Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Identification of B. deamine kh3
2.2. Establishment of Optimal Method for the Fermented Sheep Placenta
2.3. Preference and Ranking Determination via Sensory Evaluation
2.4. Determination of Aroma Profile via Sensory Attribute
2.5. Determination and Identification of Volatile Compounds in the Fermented Placenta via SPME-GC
3. Materials and Methods
3.1. Reagents
3.2. Isolation and Identification of the Novel Yeast Strain from Fermented Apple Vinegar
3.3. Evaluation of Physiological Characteristics and Enzymatic Activity of the Novel Yeast Strain
3.4. Establishment of Optimal Condition for Fermentation of Sheep Placenta
3.5. Sensory Evaluation
3.6. SPME-GC Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gude, N.M.; Roberts, C.T.; Kalionis, B.; King, R.G. Growth and function of the normal human placenta. Thromb. Res. 2004, 114, 397–407. [Google Scholar] [CrossRef]
- Liu, J.; Luo, S.; Yang, J.; Ren, F.; Zhao, Y.; Luo, H.; Ge, K.; Zhang, H. The protective effect of sheep placental extract on concanavalin A-induced liver injury in mice. Molecules 2019, 24, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Park, H.J.; Seo, H.G.; Kim, J.H.; Lim, G.S.; Lee, W.Y.; Kim, N.H.; Kim, J.H.; Lee, J.H.; Jung, H.S.; et al. Immune modulation effect of porcine placenta extracts in weaned the pig. J. Anim. Sci. 2013, 91, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chan, M.K.; Wong, M.B.; Klokol, D.; Chernykh, V. Placental therapy: An insight to their biological and therapeutic properties. J. Med. Ther. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bak, D.H.; Na, J.; Im, S.I.; Oh, C.T.; Kim, J.Y.; Park, S.K.; Han, H.J.; Seok, J.; Choi, S.Y.; Ko, E.J.; et al. Antioxidant effect of human placenta hydrolysate against oxidative stress on muscle atrophy. J. Cell Physiol. 2019, 234, 1643–1658. [Google Scholar] [CrossRef]
- Chakraborty, P.D.; Bhattacharyya, D. In vitro growth inhibition of microbes by human placental extract. Curr. Sci. 2005, 88, 782–786. [Google Scholar]
- Sur, T.K.; Biswas, T.K.; Ali, L.; Mukherjee, B. Anti-inflammatory and anti-platelet aggregation activity of human placental extract. Acta Pharm. Sin. 2003, 24, 187–192. [Google Scholar]
- Heo, J.H.; Heo, Y.; Lee, H.J.; Kim, M.; Shin, H.Y. Topical anti-inflammatory and anti-oxidative effects of porcine placenta extracts on 2,4-dinitrochlorobenzene-induced contact dermatitis. BMC Complement. Altern. Med. 2018, 18, 331. [Google Scholar] [CrossRef]
- Kim, B.Y.; Park, H.R.; Shin, J.H.; Kim, S.W.; Kim, S.W. Human placental extract reduces allergic inflammation in a murine allergic rhinitis model. Laryngoscope 2014, 124, E399–E404. [Google Scholar] [CrossRef]
- Kang-Kon, L.; Whan-Seok, C.; Keun-Sang, Y.; Sang-Wook, S.; Sun-Myeong, O.; Sat-Byul, P.; Moon-Jong, K. Efficacy and safety of human placental extract solution on fatigue: A double-blind, randomized, placebo-controlled study. Evid. Based Complement. Altern. Med. 2012, 2012, 130875. [Google Scholar] [CrossRef]
- Kato, Y.; Sugiyama, Y.; Liu, K.X.; Kaku, T.I. Human placental extract stimulates liver regeneration in rats. Biol. Pharm. Bull. 1998, 21, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, P.D.; Bhattacharyya, D.; Pal, S.; Ali, N. In vitro induction of nitric oxide by mouse peritoneal macrophages treated with human placental extract. Int. Immunopharmacol. 2006, 6, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.D.; Bhattacharyya, D. Aqueous extract of human placenta as a therapeutic agent. In Recent Advances in Research on the Human Placenta; Zheng, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 77–92. [Google Scholar] [CrossRef] [Green Version]
- Selander, J.; Cantor, A.; Young, S.M.; Benyshek, D.C. Human maternal placentophagy: A survey of self-reported motivations and experiences associated with placenta consumption. Ecol. Food Nutr. 2013, 52, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Barbusinski, K.; Kalemba, K.; Kasperczyk, D.; Urbaniec, K.; Kozik, V. Biological methods for odor treatment—A review. J. Clean Prod. 2017, 152, 223–241. [Google Scholar] [CrossRef]
- Iranpour, R.; Cox, H.H.J.; Deshusses, M.A.; Schroeder, E.D. Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ. Prog. 2005, 24, 254–267. [Google Scholar] [CrossRef]
- Nedele, A.-K.; Gross, S.; Rigling, M.; Zhang, Y. Reduction of green off-flavor compounds: Comparison of key odorants during fermentation of soy drink with Lycoperdon pyriforme. Food Chem. 2021, 334, 127591. [Google Scholar] [CrossRef]
- Kaczmarska, K.T.; Chandra-Hioe, M.V.; Frank, D.; Arcot, J. Aroma characteristics of lupin and soybean after germination and effect of fermentation on lupin aroma. LWT Food Sci. Technol. 2018, 87, 225–233. [Google Scholar] [CrossRef]
- El Youssef, C.; Bonnarme, P.; Fraud, S.; Péron, A.-C.; Helinck, S.; Landaud, S. Sensory improvement of a pea protein-based product using microbial co-cultures of lactic acid bacteria and yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.-S.; Bae, H.-N.; Eom, S.-H.; Lim, K.-S.; Yun, I.-H.; Chung, Y.-H.; Jeon, J.-M.; Kim, H.-W.; Lee, M.-S.; Lee, Y.-B.; et al. Removal of off-flavors from sea tangle (Laminaria japonica) extract by fermentation with Aspergillus oryzae. Bioresour. Technol. 2012, 121, 475–479. [Google Scholar] [CrossRef]
- Agnolucci, M.; Tirelli, A.; Cocolin, L.; Toffanin, A. Brettanomyces bruxellensis yeasts: Impact on wine and winemaking. World J. Microbiol. Biotechnol. 2017, 33, 180. [Google Scholar] [CrossRef]
- Serra Colomer, M.; Funch, B.; Forster, J. The raise of Brettanomyces yeast species for beer production. Curr. Opin. Biotechnol. 2019, 56, 30–35. [Google Scholar] [CrossRef]
- Gray, S.R.; Rawsthorne, H.; Dirks, B.; Phister, T.G. Detection and enumeration of Dekkera anomala in beer, cola, and cider using real-time PCR. Lett. Appl. Microbiol. 2011, 52, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Suzuki, M.; Takagaki, A.; Nanjo, F. Deodorizing effects of tea catechins on amines and ammonia. Biosci. Biotechnol. Biochem. 2002, 66, 373–377. [Google Scholar] [CrossRef]
- Pohjanheimo, T.A.; Sandell, M.A. Headspace volatiles contributing to flavour and consumer liking of wellness beverages. Food Chem. 2009, 115, 843–851. [Google Scholar] [CrossRef]
- Ito, T.; Miyaji, T.; Nakagawa, T.; Tomizuka, N. Degradation of dimethyl disulfide by Pseudomonas fluorescens strain 76. Biosci. Biotechnol. Biochem. 2007, 71, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, M.; Marton, D.; Tapparo, A. Headspace gas chromatographic determination of 2-alkyl-5,5-dimethyl-1,3-dioxane derivatives in wastewaters of a polyester resin plant. Analyst 2001, 126, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H.; Majcher, M.; Dziadas, M.; Zawirska-Wojtasiak, R.; Czaczyk, K.; Wąsowicz, E. Volatile compounds responsible for aroma of Jutrzenka liquer wine. J. Chromatogr. A 2011, 1218, 7566–7573. [Google Scholar] [CrossRef]
- Rebière, L.; Clark, A.C.; Schmidtke, L.M.; Prenzler, P.D.; Scollary, G.R. A robust method for quantification of volatile compounds within and between vintages using headspace-solid-phase micro-extraction coupled with GC–MS—Application on Semillon wines. Anal. Chim. Acta 2010, 660, 149–157. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Huq, M.A. Microvirga rosea sp. nov.: A nanoparticle producing bacterium isolated from soil of rose garden. Arch. Microbiol. 2018, 200, 1439–1445. [Google Scholar] [CrossRef]
- Escribano, R.; Gonzalez-Arenzana, L.; Garijo, P.; Berlanas, C.; Lopez-Alfaro, I.; Lopez, R.; Gutierrez, A.R.; Santamaria, P. Screening of enzymatic activities within different enological non-Saccharomyces yeasts. J. Food Sci. Technol. 2017, 54, 1555–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. In JAMA; World Medical Association: Ferney-Voltaire, France, 2013; Volume 310, pp. 2191–2194. [Google Scholar]
- Lecanu, L.; Ducruet, V.; Jouquand, C.; Gratadoux, J.J.; Feigenbaum, A. Optimization of headspace solid-phase microextraction (SPME) for the odor analysis of surface-ripened cheese. J. Agric. Food Chem. 2002, 50, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
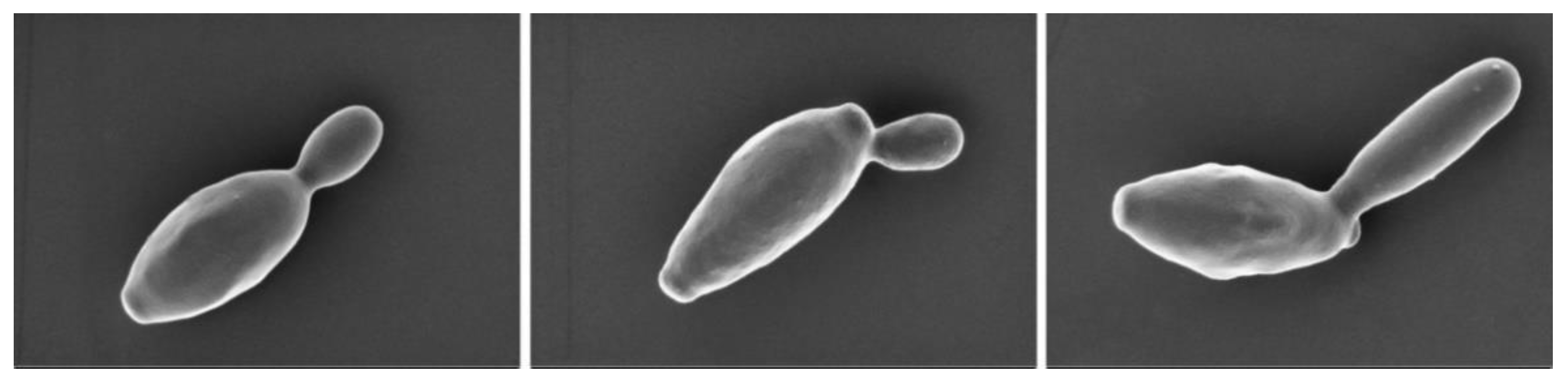
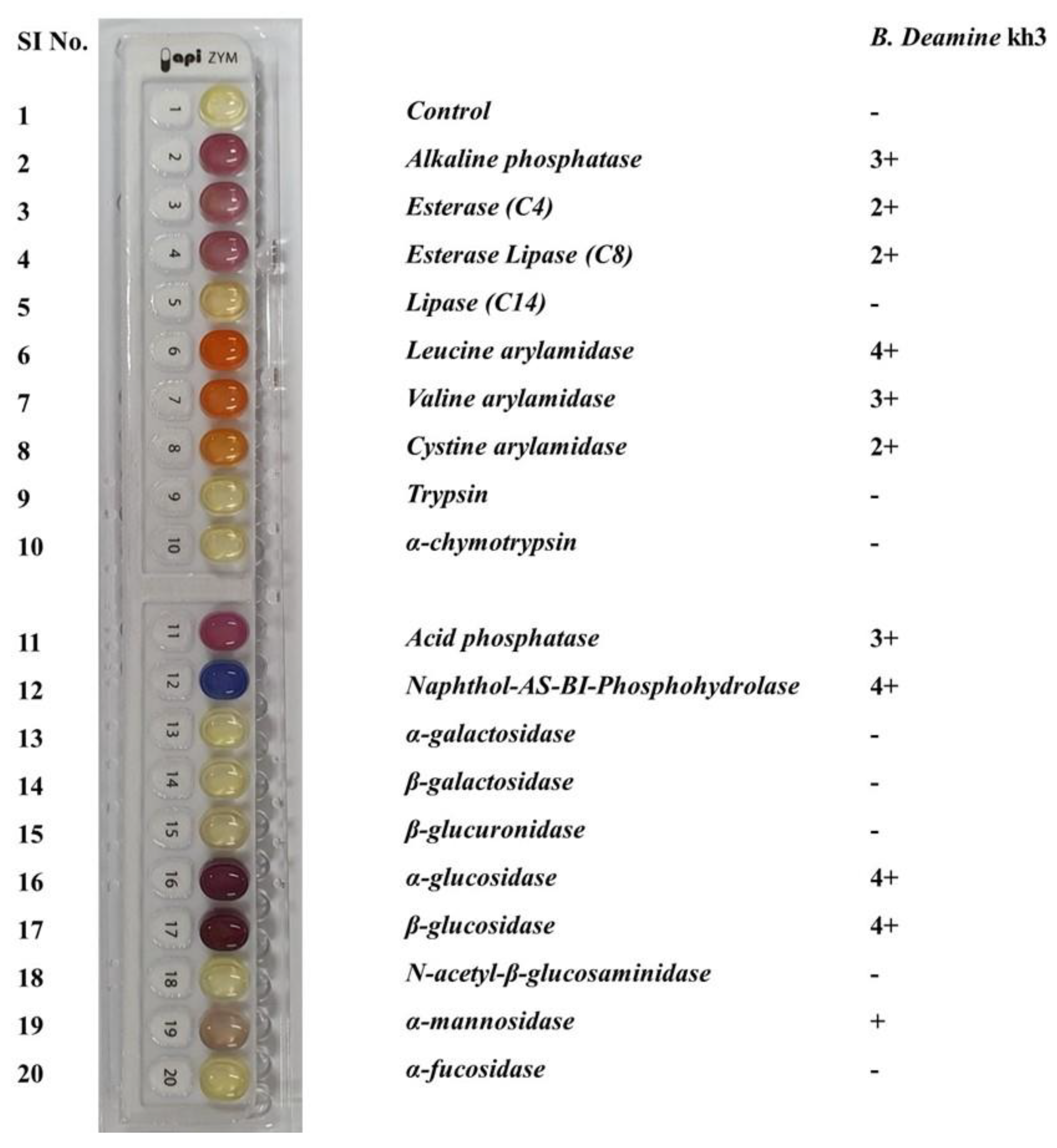
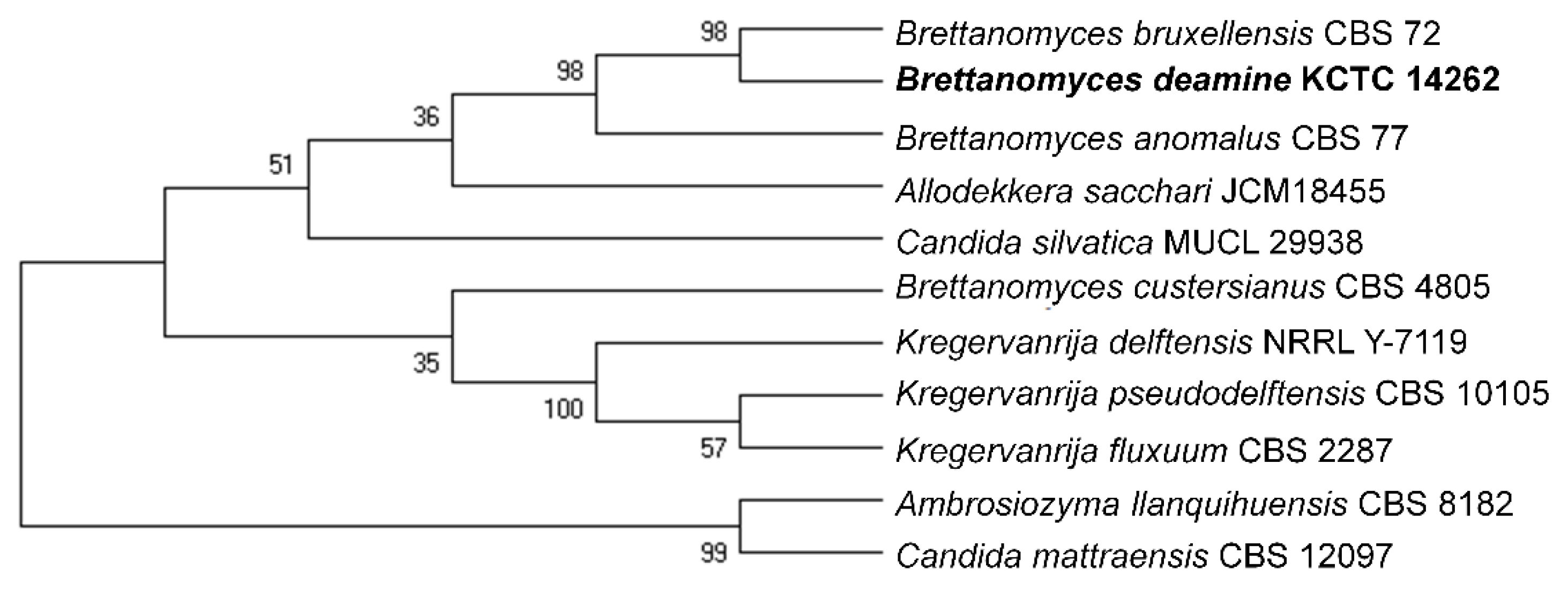
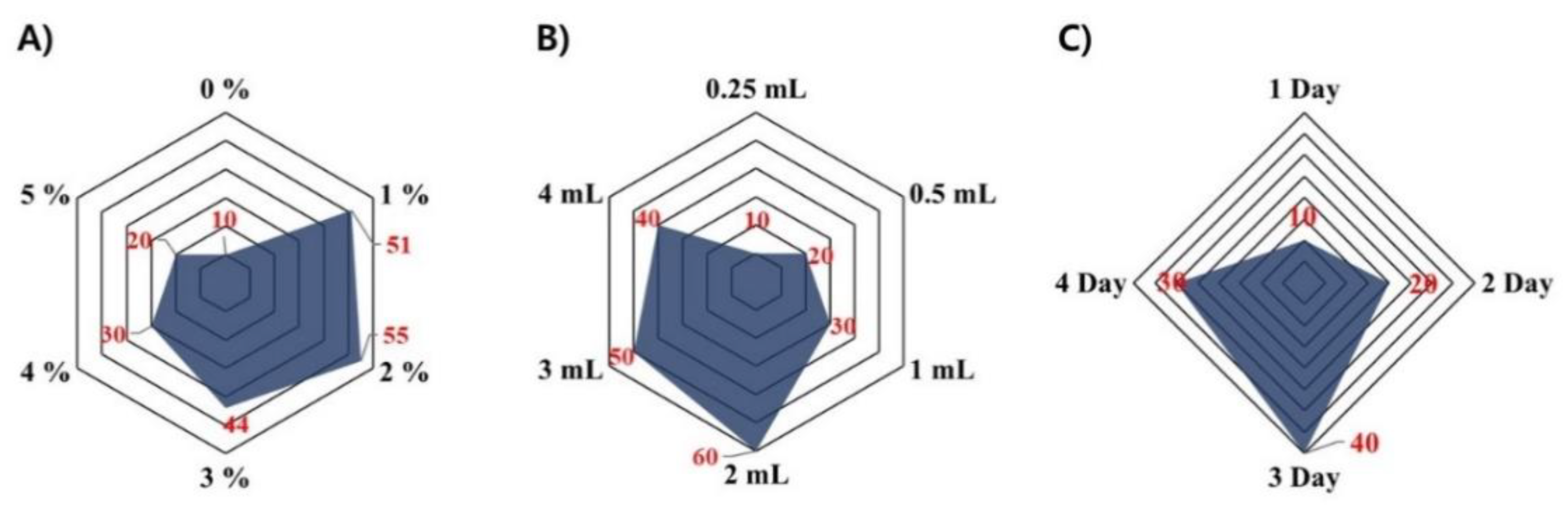

| Group | Sample Information | Sheep Placenta | Yeast Strain | Abbreviation |
|---|---|---|---|---|
| 1 | Sheep placenta | + | - | SP |
| 2 | Sheep placenta fermented with B. deamine | + | B. deamine | SP-BD |
| 3 | Sheep placenta fermented with B. bruxellesis | + | B. bruxellesis | SP-BB |
| Group | N | Mean | Z | H | p-Value | Rank |
|---|---|---|---|---|---|---|
| SP | 42 | 4.48 | −2.12 | 13.44 | 0.004 | 3 |
| SP-BD | 42 | 5.33 * | 1.26 | 1 | ||
| SP-BB | 42 | 4.57 | −1.77 | 2 |
| Group | N | Mean | Z | H | p Value | Rank |
|---|---|---|---|---|---|---|
| SP | 42 | 1.98 | −0.22 | 4.04 | 0.133 | 2 |
| SP-BD | 42 | 2.19 | 1.74 | 1 | ||
| SP-BB | 42 | 1.83 | −1.52 | 3 |
| NO. | Chemical Compound | Formula | Area (%) | |
|---|---|---|---|---|
| SP | SP-BD | |||
| 1 | Hexane | C6H14 | 11.0 | 12.1 |
| 2 | Methylcyclopentane | C6H12 | n.d. | 7.8 |
| 3 | 1,3-dioxolane | C3H6O2 | 10.4 | n.d. |
| 4 | 4-Methylheptane | C8H18 | n.d. | 4.0 |
| 5 | 2,4-Dimethylheptane | C9H20 | n.d. | 2.6 |
| 6 | Acetone | C3H6O | 10.5 | 6.2 |
| 7 | 2,4-Dimethyl-1-heptene | C9H18 | 8.9 | 3.0 |
| 8 | 2-Butanone | C4H8O | 2.8 | n.d. |
| 9 | 2-Pentanone | C5H10O | 1.5 | n.d. |
| 10 | Dimethyl disulfide | C2H6S2 | 13.5 | n.d. |
| 11 | Ammonia | NH3 | 16.7 | n.d. |
| 12 | 2-Isobutyl-4,4-dimethyl-1,3-dioxan | C10H20O2 | n.d. | 26.0 |
| 13 | Decamethyl-cyclopentasiloxane | C10H30O5Si5 | 0.5 | n.d. |
| 14 | 4-Methyl-2-heptanone | C8H16O | 11.7 | 1.1 |
| 15 | 3-Methyl-1-butanol | C5H12O | n.d. | 16.6 |
| 16 | 2-Hexanol | C6H14O | 11.6 | n.d. |
| 17 | α-Methyl-β-oxo-2-pyridinepropanoic acid ethyl ester | C9H9NO3 | 1.3 | n.d. |
| 18 | 4,6-Dimethyl-2-heptanone | C9H18O | 1.6 | 1.3 |
| 19 | 1,3-Bis(1,1-dimethylethyl) benzene | C14H22 | 2.7 | 3.5 |
| 20 | Benzene-ethanol | C8H12O | n.d. | 7.5 |
| 21 | Trace materials | - | 6.3 | 6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S.; Ha, K.-Y.; Xu, X.-Y.; Kang, H.-C.; Kim, H.; Kim, Y.-J. Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar. Molecules 2021, 26, 5835. https://doi.org/10.3390/molecules26195835
Choi H-S, Ha K-Y, Xu X-Y, Kang H-C, Kim H, Kim Y-J. Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar. Molecules. 2021; 26(19):5835. https://doi.org/10.3390/molecules26195835
Chicago/Turabian StyleChoi, Han-Sol, Keum-Yun Ha, Xing-Yue Xu, Hee-Cheol Kang, Hoon Kim, and Yeon-Ju Kim. 2021. "Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar" Molecules 26, no. 19: 5835. https://doi.org/10.3390/molecules26195835
APA StyleChoi, H.-S., Ha, K.-Y., Xu, X.-Y., Kang, H.-C., Kim, H., & Kim, Y.-J. (2021). Off-Flavor Removal from Sheep Placenta via Fermentation with Novel Yeast Strain Brettanomyces deamine kh3 Isolated from Traditional Apple Vinegar. Molecules, 26(19), 5835. https://doi.org/10.3390/molecules26195835








