Development of CE-C4D Method for Determination Tropane Alkaloids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Separation Conditions
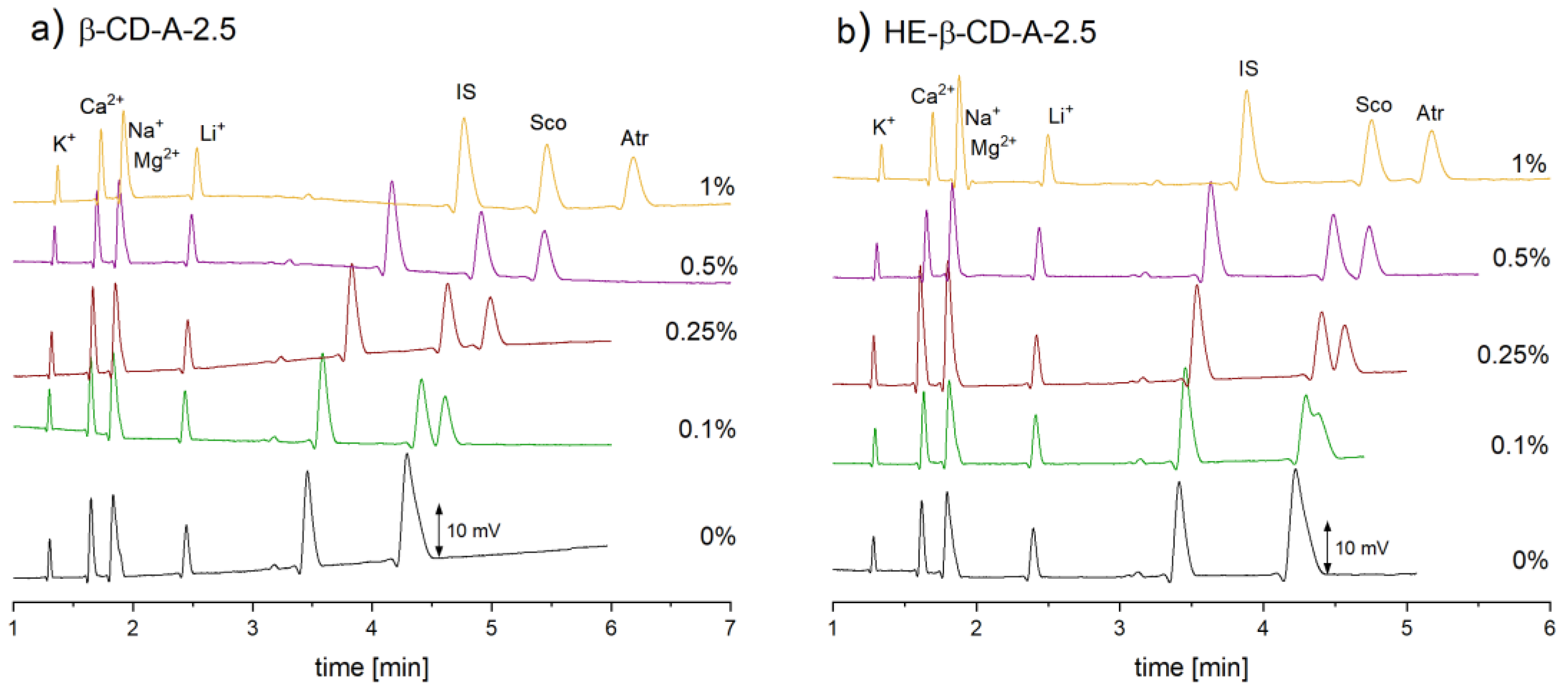
2.1.1. Selection of the Background Electrolyte
2.1.2. Instrumental Separation Conditions
2.2. Method Validation
2.3. Analysis of Solanaceae Plant Samples
2.4. Analysis of Pharmaceuticals
2.5. Assesment of the Optimized Method with RGB Model
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Stock and Standard Solutions
3.3. Extraction Protocol
3.4. Instrumentation
3.5. Data Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Grynkiewicz, G.; Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008, 60, 439–463. [Google Scholar] [PubMed]
- Ullrich, S.F.; Hagels, H.; Kayser, O. Scopolamine: A journey from the field to clinics. Phytochem. Rev. 2017, 16, 333–353. [Google Scholar] [CrossRef]
- Carlier, J.; Escard, E.; Péoc’h, M.; Boyer, B.; Romeuf, L.; Faict, T.; Guitton, J.; Gaillard, Y. Atropine eye drops: An unusual homicidal poisoning. J. Forensic Sci. 2014, 59, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Haughey, S.A.; Chevallier, O.P.; McVey, C.; Elliott, C.T. Laboratory investigations into the cause of multiple serious and fatal food poisoning incidents in Uganda during 2019. Food Control 2021, 121, 107648. [Google Scholar] [CrossRef]
- Castilla-Fernández, D.; Moreno-González, D.; García-Reyes, J.F.; Ballesteros, E.; Molina-Díaz, A. Determination of atropine and scopolamine in spinach-based products contaminated with genus Datura by UHPLC–MS/MS. Food Chem. 2021, 347, 129020. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Cubero-Leon, E.; Stroka, J. Determination of tropane alkaloids in cereals, tea and herbal infusions: Exploiting proficiency testing data as a basis to derive interlaboratory performance characteristics of an improved LC-MS/MS method. Food Chem. 2020, 331, 127260. [Google Scholar] [CrossRef]
- Sawabe, Y.; Yamasaki, K.; Tagami, T.; Kawaguchi, M.; Taguchi, S. Rapid determination of atropine and scopolamine content in scopolia extract powder by hplc. J. Nat. Med. 2011, 65, 395–399. [Google Scholar] [CrossRef]
- Jakabová, S.; Vincze, L.; Farkas, Á.; Kilár, F.; Boros, B.; Felinger, A. Determination of tropane alkaloids atropine and scopolamine by liquid chromatography-mass spectrometry in plant organs of Datura species. J. Chromatogr. A. 2012, 1232, 295–301. [Google Scholar] [CrossRef]
- Steenkamp, P.A.; Harding, N.M.; Van Heerden, F.R.; Van Wyk, B.E. Fatal Datura poisoning: Identification of atropine and scopolamine by high performance liquid chromatography/photodiode array/mass spectrometry. Forensic Sci. Int. 2004, 145, 31–39. [Google Scholar] [CrossRef]
- Ciechomska, M.; Woźniakiewicz, M.; Nowa, J.; Świadek, K.; Bazylewicz, B.; Kościelniak, P. Development of a microwave-assisted extraction of atropine and scopolamine from Solanaceae family plants followed by a QuEChERS cleanup procedure. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 538–548. [Google Scholar] [CrossRef]
- Caligiani, A.; Palla, G.; Bonzanini, F.; Bianchi, A.; Bruni, R. A validated GC-MS method for the detection of tropane alkaloids in buckwheat (Fagopyron esculentum L.) fruits, flours and commercial foods. Food Chem. 2011, 127, 204–209. [Google Scholar] [CrossRef]
- Temerdashev, A.Z.; Kolychev, I.A.; Kiseleva, N.V. Chromatographic determination of some tropane alkaloids in Datura metel. J. Anal. Chem. 2012, 67, 960–966. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Y.; Wang, E. Simultaneous determination of two active ingredients in Flos daturae by capillary electrophoresis with electrochemiluminescence detection. Anal. Chim. Acta. 2005, 545, 137–141. [Google Scholar] [CrossRef]
- Li, J.; Chun, Y.; Ju, H. Simultaneous electrochemiluminescence detection of anisodamine, atropine, and scopolamine in Flos daturae by capillary electrophoresis using β-cyclodextrin as additive. Electroanalysis 2007, 19, 1569–1574. [Google Scholar] [CrossRef]
- Bo, T.; Li, K.A.; Liu, H. Investigation of the effect of space environment on the contents of atropine and scopolamine in Datura metel by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 2003, 31, 885–891. [Google Scholar] [CrossRef]
- Eeva, M.; Salo, J.P.; Oksman-Caldentey, K.M. Determination of the main tropane alkaloids from transformed Hyoscyamus muticus plants by capillary zone electrophoresis. J. Pharm. Biomed. Anal. 1998, 16, 717–722. [Google Scholar] [CrossRef]
- Ye, N.; Zhu, R.; Gu, X.; Zou, H. Determination of scopolamine, atropine and anisodamine in Flos Daturae by capillary electrophoresis. Biomed. Chromatogr. 2001, 15, 509–512. [Google Scholar] [CrossRef]
- Mateus, L.; Cherkaoui, S.; Christen, P.; Oksman-Caldentey, K.M. Simultaneous determination of scopolamine, hyoscyamine and littorine in plants and different hairy root clones of Hyoscyamus muticus by micellar electrokinetic chromatography. Phytochemistry 2000, 54, 517–523. [Google Scholar] [CrossRef]
- Wu, H.L.; Huang, C.H.; Chen, S.H.; Wu, S.M. Micellar electrokinetic chromatography of scopolamine-related anticholinergics. J. Chromatogr. A 1998, 802, 107–113. [Google Scholar] [CrossRef]
- Sáiz, J.; Mai, T.D.; López, M.L.; Bartolomé, C.; Hauser, P.C.; García-Ruiz, C. Rapid determination of scopolamine in evidence of recreational and predatory use. Sci. Justice 2013, 53, 409–414. [Google Scholar] [CrossRef]
- Marra, M.C.; Silva, P.L.; Muñoz, R.A.A.; Richter, E.M. Ultra-fast determination of scopolamine, orphenadrine, mepyramine, caffeine, dipyrone, and ascorbic acid by capillary electrophoresis with capacitively coupled contactless conductivity detection. J. Braz. Chem. Soc. 2014, 25, 913–919. [Google Scholar] [CrossRef]
- Tůma, P. Determination of amino acids by capillary and microchip electrophoresis with contactless conductivity detection–Theory, instrumentation and applications. Talanta 2021, 224, 121922. [Google Scholar] [CrossRef]
- Kubáň, P.; Hauser, P.C. A review of the recent achievements in capacitively coupled contactless conductivity detection. Anal. Chim. Acta 2008, 607, 15–29. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniak, P. What color is your method? Adaptation of the rgb additive color model to analytical method evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC-Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Asztemborska, M.; Ceborska, M.; Pietrzak, M. Complexation of tropane alkaloids by cyclodextrins. Carbohydr. Polym. 2019, 209, 74–81. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline. Bioanalytical Method Validation. Eur. Med. Agency 2019. [Google Scholar]
- AOAC International. Appendix F: Guidelines for Standard Method Performance Requirements. AOAC Off. Methods Anal. 2016. [Google Scholar]


| Sample ID 1 | Atropine 2 | Scopolamine 2 |
|---|---|---|
| DM1 | 3994 ± 49 | 2637 ± 26 |
| DM2 | 4270 ± 20 | 2677 ± 16 |
| HN1 | 609.6 ± 21.7 | 251.2 ± 14.3 |
| HN2 | 602.4 ± 13.4 | 214.8 ± 13.7 |
| DS1 | 985.5 ± 13.1 | <LOQ |
| DS2 | 1096 ± 13 | <LOQ |
| DA1 | 467.3 ± 3.0 | 1827 ± 26 |
| DA2 | 521.8 ± 4.5 | 2080 ± 22 |
| AB1 | 8906 ± 20 | ND |
| AB2 | 9810 ± 83 | ND |
| SL1 | 3004 ± 64 | 1085 ± 17 |
| SL2 | 3135 ± 60 | 1122 ± 26 |
| Parameter | Atropine | Scopolamine |
|---|---|---|
| Linearity (μg/mL) | 5–50 | 5–50 |
| Slope | 0.027 | 0.032 |
| Intercept | −0.034 | −0.023 |
| R-squared | 0.999 | 0.996 |
| LOD (μg/mL) | 0.5 | 0.5 |
| LOQ (μg/mL) | 1.5 | 1.5 |
| Precision 1, CV (%): | ||
| 5 μg/mL | 1.88 | 1.66 |
| 20 μg/mL | 2.08 | 1.07 |
| 50 μg/mL | 1.18 | 1.73 |
| Intermediate precision 1, CV (%): | ||
| 5 μg/mL | 1.69 | 2.28 |
| 20 μg/mL | 2.13 | 2.77 |
| 50 μg/mL | 1.16 | 1.59 |
| Recovery, RE (%): | ||
| +12.5 μg/mL | 102.7 | 101.0 |
| +25 μg/mL | 101.3 | 101.8 |
| Matrix effect, ME (%): | ||
| +12.5 μg/mL | 93.56 | 92.99 |
| +25 μg/mL | 94.10 | 94.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gołąb, M.; Przybyłowska, M.; Kubáň, P.; Itterheimová, P.; Woźniakiewicz, M. Development of CE-C4D Method for Determination Tropane Alkaloids. Molecules 2021, 26, 5749. https://doi.org/10.3390/molecules26195749
Gołąb M, Przybyłowska M, Kubáň P, Itterheimová P, Woźniakiewicz M. Development of CE-C4D Method for Determination Tropane Alkaloids. Molecules. 2021; 26(19):5749. https://doi.org/10.3390/molecules26195749
Chicago/Turabian StyleGołąb, Małgorzata, Martyna Przybyłowska, Petr Kubáň, Petra Itterheimová, and Michał Woźniakiewicz. 2021. "Development of CE-C4D Method for Determination Tropane Alkaloids" Molecules 26, no. 19: 5749. https://doi.org/10.3390/molecules26195749
APA StyleGołąb, M., Przybyłowska, M., Kubáň, P., Itterheimová, P., & Woźniakiewicz, M. (2021). Development of CE-C4D Method for Determination Tropane Alkaloids. Molecules, 26(19), 5749. https://doi.org/10.3390/molecules26195749







