1. Introduction
Perovskite Solar Cells (PSCs) have been in the spotlight of research over the past 10 years, which has led to significant improvements in the technology’s output. In particular, PSCs were first reported in 2009, as an evolution of the Dye Sensitized Solar Cell (DSSC) technology. The newly introduced solar cells achieved Power Conversion Efficiency (PCE) of 3.9% [
1] and after intensive experimental work on the perovskite structure, the device configuration and fundamental studies on the working principles and physical properties of these materials [
2,
3,
4,
5,
6,
7], PCEs of 25.6% have now been recorded in monolithic devices [
8].The factors that contribute to the high efficiency of perovskites, as active materials for solar cells, are their unique optoelectronic properties, including enhanced light absorption, high charge carrier mobility and long carrier lifetimes [
9,
10]. Even though huge progress has been made, PSCs still lag behind their theoretical PCE, determined by the Shockley-Queisser limit, which is about 31% [
11].
The main reason for this power conversion loss is the recombination processes that take place during the operation of PSCs, which affect negatively the open circuit voltage (V
oc) and the fill factor (FF) obtained, reducing correspondingly the overall device efficiency [
12]. In particular, non-radiative recombination, also known as Shockley–Read–Hall (SRH), that accounts for the largest portion of losses, occurs both at the interfaces between the perovskite and the charge transport layers, as well as the perovskite bulk and the grain boundaries [
13,
14]. The most promising approaches that have been used in order to minimize the trap-assisted non-radiative recombination losses are the control of the perovskite crystallization, through the engineering of the perovskite composition and the use of additives, the defect passivation and interface engineering and the formation of graded 2D/3D junctions, by applying post-treatment methods [
15,
16,
17]. By applying the aforementioned techniques, increases in the PCE have been achieved, reducing the gap between the theoretical and realistic PCEs obtained.
Organic molecules with several functional groups, the most prominent being ammonium iodide salts [
18], have been used to successfully passivate defects. Alkyl- and arylammonium halides have been effectively incorporated as additives into the perovskite precursor solution and have been proven to be beneficial for the film formation, resulting in improved surface morphology and enhanced crystallinity of the active phase. These organic amine salts (R
+NH
3 X
−), which possess electron-deficient H
+ with the ability to accept electrons, interact with the perovskite film surface via hydrogen bonding between the
+NH
4 group and the highly nucleophilic [PbI
6]
4− octahedra of the halide-based perovskite structure. The strong binding between the organic and inorganic frameworks is believed to regulate the grain size, consequently affecting the film coverage and crystal growth in the mesoporous scaffolds. Furthermore, it is well known that the counter ion on these organic ammonium halide modulators, usually I
−, can passivate halide defects, thus mitigating the potential recombination of the charge carrier at the interface between the perovskite absorber and the load carrying layers [
19].
Among these, phenethylammonium iodide (PEAI) and tetrabutyl ammonium iodide (TBAI) have emerged as highly efficient, achieving PCEs of 23.3%, with Voc of 1.18 V [
20] and 21.6 mA/cm
2 with Voc of 1.12 V respectively [
21,
22], which is over 90% of the Shockley-Queisser Voc limit. The working mechanism and the role of ammonium iodides, both as additives and as passivation agents, has been widely studied in typical PSCs [
18,
19,
23,
24,
25,
26,
27].
By introducing the ammonium iodides as additives in the perovskite precursor solution, chemical interactions between the additive and the perovskite occur, with the most prominent being the hydrogen bonding between the inorganic framework and the ammonium group. This interaction is responsible for the ordering and packing of the molecular domains, while it additionally determines the geometry and properties of the perovskite crystal, by regulating the crystal growth [
28,
29].
On the other hand, the post-treatment of the perovskite film, in the annealed perovskite phase, using the ammonium iodides as post-treatment agents is expected to reduce the trap density at the surface of the perovskite layer and consequently improve the performance and stability [
16,
30]. By using this method, a passivation occurs at the perovskite surface, which makes the material less prone to external environment effects and a suppress of the ion movement, together with reduction of defects, is achieved [
31].
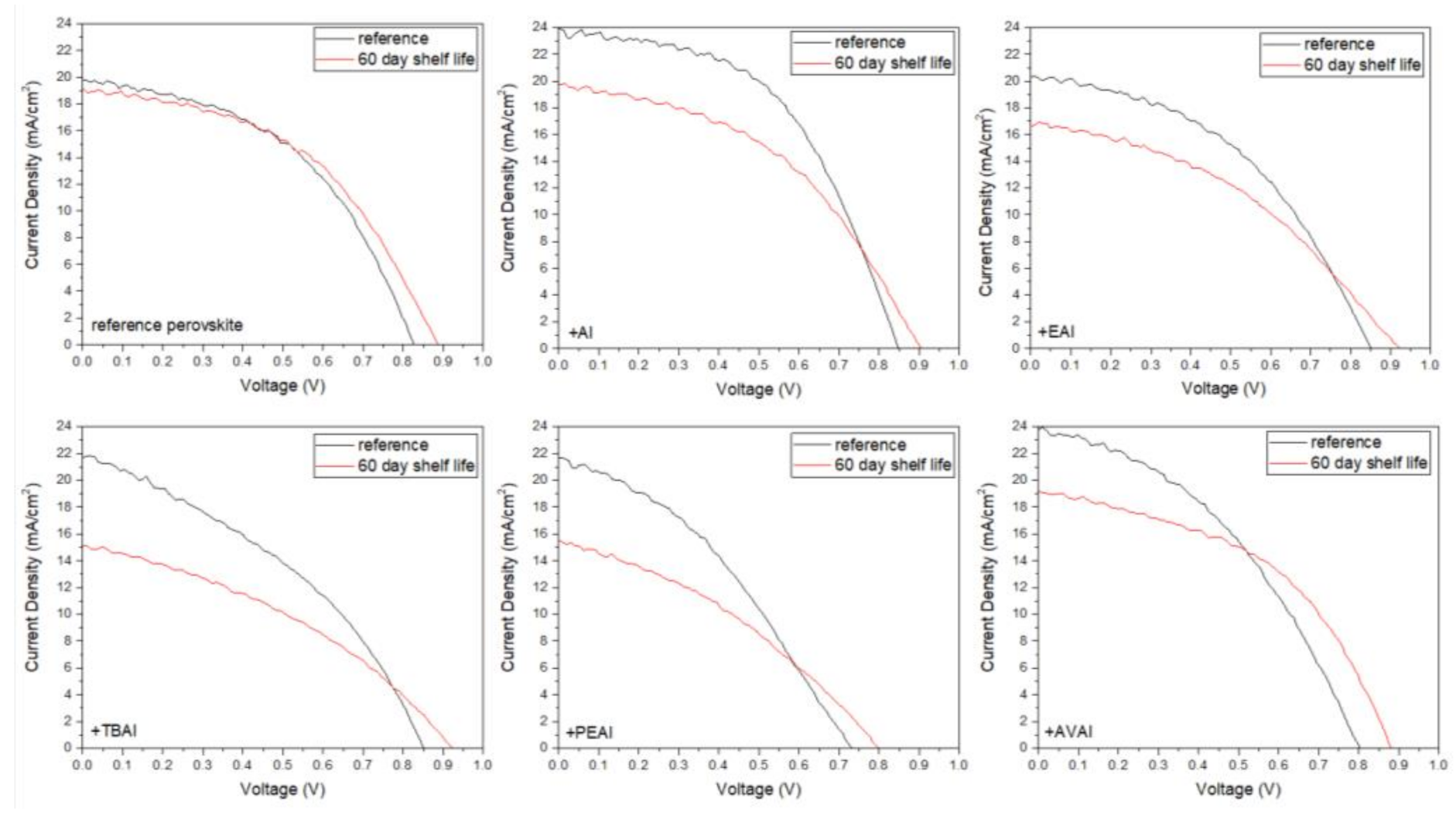
Herein, a series of ammonium iodide salts have been implemented in PSCs, which use carbon (C) as the counter electrode and are referred to as Carbon-based Perovskite Solar Cells (C-PSCs). This type of device differs from the metal electrode-based PSCs, since there is a complete elimination of the typical gold (Au) and/or silver (Ag) metal electrode, which is substituted by a C electrode, prepared from a C paste. In addition, owing to the bipolar charge carrier transport properties of perovskites, the expensive and unstable hole transport layer (HTL) can be omitted from these devices. C-PSCs offer the advantages of low cost and scalability, combined with ambient processability and increased device stability, which make them highly promising for commercialization.
In particular, in this work, the ammonium salts of ammonium iodide (AI), ethylammonium iodide (EAI), tetra butylammonium iodide (TBAI), phenethylammonium iodide (PEAI) and 5-ammonium valeric acid iodide (AVAI) have been used as additives in the multiple cation-mixed halide perovskite precursor solution, in order to control the crystallization process. Moreover, the same organic compounds have been used to post-treat the perovskite film, with the scope of serving as passivating agents. Both their use as additives and as passivators has been applied and evaluated in C-PSCs of the triple mesoscopic structure. A study of the ammonium iodide addition effects has been performed, together with a comparison among the different anions used. Being targeted in the triple mesoscopic C electrode-based PSC architecture, which is the most promising for large area applications but also the most challenging in terms of the fabrication methods and processing, this study provides a useful insight into the different routes towards the increase of the PCE and at the same time highlights the peculiarity and uniqueness of this system compared to the conventional metal electrode PSCs.
3. Materials and Methods
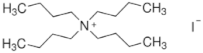
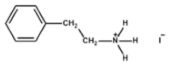
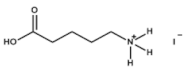
The chemical formulas and structures of all ammonium iodide additives that have been used in this study are presented in
Table 9.
Lead bromide (PbBr
2) and methyl ammonium bromide (MABr) were purchased from TCI (Tokyo Chemical Industry). Formamidinium iodide was purchased from Dyenamo. All the other chemicals were purchased from Sigma-Aldrich. Commercial nanocrystalline Titania paste 18-NRT was purchased from Greatcell and SnO
2: F transparent conductive electrodes (FTO, Resistance 10 Ohm/square) were purchased from Pilkington. The carbon and ZrO
2 pastes were prepared according to the method described elsewhere [
69].
The reference perovskite Csy[(FAPbI3)1−x (MAPbBr3)x]1−y (x = 0.15 and y = 0.05) was prepared by mixing FAI (1.27 M), MABr (0.225 M), PbI2 (1.39 M) and PbBr2 (0.225 M) in DMF:DMSO (4:1 v/v) with subsequent addition of 5% v/v of CsI (1.5 M in DMSO). The solution was kept under stirring at 70 °C, for 2 h before deposition. The organic ammonium iodides used as additives (NH4I, EAI, TBAI, PEAI, 5-AVAI) were incorporated in the reference perovskite precursor solution by adding the proper amount of stock solution (0.8 M in DMF) in order to reach the desired final additive concentration (5 mol% with respect to FAI content).
Fluorine-doped tin oxide (FTO, 10 ohm/square) glass substrates were chemically etched by reacting Zn powder with 4 M HCl, to form the desired pattern. The etched substrates were subsequently immersed in surfactant solution (Triton-X 10% wt in distilled water), deionized water, acetone and isopropanol and placed in ultrasonic bath for 15 min per cleaning step. After the last rinse with isopropanol, the clean substrates were annealed at 500 °C, in order to remove any organic residuals. The compact TiO
2 layer (c-TiO
2) was deposited by spin coating 40 μL of Ti-diisopropoxide bis (acetyl) acetonate (75% in isopropanol, diluted by 1:9
v/v in
N-propanol) at 2000 rpm for 10 s. The spin-coated films were dried at 125 °C and then annealed at 500 °C for 10 min and the procedure was repeated 4 times. The mesoporous TiO
2 layer (m-TiO
2) was then deposited, by spin-coating 40 μL of TiO
2 paste (18-NRT, diluted in ethanol 1:6
w/v) at 4000 rpm for 20 s, followed by drying at 125 °C and annealing at 525 °C for 30 min. After cooling down, the ZrO
2 was deposited on the substrates, by doctor blading a quantity of the as-prepared paste, followed by spin-coating at 5000 rpm for 30 s, in order to obtain a homogeneous film with full coverage. The films were then annealed at 450 °C for 20 min. Finally, the C counter electrodes were deposited by doctor-blading a quantity of the as-prepared paste, followed by annealing at 400 °C for 30 min. The perovskite solution was then infiltrated through the C electrode, followed by two-step spin coating at 1000 rpm for 10 s and 5000 rpm for 20 s. The perovskite-loaded substrates were then placed for annealing at 100 °C, for 60 min, capped with a petri dish to mitigate the impact of ambient oxygen and moisture [
53]. No Hole Transport Material and no antisolvent dripping were used, while the whole process was performed entirely under ambient conditions (RT 20 °C and RH 25–30%). For the post-treatment of the perovskite films, after cooling down to room temperature, 80 μL of the ammonium iodide stock solutions (5 mg/mL in isopropanol) were dropped on the surface of the film, spin-coated at 5000 rpm for 20 s, and annealed at 100° for 10 min. A total of 8 C-PSCs for each scenario have been prepared and the champion devices’ performance is demonstrated.
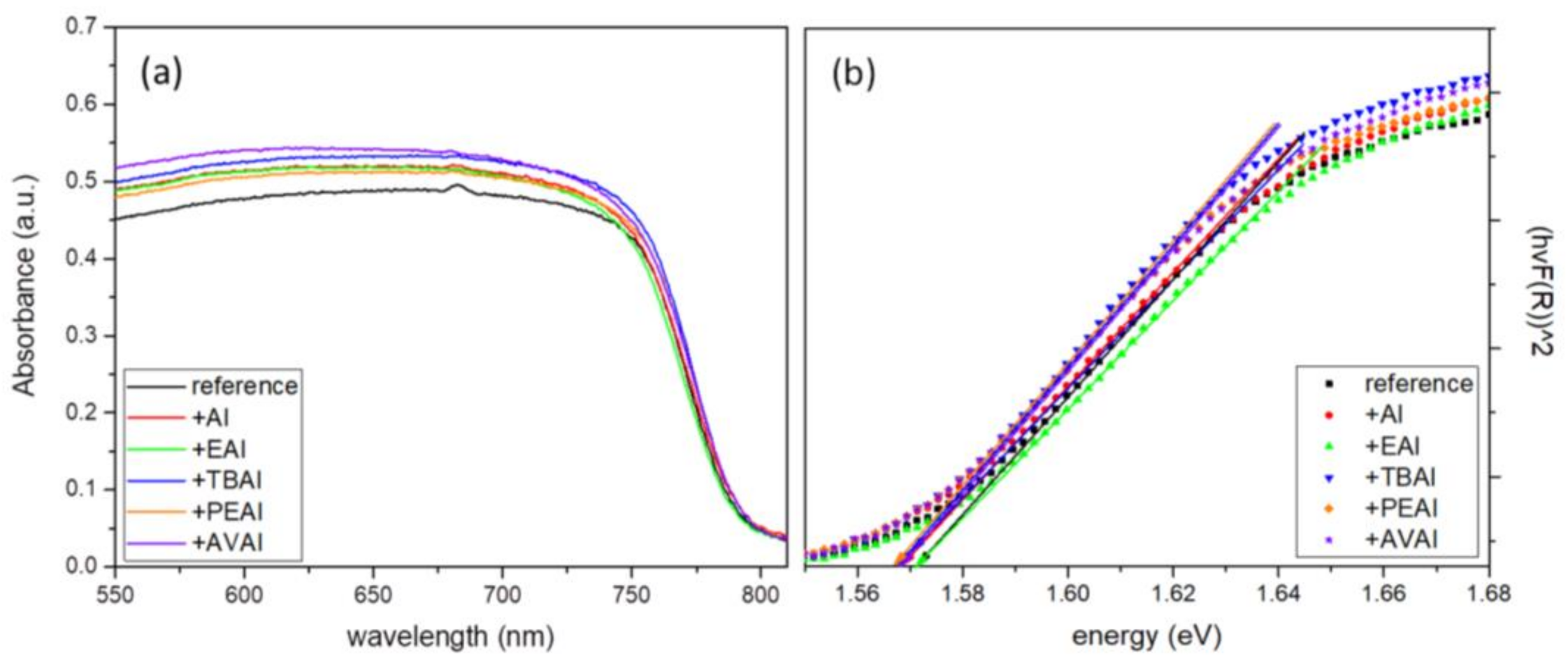
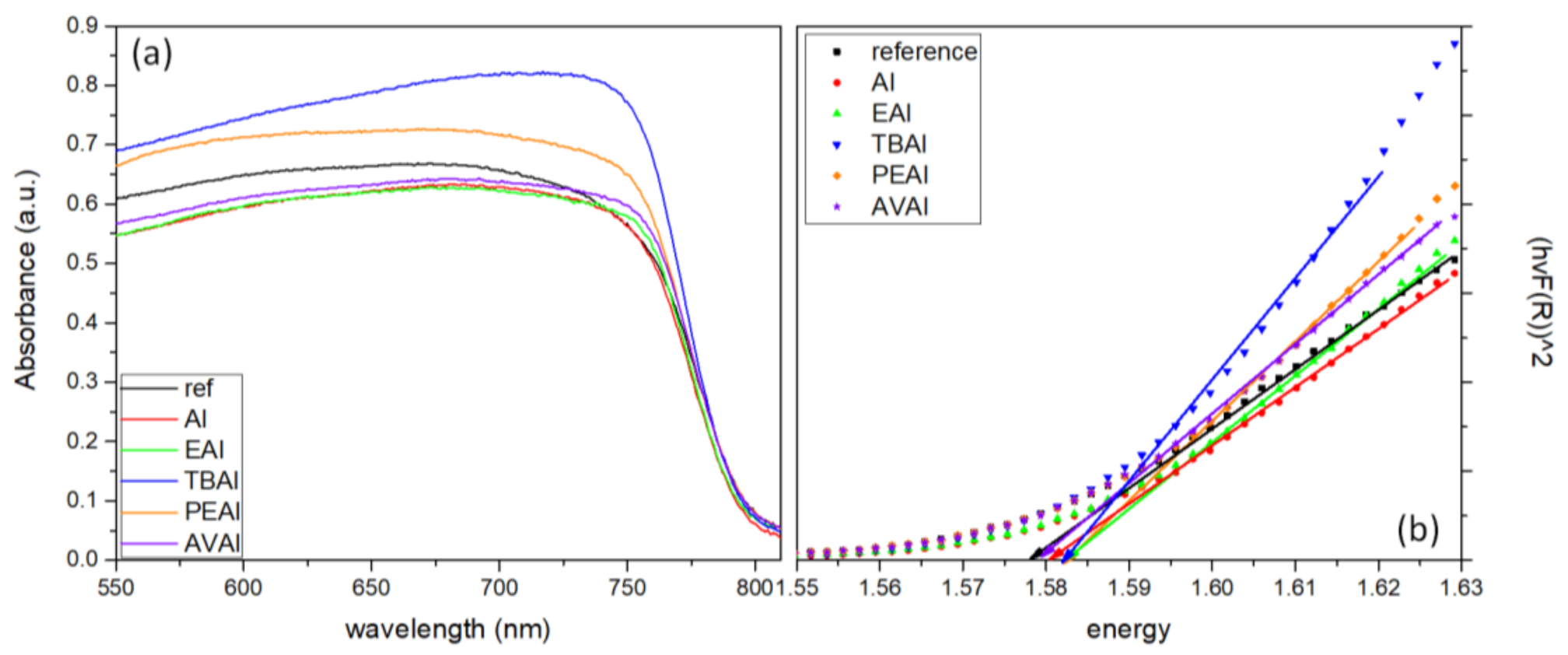
The Ultraviolet-Visible/NIR absorption diffuse reflectance spectra of the perovskite films were obtained in a range of 300 nm to 850 nm using a Jasco V-770 spectrophotometer equipped with a 60 mm integrating sphere embedding a PbS Detector (ISN-923).
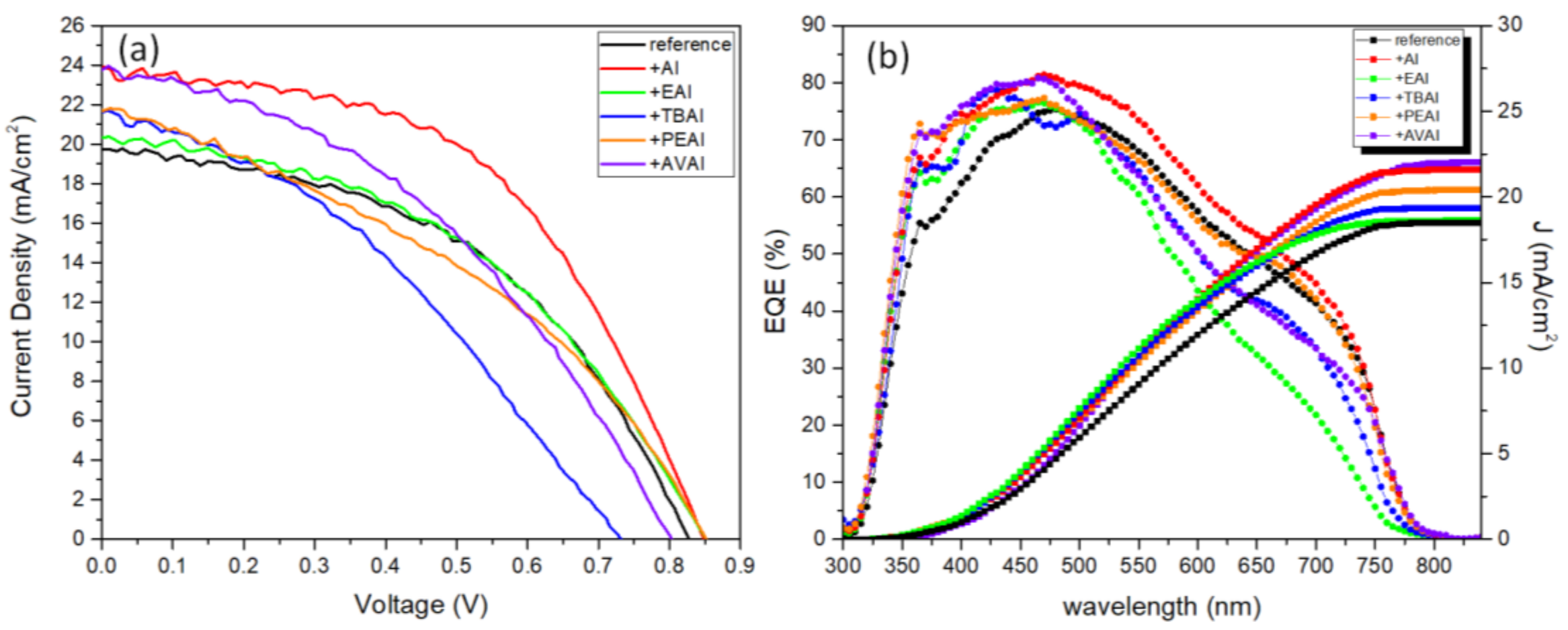
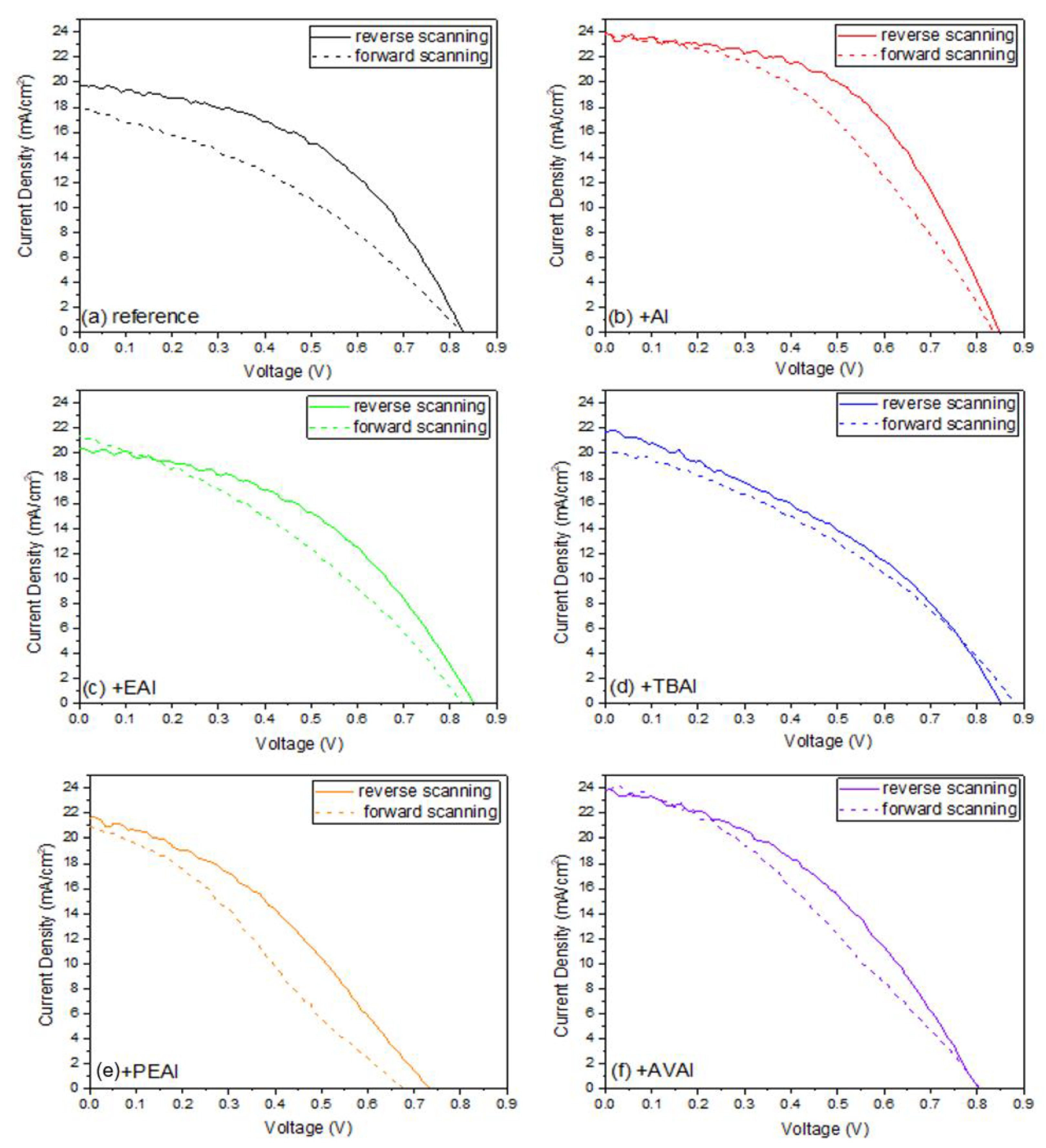
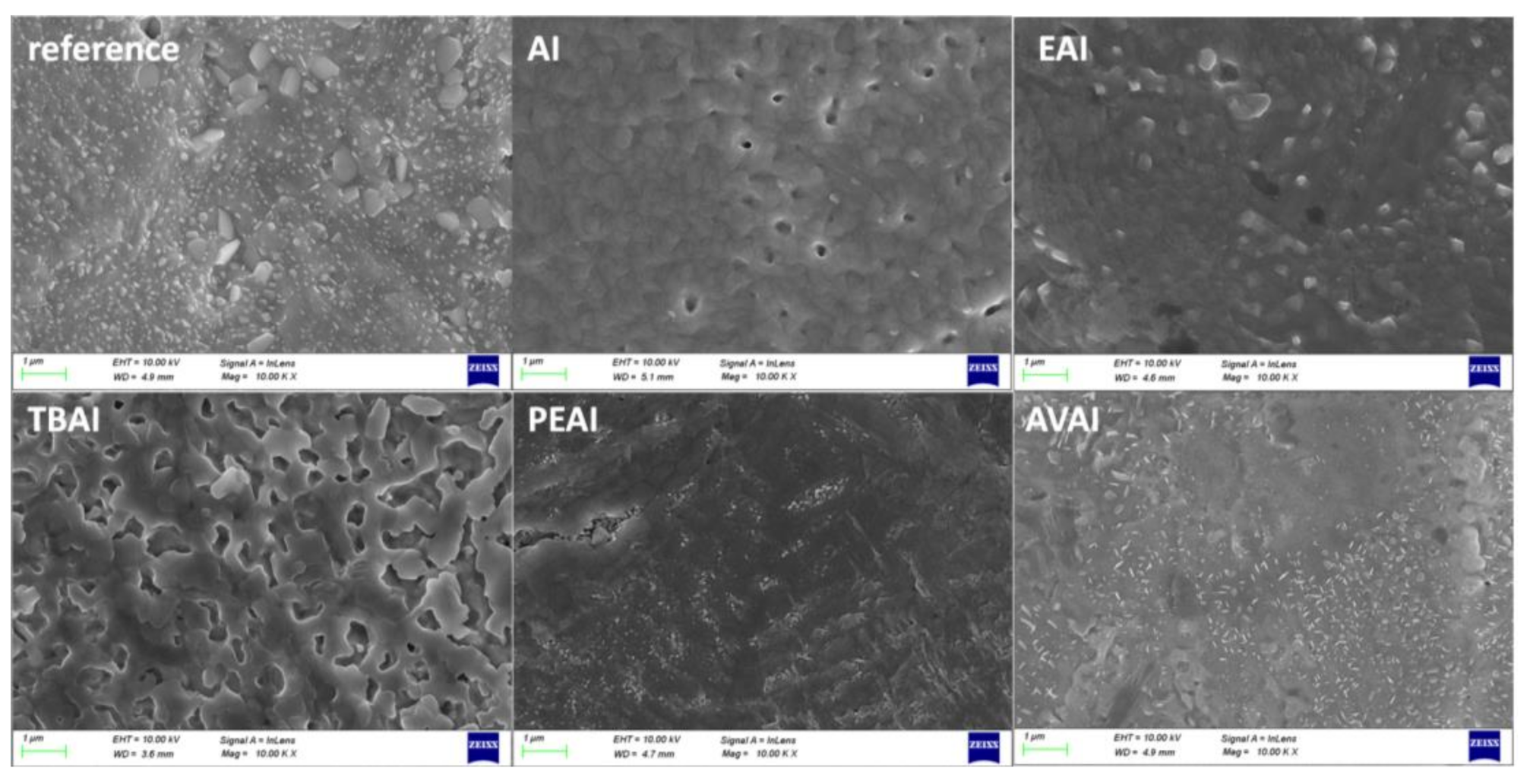
Photocurrent density-voltage (J-V) curves were recorded by using a Keithley 2601 source meter, with an applied potential range from 1.1 to −0.1 V and a scan rate of 125 mV/s, by illumination with a Solar Light simulator, providing a beam of 100 mWcm
−2 light intensity. In order to determine the active area of the devices, a black metallic mask of aperture size of 0.145 cm
2, which is very close to the actual area of the devices, was used [
70]. External quantum efficiency (EQE) was determined by incident photo to current efficiency (IPCE) measurement by Thetametrisis PM−QE equipped with Xenon (Xe) light source using a filter monochromator (Oriel CornerstoneTM 260 1/4 m, Newport), which was controlled by PM−Monitor
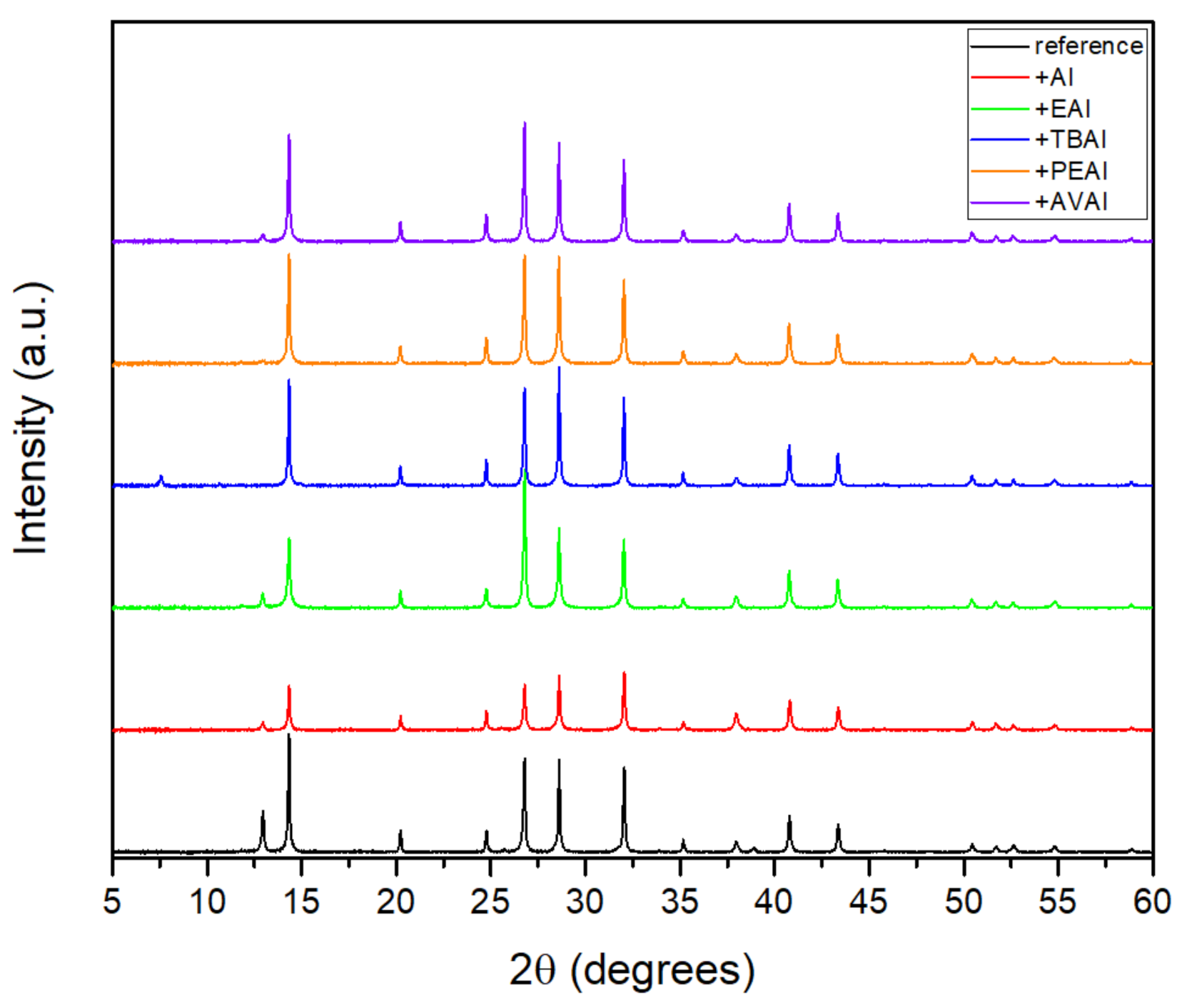
® software. Electrochemical Impedance Spectroscopy (EIS) characterization was performed on PSC devices with a potentiostat/galvanostat (PGSTAT128 N, Autolab B.V., Netherlands) under AM-1.5G-illuminated conditions. The morphology was investigated with a high resolution field-emission scanning electron microscope (FE-SEM) instrument (Zeiss, SUPRA 35VP) operating at 10–15 kV. The crystal structure was investigated using X-ray Diffraction (XRD, Bruker D8 Advance) utilizing a nickel-filtered CuKα (1.5406 Å) radiation source operated at 40 kV and 40 mA with a fixed step size of 0.02° and 5 s/step in the range of 5° ≤ 2
θ ≤ 60°.
4. Conclusions
In this work, the effect of ammonium iodides in PSCs with a C electrode and the triple mesoscopic structure has been evaluated. The study has been conducted in two directions: the incorporation of ammonium iodides as additives in the perovskite precursor solution and the application of ammonium iodides as post-treatment agents. In the direction of ammonium iodides as additives, after the incorporation of ammonium iodide (AI) in the precursor solution, an increase in the absorbance, combined with a decreased charge transfer resistance, has led to C-PSCs with PCE of 10.3%, increased by 33% compared to the reference perovskite, and with a low hysteresis index of 0.18, compared to 0.31 for the reference. In the direction of post-treatment with ammonium iodides, the two iodides with the shortest alkyl chain, ammonium iodide (AI) and ethylammonium iodide (EAI), have yielded the highest PCEs of 9.77% and 9.15% respectively, mainly attributed to the increased photocurrent density obtained, with a significant simultaneous increase in the FF values, which has confirmed the beneficial effect of the post-treatments in the optimizing of the perovskite/charge extraction layer interfaces. A common result of these parallel studies has been the influence of the alkyl chain length in the resulting properties of the C-PSCs. In particular, it has been noted in both cases that the longest alkyl chain is incapable of penetrating through the mesoporous C electrode, and therefore any favorable effect that the ammonium iodide could have had on the physical, chemical, structural and electrical properties is inhibited. The results of this work have demonstrated the importance of choosing the appropriate materials that will be compatible with each one of the individual parts that the C-PSCs comprise of, in order to obtain high performing devices. Finally, this study has revealed the key role that both the precursor solution and the post-treatment solution have when applying the ammonium iodides in the triple mesoscopic C electrode configuration, and has highlighted the great difference compared to the typical metal electrode PSCs, emphasizing the importance of independent research in this field.