibre and Enhancement Properties in Biopolymer Composites" Molecules 26, no. 17: 5276. https://doi.org/10.3390/molecules26175276
APA StyleN. G., O., H. P. S., A. K., El-Bahy, S. M., Rafatullah, M., Abdullah, C. K., El-Bahy, Z. M., & Grace, O. F. (2021). Supercritical Carbon Dioxide Isolation of Cellulose Nanofibre and Enhancement Properties in Biopolymer Composites. Molecules, 26(17), 5276. https://doi.org/10.3390/molecules26175276