New Series of Pyrazoles and Imidazo-Pyrazoles Targeting Different Cancer and Inflammation Pathways
Abstract
:1. Introduction
- (1)
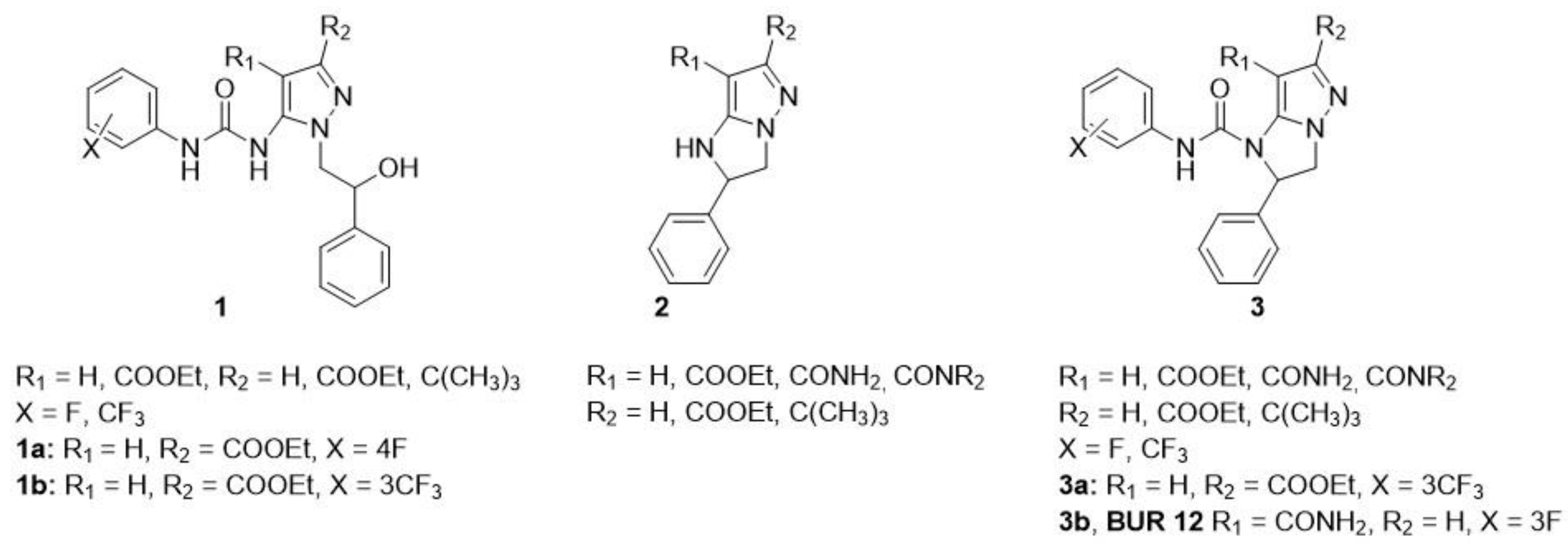
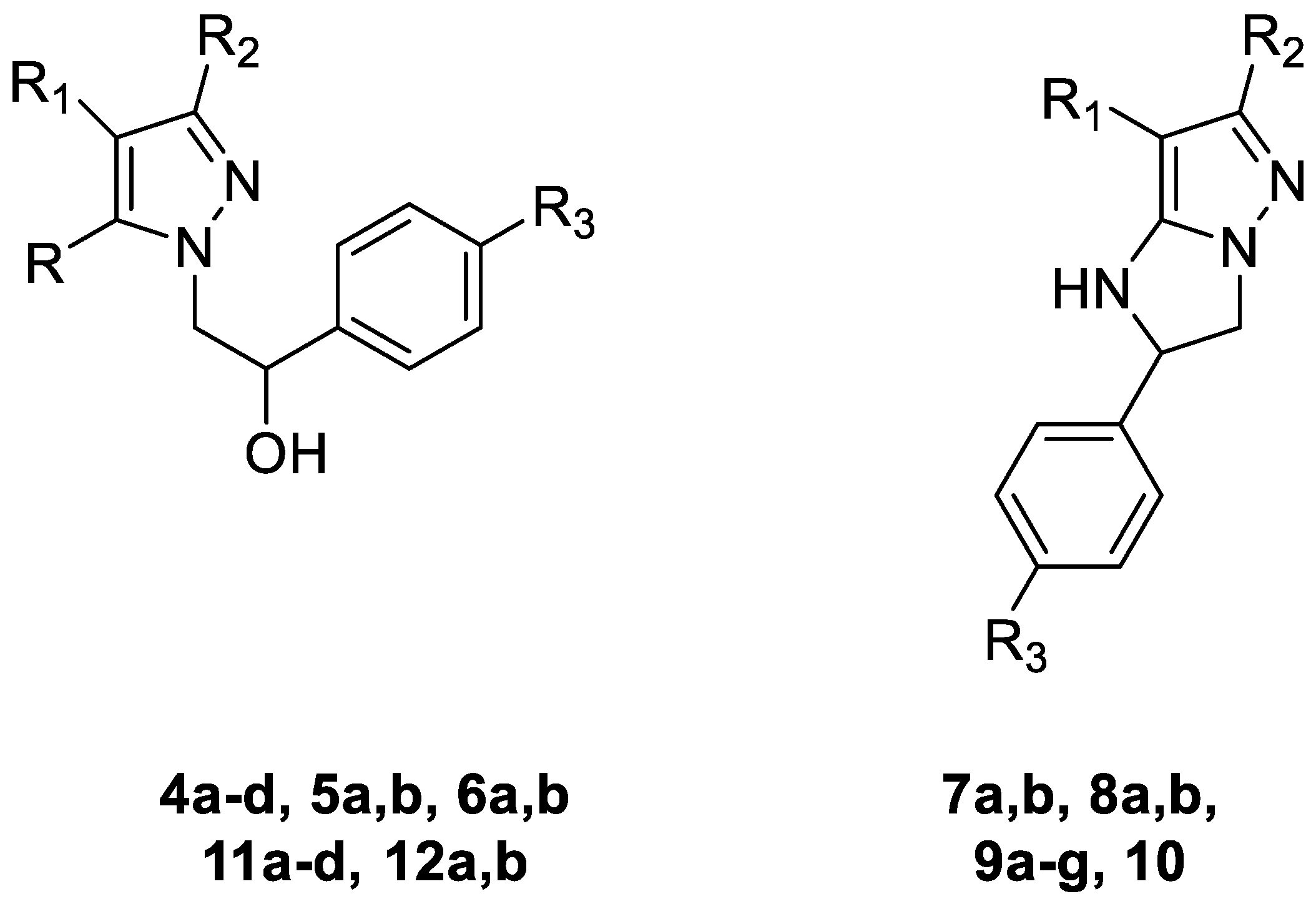
- firstly, we synthesized both pyrazoles (compounds 4 and 6), pyrrolyl-pyrazoles (compound 5) and more rigid imidazo-pyrazoles (compounds 7–10) to verify the role of molecule rigidity and the importance of the nitrogen basic atom able to form H bonds.
- (2)
- in some cases in these chemical scaffolds, we introduced several amide groups (which, in the previous compound 2, showed a good ability to block neutrophil chemotaxis and angiogenesis [19,21]) at different positions of the pyrazole nucleus (position 4, compounds 4 and 5) and the imidazo-pyrazole one (position 6 or 7, compound 9); intermediates with carboxyethyl and carboxylic groups at different positions of pyrazoles, pyrrolyl-pyrazoles and imidazo-pyrazoles were also tested to verify the role of these substituents and their position for biological activity (compounds 7, 8, 11d, 12b).
- (3)
- since in all previous series, fluorine derivatives showed the most efficient potency, probably due to an increasing cell membrane crossing, in some cases, we introduced a more lipophilic fluorine atom in the para-position of the phenyl ring (compounds 6a, 6b, 7a, 8a, 9a,b and 10).
2. Results and Discussion
2.1. Chemistry
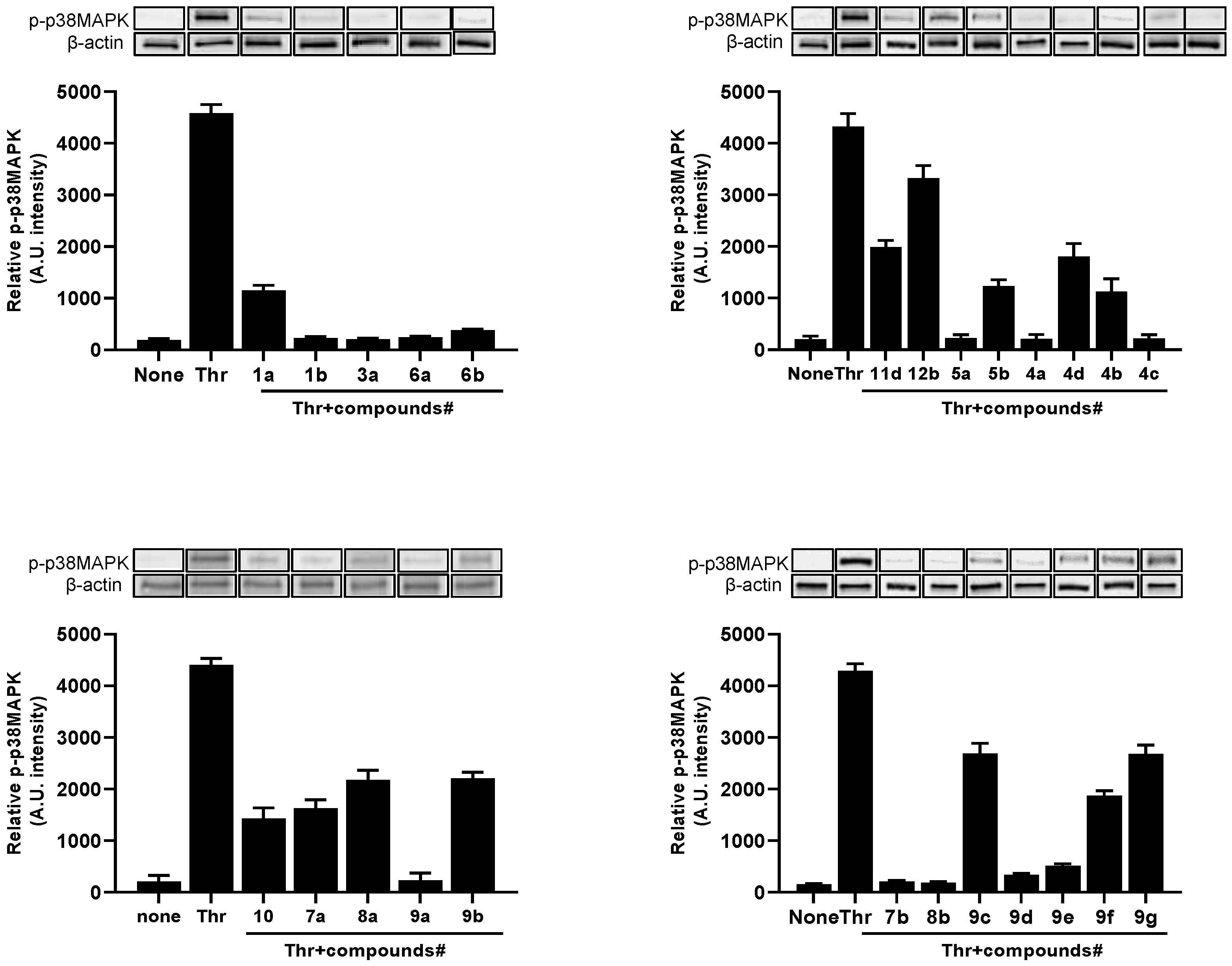
2.2. Inhibition Effect on Human Platelet Aggregation, ROS Production and p38MAPK Phosphorylation
2.3. SAR Considerations
2.4. Anti-Proliferative Activity Evaluation
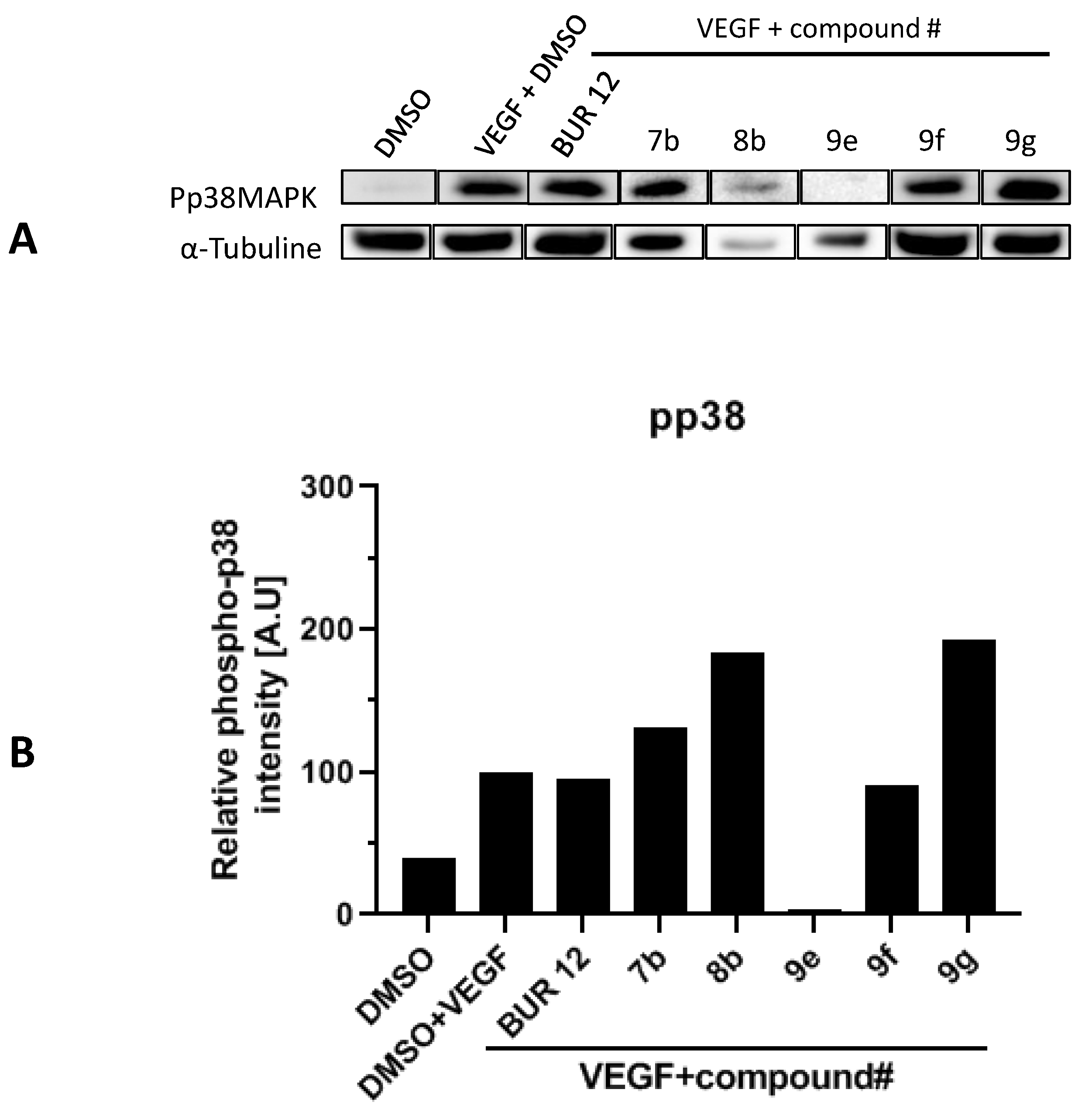
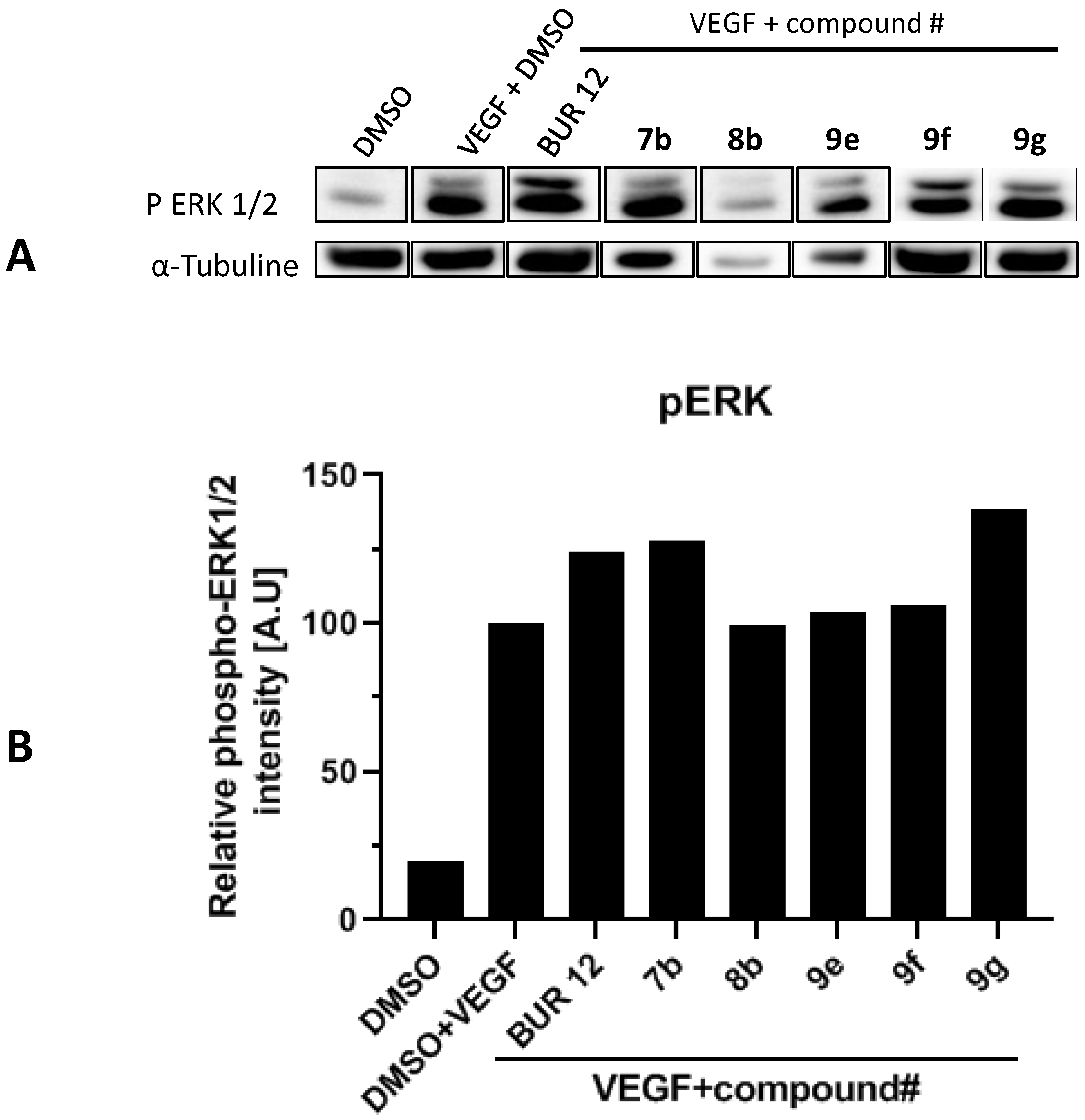
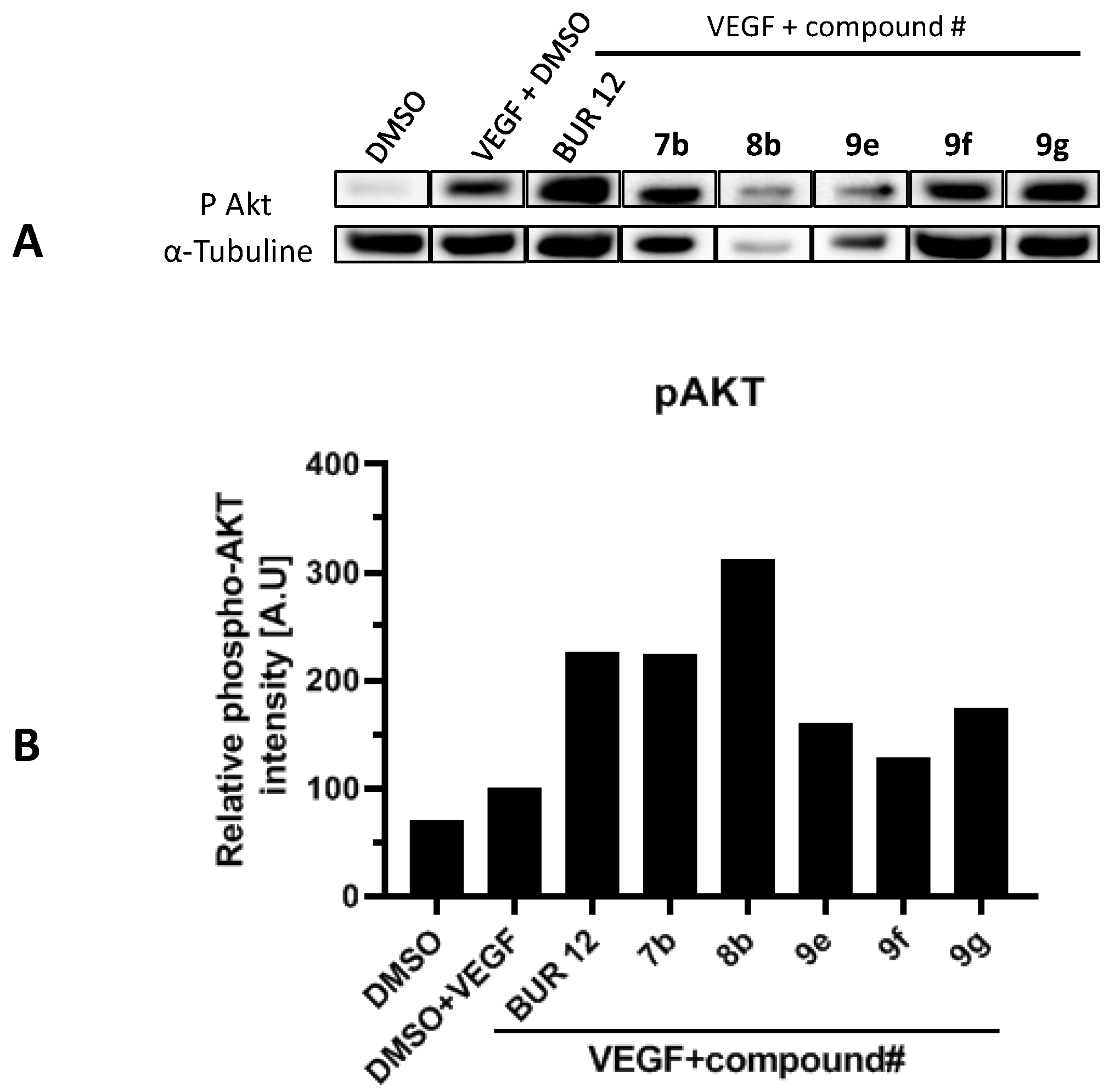
2.5. p38 Phosphorylation Inhibition on HUVEC Cells VEGF-Stimulated
3. Materials and Methods
3.1. General Information
3.2. Synthesis
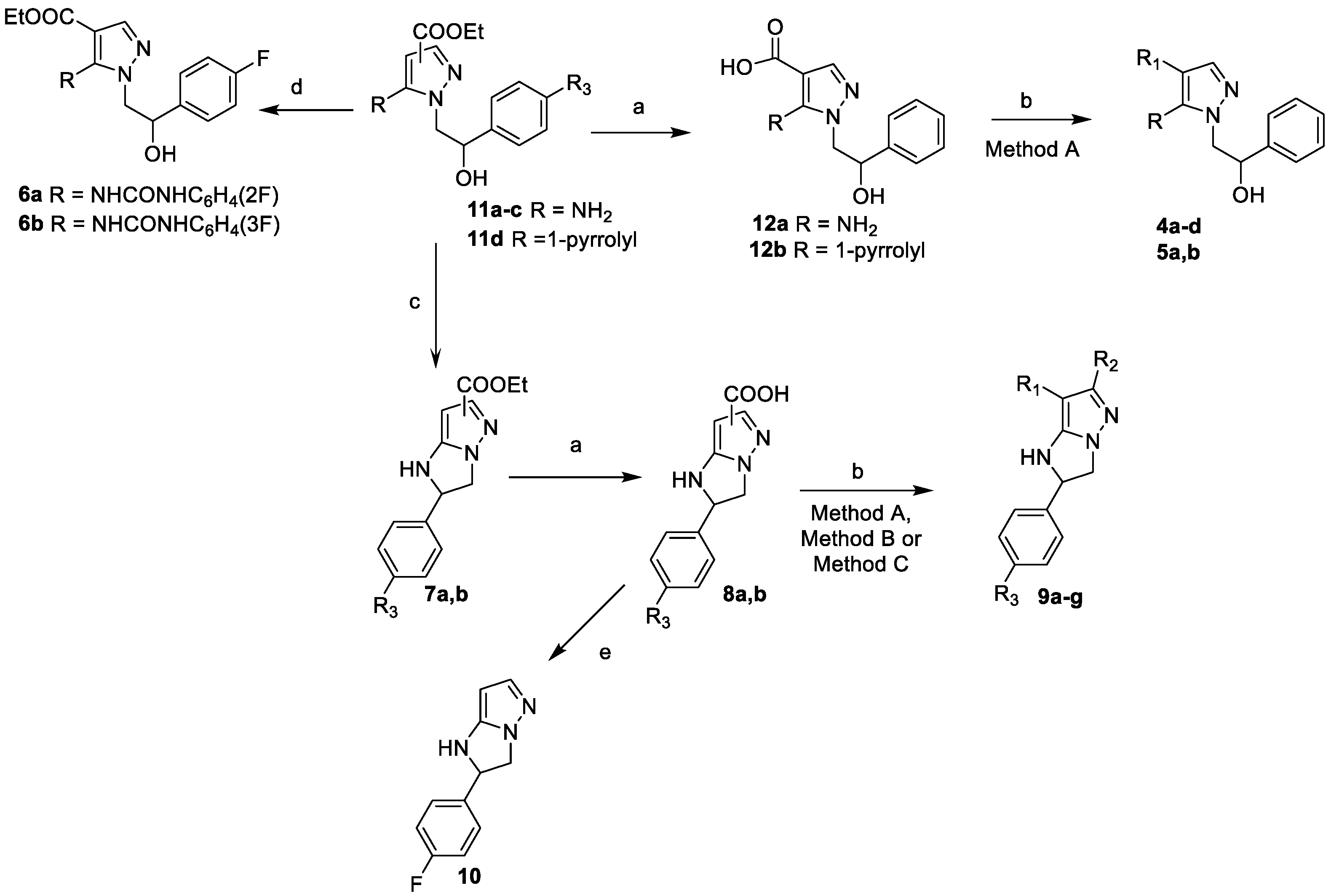
3.2.1. General Procedure for Synthesis of Compounds 4a–d and 5a,b
5-Amino-N-cyclopropyl-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carboxamide (4a)
(5-Amino-1-(2-hydroxy-2-phenylethyl)-1H-pyrazol-4-yl)(piperidin-1-yl)methanone (4b)
(5-Amino-1-(2-hydroxy-2-phenylethyl)-1H-pyrazol-4-yl)(morpholino)methanone (4c)
5-Amino-N-cyclopentyl-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carboxamide (4d)
N-Cyclopropyl-1-(2-hydroxy-2-phenylethyl)-5-(1H-pyrrol-1-yl)-1H-pyrazole-4-carboxamide (5a)
(1-(2-Hydroxy-2-phenylethyl)-5-(1H-pyrrol-1-yl)-1H-pyrazol-4-yl)(piperidin-1-yl)methanone (5b)
3.2.2. Synthesis of Ethyl 1-(2-(4-fluorophenyl)-2-hydroxyethyl)-5-(3-(2-fluorophenyl)ureido)-1H-pyrazole-4-carboxylate (6a) and 1-(2-(4-fluorophenyl)-2-hydroxyethyl)-5-(3-(3-fluorophenyl)ureido)-1H-pyrazole-4-carboxylate 6b
Ethyl 1-(2-(4-fluorophenyl)-2-hydroxyethyl)-5-(3-(2-fluorophenyl)ureido)-1H-pyrazole-4-carboxylate (6a)
Ethyl 1-(2-(4-fluorophenyl)-2-hydroxyethyl)-5-(3-(3-fluorophenyl)ureido)-1H-pyrazole-4-carboxylate (6b)
3.2.3. General Procedure for Synthesis of Compounds 7a,b
Ethyl 2-(4-fluorophenyl)-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carboxylate (7a)
3.2.4. General Procedure for Synthesis of Compounds 8a,b
2-(4-Fluorophenyl)-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carboxylic acid (8a)
2-Phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-6-carboxylic acid (8b)
3.2.5. General Procedure for Synthesis of Compounds 9a–g
Method A (Compounds 9b and 9d)
Method B (Compounds 9f and 9g)
Method C (Compounds 9a, 9c and 9e)
N-Cyclopropyl-2-(4-fluorophenyl)-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-7-carboxamide (9a)
(2-(4-Fluorophenyl)-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-7-yl)(piperidin-1-yl)methanone (9b)
N-Isopropyl-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-6-carboxamide (9c)
N-Isobutyl-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-6-carboxamide (9d)
N-Cyclopropyl-2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole-6-carboxamide (9e)
(2-Phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-6-yl)(piperidin-1-yl)methanone (9f)
Morpholino(2-phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazol-6-yl)methanone (9g)
3.2.6. Synthesis of 2-(4-fluorophenyl)-2,3-dihydro-1H-imidazo[1,2-b]pyrazole (10)
3.3. Biological Studies
3.3.1. Material
3.3.2. Blood Collection and Preparative Procedures
3.3.3. p38MAPK Phosphorylation in Human Platelet
3.3.4. ROS Assay
3.3.5. Platelet Aggregation
3.3.6. Anti-Proliferative Activity
3.3.7. Western Blotting on HUVEC Cells—VEGF Stimulated
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AKT | protein kinase B |
| DPPA | diphenylphosphorylazide |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| ERK | extracellular signal regulated kinase |
| fMLP | Formyl-methyl-leucyl-phenylalanine |
| HUVEC | Human umbilical vein endothelial |
| IL-8 | Interleukine-8 |
| NAC | n-acetylcysteine |
| PI3K | phosphatidylinositol 3-kinase |
| p38 MAPK | p38 Mitogen-Activated Protein Kinase |
| ROS | Reactive oxygen species |
| SAR | Structure–activity relationship |
| Thr | Thrombin |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Available online: http://www.cancer.gov/about-cancer/understanding/statistics (accessed on 1 May 2021).
- Kyriakis, J.M.; Avruch, J. Mammalian mapk signal transduction pathways activated by stress and inflammation: A 10-year update. Physiol. Rev. 2012, 92, 689–737. [Google Scholar] [CrossRef] [Green Version]
- Chapnick, D.A.; Warner, L.; Bernet, J.; Rao, T.; Liu, X. Partners in crime: The TGFα and MAPK pathways in cancer progression. Cell Biosci. 2011, 1, 42. [Google Scholar] [CrossRef] [Green Version]
- Gupta, J.; Nebreda, A.R. Roles of p38α mitogen-activated protein kinase in mouse models of inflammatory diseases and cancer. FEBS J. 2015, 282, 1841–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Kucuksayan, E.; Ozben, T. Hybrid Compounds as Multitarget Directed Anticancer Agents. Curr. Top. Med. Chem. 2017, 17, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, L.; Xu, M.; Xu, Y.; Qian, X. Rationally designed multitarget anticancer agents. Curr. Med. Chem. 2013, 20, 1694–1714. [Google Scholar] [CrossRef]
- Liu, F.; Yang, X.; Geng, M.; Huang, M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sini. B 2018, 8, 552–562. [Google Scholar] [CrossRef]
- Franco, A.T.; Corken, A.; Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood J. Am. Soc. Hematol. 2015, 126, 582–588. [Google Scholar] [CrossRef] [Green Version]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory response and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [Green Version]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Sierko, E.; Wojtukiewicz, M.Z. Platelets and angiogenesis in malignancy. Semin. Thromb. Hemost. 2004, 30, 95–108. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Sakurai, K.; Matsuo, Y.; Sudo, T.; Takuwa, Y.; Kimura, S.; Kasuya, Y. Role of p38 Mitogen-Activated Protein Kinase in Thrombus Formation. J Recept. Signal Transduct. 2004, 24, 283–296. [Google Scholar] [CrossRef]
- Borst, O.; Walker, B.; Muenzer, P.; Russo, A.; Schmid, E.; Faggio, C.; Bigalke, B.; Laufer, S.; Gawaz, M.; Lang, F. Skepinone-L. A Novel Potent and Highly Selective Inhibitor of p38 MAP Kinase, Effectively Impairs Platelet Activation and Thrombus Formation. Cell. Physiol. Biochem. 2013, 31, 914–924. [Google Scholar] [CrossRef]
- Kuliopulos, A.; Mohanlal, R.; Covic, L. Effect of selective inhibition of the p38 MAP kinase pathway on platelet aggregation. Thromb. Haemost. 2004, 92, 1387–1393. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zuo, X.; Zhao, Y.; Li, Q.; Tian, Z.; Yang, Y. Betanin-enriched red beet extract attenuated platelet activation and aggregation by suppressing Akt and P38 Mitogen-activated protein kinases phosphorylation. J. Funct. Foods 2019, 61, 103491. [Google Scholar] [CrossRef]
- Bruno, O.; Brullo, C.; Bondavalli, F.; Ranise, A.; Schenone, S.; Falzarano, M.S.; Varani, K.; Spisani, S. 2-Phenyl-2,3-dihydro-1H-imidazo[1,2-b]pyrazole derivatives: New potent inhibitors of fMLP-OMe-induced neutrophil chemotaxis. Bioorg. Med. Chem. Lett. 2007, 17, 3696–3701. [Google Scholar] [CrossRef]
- Bruno, O.; Brullo, C.; Bondavalli, F.; Schenone, S.; Spisani, S.; Falzarano, M.S.; Varani, K.; Barocelli, E.; Ballabeni, V.; Giorgio, C.; et al. 1-Methyl and 1-(2-hydroxyalkyl)-5-(3-alkyl/cycloalkyl/phenyl/naphthylureido)-1H-pyrazole-4-carboxylic acid ethyl esters as potent human neutrophil chemotaxis inhibitors. Bioorg. Med. Chem. 2009, 17, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Meta, E.; Brullo, C.; Sidibè, A.; Imhof, B.A.; Bruno, O. Design, synthesis, and biological evaluation of new pyrazolyl-ureas and imidazopyrazolecarboxamides able to interfere with MAPK and PI3K upstream signalling involved in the angiogenesis. Eur. J. Med. Chem. 2017, 133, 24–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marengo, B.; Meta, E.; Brullo, C.; De Ciucis, C.; Colla, R.; Speciale, A.; Garbarino, O.; Bruno, O.; Domenicotti, C. Biological evaluation of pyrazolyl-urea and dihydro-imidazo-pyrazolyl-urea derivatives as potential anti-angiogenetic agents in the treatment of neuroblastoma. Oncotarget 2020, 11, 3459–3472. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Bruno, O.; Fossa, P.; Ranise, A.; Menozzi, G.; Mosti, L.; Bondavalli, F.; Martini, C.; Trincavelli, L. Synthesis and biological data of 4-amino-1-(2-chloro-2-phenylethyl)-1H-pyrazolo[3,4-b]pyridine-5-carboxylic acid ethyl esters, a new series of A1-adenosine receptor (A1AR) ligands. Bioorg. Med. Chem. Lett. 2001, 11, 2529–2531. [Google Scholar] [CrossRef]
- Manetti, F.; Santucci, A.; Locatelli, G.A.; Maga, G.; Spreafico, A.; Serchi, T.; Orlandini, M.; Bernardini, G.; Caradonna, N.P.; Spallarossa, A.; et al. Identification of a Novel Pyrazolo[3,4-d]pyrimidine Able To Inhibit Cell Proliferation of a Human Osteogenic Sarcoma in Vitro and in a Xenograft Model in Mice. J. Med. Chem. 2007, 50, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Meta, E.; Brullo, C.; Tonelli, M.; Franzblau, S.G.; Wang, Y.; Ma, R.; Baojie, W.; Orena, B.S.; Pasca, M.R.; Bruno, O. Pyrazole and imidazo[1,2-b]pyrazole derivatives as new potential anti-tuberculosis agents. Med. Chem. 2019, 15, 17–27. [Google Scholar] [CrossRef]
- Bondavalli, F.; Botta, M.; Bruno, O.; Ciacci, A.; Corelli, F.; Fossa, P.; Lucacchini, A.; Manetti, F.; Martini, C.; Menozzi, G.; et al. Synthesis, Molecular Modeling Studies, and Pharmacological Activity of Selective A1 Receptor Antagonists. J. Med. Chem. 2002, 45, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Y.; Bao, L.; Chen, B.; Zhao, Y.; Qi, R. Ginkgolide B inhibits platelet release by blocking Syk and p38 MAPK phosphorylation in thrombin-stimulated platelets. Thromb Res. 2014, 134, 1066–1073. [Google Scholar] [CrossRef]
- Zhang, P.; Du, J.; Zhao, L.; Wang, X.; Zhang, Y.; Yan, R.; Dai, J.; Liu, G.; Zhang, F.; Dai, K. The role of intraplatelet reactive oxygen species in the regulation of platelet glycoprotein Ibα ectodomain shedding. Thromb Res. 2013, 132, 696–701. [Google Scholar] [CrossRef]
- Brandt, R.; Keston, A.S. Synthesis of diacetyldichlorofluorescin: A stable reagent for fluorimetric analysis. Anal. Biochem. 1965, 11, 6–9. [Google Scholar] [CrossRef]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef]
- Available online: http://dtp.cancer.gov (accessed on 20 September 2021).
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
| Compd. | R | R1 | R2 | R3 |
|---|---|---|---|---|
| 4a | NH2 | CO-cyclopropylamino | H | H |
| 4b | NH2 | CO-piperidino | H | H |
| 4c | NH2 | CO-morpholino | H | H |
| 4d | NH2 | CO-cyclopentylamino | H | H |
| 5a | 1-pyrrolyl | CO-cyclopropylamino | H | H |
| 5b | 1-pyrrolyl | CO-piperidino | H | H |
| 6a | NHCONHC6H4(2)F | COOEt | H | F |
| 6b | NHCONHC6H4(3)F | COOEt | H | F |
| 7a | // | COOEt | H | F |
| 7b | // | H | COOEt | H |
| 8a | // | COOH | H | F |
| 8b | // | H | COOH | H |
| 9a | // | CO-cyclopropylamino | H | F |
| 9b | // | CO-piperidino | H | F |
| 9c | // | H | CO-isopropylamino | H |
| 9d | // | H | CO-isobutylamino | H |
| 9e | // | H | CO-cyclopropylamino | H |
| 9f | // | H | CO-piperidino | H |
| 9g | // | H | CO-morpholino | H |
| 10 | // | H | H | F |
| 11a | NH2 | COOEt | H | H |
| 11b | NH2 | COOEt | H | F |
| 11c | NH2 | H | COOEt | H |
| 11d | 1-pyrrolyl | COOEt | H | H |
| 12a | NH2 | COOH | H | H |
| 12b | 1-pyrrolyl | COOH | H | H |
| Compound | Aggregation Inhibition IC50 (μM) | ROS Production Inhibition IC50 (μM) | p-p38MAPK Inhibition IC50 (μM) |
|---|---|---|---|
| 1a | 157.45 ± 10.38 | 156.24 ± 25.71 | 133.66 ± 13.52 |
| 1b | 85.11 ± 6.15 | 73.00 ± 23.82 | 95.09 ± 8.92 |
| 3a | 95.36 ± 7.65 | 71.17 ± 12.07 | 89.35 ± 5.21 |
| 4a | 83.89 ± 3.36 | 74.86 ± 6.18 | 95.73 ± 4.31 |
| 4b | 124.12 ± 6.56 | 150.87 ± 0.38 | 135.34 ± 10.47 |
| 4c | 99.07 ± 1.60 | 71.53 ± 8.65 | 105.20 ± 8.45 |
| 4d | 146.77 ± 17.27 | 158.96 ± 6.09 | 171.10 ± 17.21 |
| 5a | 104.72 ± 4.22 | 69.91 ± 12.06 | 108.76 ± 6.76 |
| 5b | 136.57 ± 13.85 | 173.22 ± 8.37 | 139.22 ± 7.94 |
| 6a | 97.45 ± 5.58 | 86.44 ± 15.07 | 95.83 ± 6.45 |
| 6b | 98.25 ± 7.25 | 92.22 ± 8.64 | 90.09 ± 1053 |
| 7a | 156.55 ± 21.19 | 174.08 ± 0.32 | 157.80 ± 9.58 |
| 7b | 107.74 ± 2.18 | 65.65 ± 18.10 | 97.52 ± 10.52 |
| 8a | 195.79 ± 14.69 | 210.82 ± 18.86 | 194.85 ± 5.87 |
| 8b | 76.37 ± 2.47 | 77.83 ± 6.53 | 90.07 ± 5.14 |
| 9a | 109.35 ± 8.18 | 82.56 ± 1.05 | 100.97 ± 8.62 |
| 9b | 205.56 ± 14.70 | 242.52 ± 14.16 | 200.65 ± 5.36 |
| 9c | 225.91 ± 15.95 | 314.87 ± 19.13 | 261.46 ± 15.68 |
| 9d | 86.39 ± 3.68 | 80.90 ± 0.53 | 109.06 ± 9.24 |
| 9e | 106.06 ± 6.49 | 86.40 ± 6.49 | 115.75 ± 4.83 |
| 9f | 148.12 ± 4.94 | 144.41 ± 8.64 | 178.29 ± 8.88 |
| 9g | 210.03 ± 8.91 | 198.90 ± 8.83 | 265.79 ± 11.62 |
| 10 | 141.94 ± 8.93 | 122.17 ± 9.46 | 146.79 ± 4.87 |
| 11d | 213.46 ± 4.59 | 248.19 ± 11.08 | 184.00 ± 9.25 |
| 12b | 305.34 ± 12.66 | 372.7 ± 19.8 | 428.76 ± 16.57 |
| SB203580 | 19.51 ± 1.83 | ND | 13.31 ± 1.64 |
| NAC | ND | 964.23 ± 48.51 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorello, M.G.; Rapetti, F.; Meta, E.; Sidibè, A.; Bruno, O.; Brullo, C. New Series of Pyrazoles and Imidazo-Pyrazoles Targeting Different Cancer and Inflammation Pathways. Molecules 2021, 26, 5735. https://doi.org/10.3390/molecules26195735
Signorello MG, Rapetti F, Meta E, Sidibè A, Bruno O, Brullo C. New Series of Pyrazoles and Imidazo-Pyrazoles Targeting Different Cancer and Inflammation Pathways. Molecules. 2021; 26(19):5735. https://doi.org/10.3390/molecules26195735
Chicago/Turabian StyleSignorello, Maria Grazia, Federica Rapetti, Elda Meta, Adama Sidibè, Olga Bruno, and Chiara Brullo. 2021. "New Series of Pyrazoles and Imidazo-Pyrazoles Targeting Different Cancer and Inflammation Pathways" Molecules 26, no. 19: 5735. https://doi.org/10.3390/molecules26195735
APA StyleSignorello, M. G., Rapetti, F., Meta, E., Sidibè, A., Bruno, O., & Brullo, C. (2021). New Series of Pyrazoles and Imidazo-Pyrazoles Targeting Different Cancer and Inflammation Pathways. Molecules, 26(19), 5735. https://doi.org/10.3390/molecules26195735








