High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of HHP Treatment on Proximate Compositions and pH
2.2. Effect of HHP Treatment on Texture Profile
2.3. Effect of HHP Treatment on Fatty Acid Composition
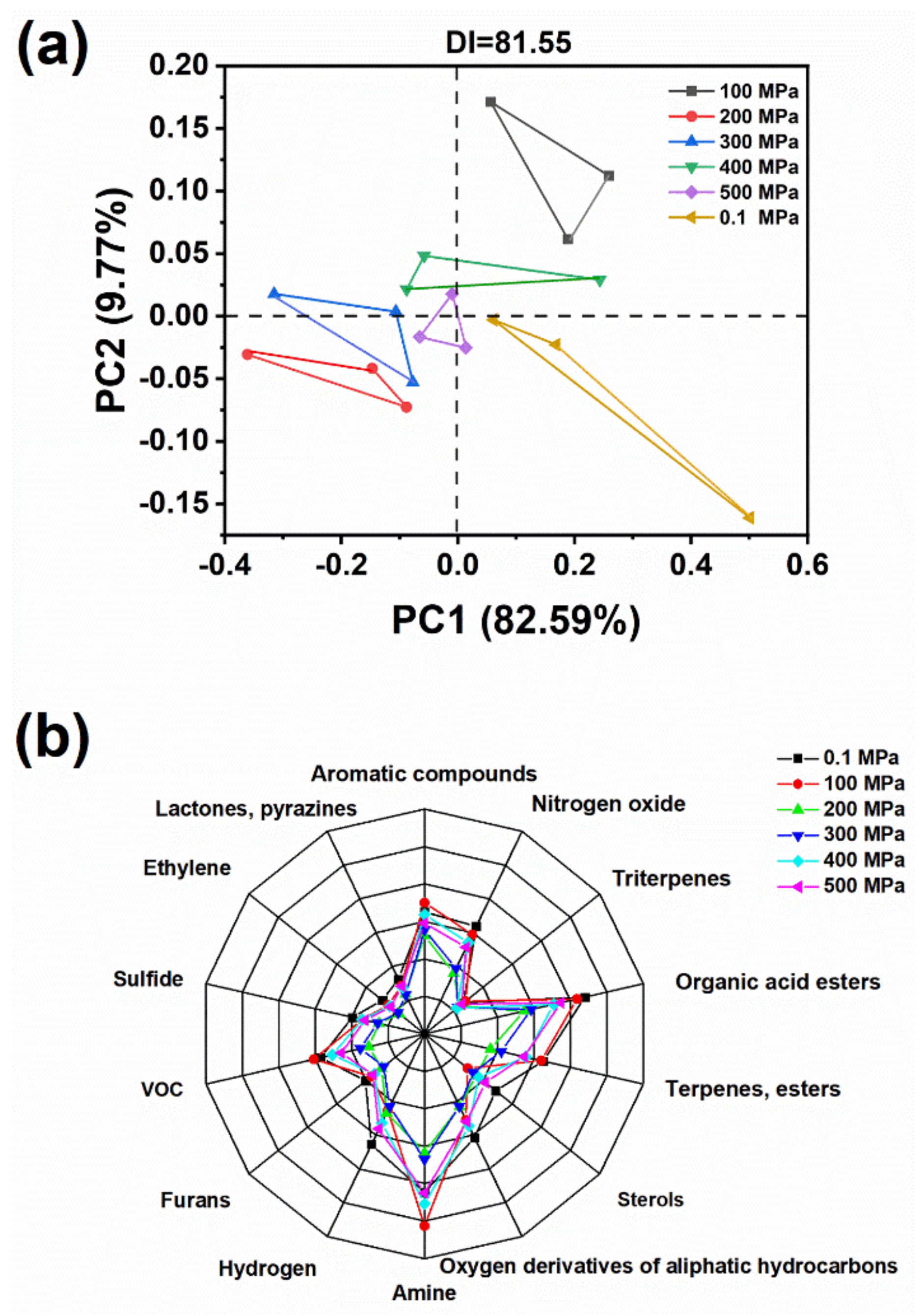
2.4. Electronic Nose Result Analysis
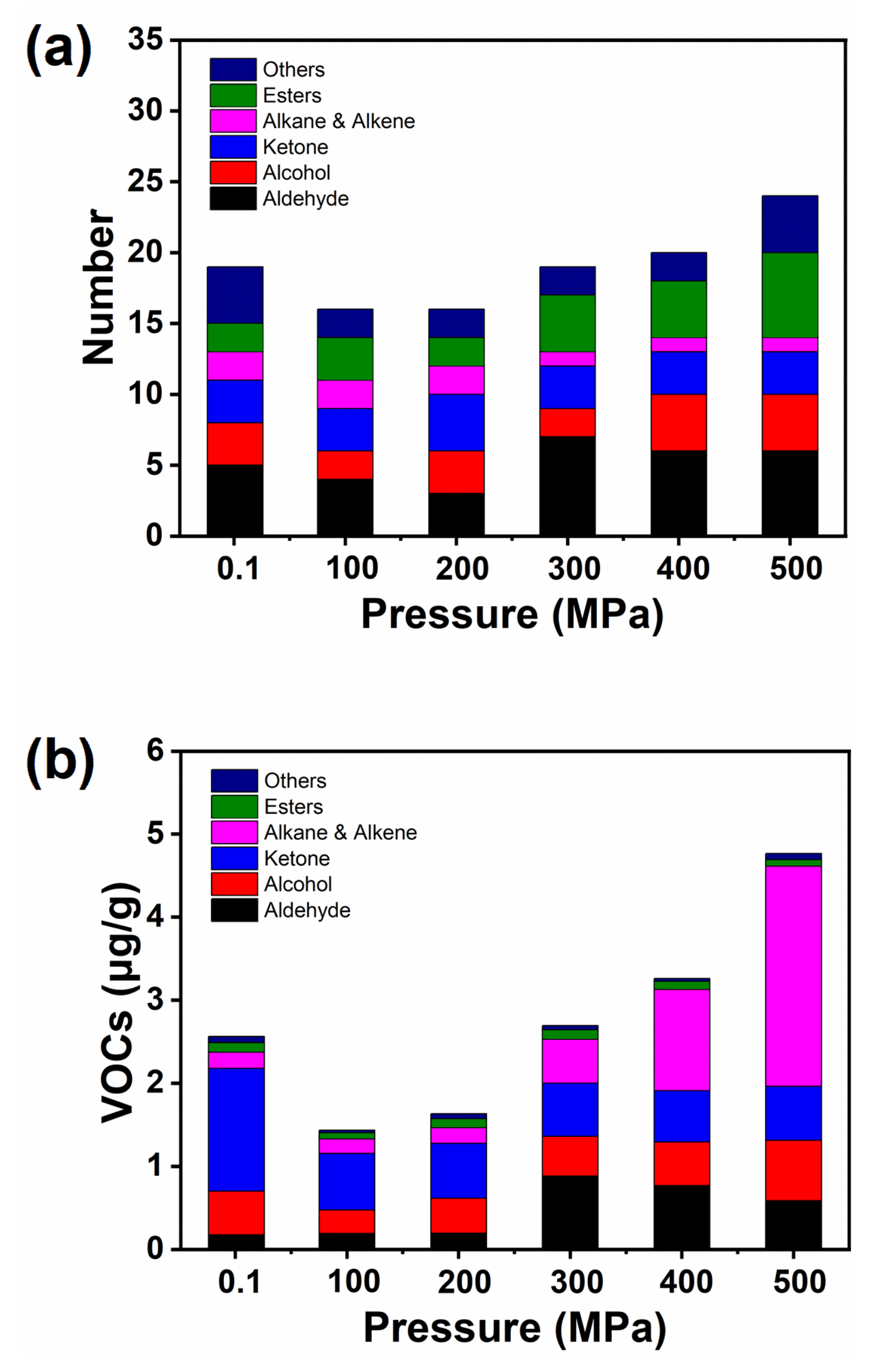
2.5. Effect of HHP Treatment on Volatile Compounds
3. Materials and Methods
3.1. Oyster Materials
3.2. HHP Treatment of Oysters
3.3. Proximate Composition Analysis
3.4. Changes in pH
3.5. Fatty Acid Analysis
3.6. Texture Profile Analysis (TPA)
3.7. Electronic Nose Analysis
3.8. Determination of Volatile Components
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Farmery, A.K.; Scott, J.M.; Brewer, T.D.; Eriksson, H.; Steenbergen, D.J.; Albert, J.; Raubani, J.; Tutuo, J.; Sharp, M.K.; Andrew, N.L. Aquatic Foods and Nutrition in the Pacific. Nutrients 2020, 12, 3705. [Google Scholar] [CrossRef]
- Twining, C.W.; Brenna, J.T.; Lawrence, P.; Winkler, D.W.; Flecker, A.S.; Hairston, N.G., Jr. Aquatic and terrestrial resources are not nutritionally reciprocal for consumers. Funct. Ecol. 2019, 33, 2042–2052. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, S.; Zeng, M. In vitro simulated digestion and fermentation characteristics of polysaccharide from oyster (Crassostrea gigas), and its effects on the gut microbiota. Food Res. Int. 2021, 149, 110646. [Google Scholar] [CrossRef]
- Qin, Y.; Li, X.; Li, J.; Zhou, Y.; Xiang, Z.; Ma, H.; Noor, Z.; Mo, R.; Zhang, Y.; Yu, Z. Seasonal variations in biochemical composition and nutritional quality of Crassostrea hongkongensis, in relation to the gametogenic cycle. Food Chem. 2021, 356, 129736. [Google Scholar] [CrossRef] [PubMed]
- Felici, A.; Vittori, S.; Meligrana, M.C.T.; Roncarati, A. Quality traits of raw and cooked cupped oysters. Eur. Food Res. Technol. 2020, 246, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Ghribi, F.; Bejaoui, S.; Rabeh, I.; Aouini, F.; Chetoui, I.; El Cafsi, M.h. Effects of Culinary Methods on Nutritional Characteristics of the Edible Shellfish Noah’s Ark (Arca noae L., 1758) from Tunisian Coasts. J. Aquat. Food Prod. Technol. 2017, 26, 1324–1336. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef] [PubMed]
- Villicaña, C.; Amarillas, L.; Soto-Castro, L.; Gómez-Gil, B.; Lizárraga-Partida, M.L.; León-Félix, J. Occurrence and Abundance of Pathogenic Vibrio Species in Raw Oysters at Retail Seafood Markets in Northwestern Mexico. J. Food Prot. 2019, 82, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Spaur, M.; Davis, B.J.K.; Kivitz, S.; De Paola, A.; Bowers, J.C.; Curriero, F.C.; Nachman, K.E. A systematic review of post-harvest interventions for Vibrio parahaemolyticus in raw oysters. Sci. Total Environ. 2020, 745, 140795. [Google Scholar] [CrossRef]
- Bui, A.; Cortese, C.; Libertin, C.R.; Porter, I.E. Minimal change disease and subacute interstitial nephritis in association with Edwardsiella tarda gastroenteritis following oyster consumption. IDCases 2021, 25, e01236. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Señorans, F.J.; Ibáñez, E.; Cifuentes, A. New Trends in Food Processing. Crit. Rev. Food Sci. Nutr. 2003, 43, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chen, Q.; Xia, X.; Liu, Q.; Kong, B.; Wang, H. High hydrostatic pressure combined with moisture regulators improves the tenderness and quality of beef jerky. Meat Sci. 2021, 181, 108617. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, E.J.; Chang, J.Y.; Song, K.B.; Chun, H.H. Effect of high hydrostatic pressure (HHP) and supercooling storage in leaf mustard (Brassica juncea L.) kimchi: Modelling of microbial activity and preservation of physicochemical properties. LWT 2021, 145, 111325. [Google Scholar] [CrossRef]
- Tan, C.; Li, D.; Wang, H.; Tong, Y.; Zhao, Y.; Deng, H.; Kong, Y.; Shu, C.; Yan, T.; Meng, X. Effects of high hydrostatic pressure on the binding capacity, interaction, and antioxidant activity of the binding products of cyanidin-3-glucoside and blueberry pectin. Food Chem. 2021, 344, 128731. [Google Scholar] [CrossRef]
- Orel, R.; Tabilo-Munizaga, G.; Cepero-Betancourt, Y.; Reyes-Parra, J.E.; Badillo-Ortiz, A.; Pérez-Won, M. Effects of high hydrostatic pressure processing and sodium reduction on physicochemical properties, sensory quality, and microbiological shelf life of ready-to-eat chicken breasts. LWT 2020, 127, 109352. [Google Scholar] [CrossRef]
- Salazar, F.A.; Yildiz, S.; Leyva, D.; Soto-Caballero, M.; Welti-Chanes, J.; Anubhav, P.S.; Lavilla, M.; Escobedo-Avellaneda, Z. 1.05-HHP Influence on Food Quality and Bioactive Compounds: A Review of the Last Decade. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 87–111. [Google Scholar]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Imamura, S.; Kanezashi, H.; Goshima, T.; Suto, A.; Ueki, Y.; Sugawara, N.; Ito, H.; Zou, B.; Uema, M.; Noda, M.; et al. Effect of High-Pressure Processing on Human Noroviruses in Laboratory-Contaminated Oysters by Bio-Accumulation. Foodborne Pathog. Dis. 2017, 14, 518–523. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Pérez-Mateos, M.; Montero, P.; Borderías, A.J. Oyster Preservation by High-Pressure Treatment. J. Food Prot. 2000, 63, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Kural, A.G.; Chen, H. Conditions for a 5-log reduction of Vibrio vulnificus in oysters through high hydrostatic pressure treatment. Int. J. Food Microbiol. 2008, 122, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Ling, Z.; Huihui, S.; Qi, L. Characterization of microbial community in high-pressure treated oysters by high-throughput sequencing technology. Innov. Food Sci. Emerg. Technol. 2018, 45, 241–248. [Google Scholar] [CrossRef]
- Zheng, J.-Y.; Tao, N.-P.; Gong, J.; Gu, S.-Q.; Xu, C.-H. Comparison of non-volatile taste-active compounds between the cooked meats of pre- and post-spawning Yangtze Coilia ectenes. Fish. Sci. 2015, 81, 559–568. [Google Scholar] [CrossRef]
- Wen, R.; Hu, Y.; Zhang, L.; Wang, Y.; Chen, Q.; Kong, B. Effect of NaCl substitutes on lipid and protein oxidation and flavor development of Harbin dry sausage. Meat Sci. 2019, 156, 33–43. [Google Scholar] [CrossRef]
- Duranton, F.; Simonin, H.; Guyon, C.; Jung, S.; de Lamballerie, M. Chapter 3-high-pressure processing of meats and seafood. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 35–63. [Google Scholar]
- Chapleau, N.; Mangavel, C.; Compoint, J.-P.; de Lamballerie-Anton, M. Effect of high-pressure processing on myofibrillar protein structure. J. Sci. Food Agric. 2004, 84, 66–74. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Smiddy, M.; Hill, C.; Kerry, J.P.; Kelly, A.L. Effects of high pressure treatment on physicochemical characteristics of fresh oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2004, 5, 161–169. [Google Scholar] [CrossRef]
- Lücke, F.K. 7-The control of pH. In Food Preservation Techniques; Zeuthen, P., Bøgh-Sørensen, L., Eds.; Woodhead Publishing: Sawston, UK, 2003; pp. 109–125. [Google Scholar]
- Chen, X.; Tume, R.K.; Xiong, Y.; Xu, X.; Zhou, G.; Chen, C.; Nishiumi, T. Structural modification of myofibrillar proteins by high-pressure processing for functionally improved, value-added, and healthy muscle gelled foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2981–3003. [Google Scholar] [CrossRef]
- Li, S.; Zhang, R.; Lei, D.; Huang, Y.; Cheng, S.; Zhu, Z.; Wu, Z.; Cravotto, G. Impact of ultrasound, microwaves and high-pressure processing on food components and their interactions. Trends Food Sci. Technol. 2021, 109, 1–15. [Google Scholar] [CrossRef]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef] [Green Version]
- Di Monaco, R.; Cavella, S.; Masi, P. Predicting sensory cohesiveness, hardness and springiness of solid foods from instrumental measurements. J. Texture Stud. 2008, 39, 129–149. [Google Scholar] [CrossRef]
- Paker, I.; Matak, K.E. Impact of sarcoplasmic proteins on texture and color of silver carp and Alaska Pollock protein gels. LWT Food Sci. Technol. 2015, 63, 985–991. [Google Scholar] [CrossRef]
- Bernasconi, A.; Szerman, N.; Vaudagna, S.R.; Speroni, F. High hydrostatic pressure and soybean protein addition to beef patties: Effects on the formation of mixed aggregates and technological parameters. Innov. Food Sci. Emerg. Technol. 2020, 66, 102503. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control. 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Wang, X.; Shan, J.; Han, S.; Zhao, J.; Zhang, Y. Optimization of Fish Quality by Evaluation of Total Volatile Basic Nitrogen (TVB-N) and Texture Profile Analysis (TPA) by Near-Infrared (NIR) Hyperspectral Imaging. Anal. Lett. 2019, 52, 1845–1859. [Google Scholar] [CrossRef]
- Peleg, M. The instrumental texture profile analysis revisited. J. Texture Stud. 2019, 50, 362–368. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Zu, S.; Wu, X.; Shi, A.; Zhang, J.; Wang, Q.; He, N. Effects of High Hydrostatic Pressure on the Conformational Structure and Gel Properties of Myofibrillar Protein and Meat Quality: A Review. Foods 2021, 10, 1872. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, S.; Li, Y.; Xu, Y.; Zhang, T.; Zhang, F.; Feng, X.; Zhao, Y.; Zeng, M. Effects of High Pressure Modification on Conformation and Digestibility Properties of Oyster Protein. Molecules 2019, 24, 3273. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, A.J.; Murphy, K.J.; Li, D. Marine lipids: Overview “news insights and lipid composition of Lyprinol”. Allergie et Immunologie 2000, 32, 261–271. [Google Scholar]
- German, J.B. Food processing and lipid oxidation. In Impact of Processing on Food Safety; Jackson, L.S., Knize, M.G., Morgan, J.N., Eds.; Springer US: Boston, MA, USA, 1999; pp. 23–50. [Google Scholar]
- Dyerberg, J. Linolenate-derived Polyunsaturated Fatty Acids and Prevention of Atherosclerosis. Nutr. Rev. 1986, 44, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Romero, M.C.; Kerry, J.P.; Kelly, A.L. Fatty acids, volatile compounds and colour changes in high-pressure-treated oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2008, 9, 54–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Wang, D.; Zhang, L.; Chen, G. Study on the volatile profile characteristics of oyster Crassostrea gigas during storage by a combination sampling method coupled with GC/MS. Food Chem. 2009, 115, 1150–1157. [Google Scholar] [CrossRef]
- Soncin, S.; Chiesa, L.M.; Panseri, S.; Biondi, P.; Cantoni, C. Determination of volatile compounds of precooked prawn (Penaeus vannamei) and cultured gilthead sea bream (Sparus aurata) stored in ice as possible spoilage markers using solid phase microextraction and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2009, 89, 436–442. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, P.; Xiao, Z.; Zhu, J.; Sun, X.; Wang, R. Evaluation of the perceptual interaction among ester aroma compounds in cherry wines by GC–MS, GC–O, odor threshold and sensory analysis: An insight at the molecular level. Food Chem. 2019, 275, 143–153. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Wu, W.-W.; Li, G.-K. A GC—MS Study of the Volatile Organic Composition of Straw and Oyster Mushrooms during Maturity and its Relation to Antioxidant Activity. J. Chromatogr. Sci. 2008, 46, 690–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takenaka, S.; Nakabayashi, R.; Ogawa, C.; Kimura, Y.; Yokota, S.; Doi, M. Characterization of surface Aspergillus community involved in traditional fermentation and ripening of katsuobushi. Int. J. Food Microbiol. 2020, 327, 108654. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Wu, C.; Wang, A.; Gu, Z. Effects of three light intensities on the survival, growth performance and biochemical composition of two size giant clams Tridacna crocea in the Southern China Sea. Aquaculture 2020, 528, 735548. [Google Scholar] [CrossRef]
- Chouhan, A.; Kaur, B.P.; Rao, P.S. Effect of high pressure processing and thermal treatment on quality of hilsa (Tenualosa ilisha) fillets during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2015, 29, 151–160. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Xu, L.; Gao, J.-M. Novel method for the producing area identification of Zhongning Goji berries by electronic nose. Food Chem. 2017, 221, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
| HHP Treatment Pressure (MPa) | ||||||
|---|---|---|---|---|---|---|
| 0.1 | 100 | 200 | 300 | 400 | 500 | |
| Moisture (%) | 82.45 ± 0.23 d | 82.35 ± 0.35 d | 82.84 ± 0.14 c | 83.43 ± 0.22 b | 84.65 ± 0.45 a | 84.71 ± 0.11 a |
| Crude protein (%) | 12.14 ± 0.25 a | 11.48 ± 0.31 b | 11.52 ± 0.22 b | 11.38 ± 0.21 b | 10.54 ± 0.29 c | 10.52 ± 0.37 c |
| Crude lipid (%) | 1.41 ± 0.15 a | 1.39 ± 0.12 a | 1.34 ± 0.26 a | 1.38 ± 0.08 a | 1.44 ± 0.09 a | 1.46 ± 0.12 a |
| Glycogen (%) | 2.61 ± 0.54 a | 2.57 ± 0.14 a | 2.64 ± 0.57 a | 2.59 ± 0.32 a | 2.64 ± 0.22 a | 2.66 ± 0.23 a |
| Ash (%) | 4.33 ± 0.19 a | 4.29 ± 0.16 a | 4.14 ± 0.22 a | 4.10 ± 0.12 a | 3.78 ± 0.16 b | 3.64 ± 0.14 c |
| pH | 6.60 ± 0.03 b | 6.61 ± 0.01 b | 6.63 ± 0.01 b | 6.66 ± 0.02 a | 6.67 ± 0.02 a | 6.66 ± 0.01 a |
| Pressure (MPa) | Hardness (N) | Springiness (mm) | Chewiness (mJ) | Cohesiveness (Ratio) |
|---|---|---|---|---|
| 0.1 | 56.68 ± 4.23 c | 0.18 ± 0.02 d | 2.35 ± 0.53 d | 0.22 ± 0.03 c |
| 100 | 60.06 ± 5.90 c | 0.20 ± 0.01 d | 2.95 ± 0.33 d | 0.24 ± 0.04 bc |
| 200 | 58.08 ± 7.31 c | 0.18 ± 0.02 d | 2.43 ± 0.59 d | 0.24 ± 0.04 bc |
| 300 | 60.59 ± 5.99 c | 0.26 ± 0.03 c | 4.53 ± 1.09 c | 0.29 ± 0.02 b |
| 400 | 101.70 ± 7.53 b | 0.32 ± 0.01 b | 12.29 ± 1.72 b | 0.38 ± 0.04 a |
| 500 | 114.06 ± 4.07 a | 0.39 ± 0.03 a | 20.06 ± 2.37 a | 0.40 ± 0.02 a |
| HHP Treatment Pressure (MPa) | ||||||
|---|---|---|---|---|---|---|
| Fatty Acid (%) | 0.1 | 100 | 200 | 300 | 400 | 500 |
| C6:0 | 1.15 ± 0.33 d | 2.11 ± 0.06 c | 2.38 ± 0.09 b | 2.52 ± 0.15 b | 2.86 ± 0.03 a | 2.94 ± 0.13 a |
| C8:0 | 3.15 ± 0.19 a | 1.38 ± 0.17 b | 1.45 ± 0.25 b | 1.36 ± 0.54 b | 1.28 ± 0.37 b | 1.31 ± 0.36 b |
| C10:0 | 2.27 ± 0.08 a | 2.19 ± 0.11 a | 1.53 ± 0.12 c | 1.59 ± 0.12 c | 1.89 ± 0.04 b | 1.87 ± 0.03 b |
| C11:0 | 2.54 ± 0.11 a | 1.85 ± 0.11 b | 1.47 ± 0.34 b | 1.53 ± 0.24 b | 1.76 ± 0.05 b | 1.82 ± 0.12 b |
| C12:0 | 1.88 ± 0.06 b | 2.53 ± 0.07 a | 1.55 ± 0.24 c | 1.64 ± 0.16 c | 1.78 ± 0.05 bc | 1.81 ± 0.11 bc |
| C14:0 | 2.78 ± 0.22 ab | 2.55 ± 0.18 b | 2.53 ± 0.19 b | 2.62 ± 0.11 b | 2.97 ± 0.07 a | 2.95 ± 0.08 a |
| C15:0 | 2.22 ± 0.09 b | 2.19 ± 0.13 b | 2.17 ± 0.15 b | 2.23 ± 0.06 b | 2.59 ± 0.11b a | 2.65 ± 0.12 a |
| C16:0 | 23.84 ± 0.55 a | 24.27 ± 0.96 a | 24.78 ± 1.65 a | 24.89 ± 1.2 a | 25.13 ± 0.68 a | 25.33 ± 0.34 a |
| C17:0 | 2.26 ± 0.41 a | 2.54 ± 0.34 b | 2.62 ± 0.44 b | 2.73 ± 0.33 a | 2.75 ± 0.43 a | 2.80 ± 0.34 a |
| C18:0 | 11.38 ± 0.87 a | 11.88 ± 1.06 a | 11.97 ± 0.48 a | 11.86 ± 1.27 a | 12.33 ± 0.28 a | 12.58 ± 1.06 a |
| C20:0 | 0.38 ± 0.13 b | 0.56 ± 0.13 b | 0.97 ± 0.21 a | 0.98 ± 0.15 a | 1.13 ± 0.22 a | 1.15 ± 0.37 a |
| ΣSFA | 54.32 ± 2.13 a | 52.70 ± 1.55 a | 55.29 ± 2.48 a | 53.85 ± 2.39 a | 54.55 ± 1.05 a | 52.82 ± 2.27 a |
| C14:1n5 | 2.32 ± 0.33 a | 2.35 ± 0.24 a | 2.33 ± 0.21 a | 2.31 ± 0.14 a | 2.37 ± 0.43 a | 2.36 ± 0.52 a |
| C15:1n9 | 1.25 ± 0.05 a | 1.24 ± 0.12 a | 1.23 ± 0.05 a | 1.25 ± 0.14 a | 1.45 ± 0.15 a | 1.47 ± 0.26 a |
| C16:1n7 | 2.62 ± 0.27 a | 2.55 ± 0.18 a | 2.35 ± 0.14 a | 2.56 ± 0.23 a | 2.93 ± 0.43 a | 2.95 ± 0.33 a |
| C18:1n-7 | 2.53 ± 0.27 a | 2.55 ± 0.06 a | 2.47 ± 0.28 a | 2.45 ± 0.18 a | 2.46 ± 0.12 a | 2.38 ± 0.24 a |
| C18:1n-5 | 3.75 ± 0.27 b | 3.45 ± 0.18 b | 3.54 ± 0.23 b | 3.58 ± 0.27 b | 4.64 ± 0.33 a | 4.68 ± 0.69 a |
| C20:1n9 | 2.49 ± 0.22 a | 2.35 ± 0.17 a | 2.38 ± 0.35 a | 2.41 ± 0.29 a | 2.54 ± 0.12 a | 2.62 ± 0.16 a |
| ∑MUFA | 13.42 ± 1.34 a | 13.67 ± 1.52 a | 13.64 ± 1.65 a | 14.13 ± 1.35 a | 14.03 ± 1.76 a | 13.67 ± 1.22 a |
| C18:2n-6 | 3.03 ± 0.28 a | 2.88 ± 0.11 a | 2.52 ± 0.32 a | 2.71 ± 0.08 a | 2.82 ± 0.22 a | 2.88 ± 0.24 a |
| C18:3n-3 | 3.14 ± 0.21 b | 3.34 ± 0.32 b | 3.35 ± 0.11 b | 3.34 ± 0.09 b | 3.98 ± 0.19 a | 4.11 ± 0.17 a |
| C20:2n6 | 3.28 ± 0.23 a | 3.06 ± 0.27 a | 3.08 ± 0.39 a | 3.08 ± 0.18 a | 3.02 ± 0.36 a | 3.05 ± 0.54 a |
| C20:3n6 | 2.46 ± 0.29 a | 2.41 ± 0.14 a | 2.37 ± 0.28 a | 2.35 ± 0.17 a | 2.24 ± 0.15 a | 2.18 ± 0.34 a |
| C20:4n6 ARA | 3.46 ± 0.35 a | 3.52 ± 0.21 a | 3.34 ± 0.09 a | 3.39 ± 0.12 a | 3.28 ± 0.15 a | 3.14 ± 0.24 a |
| C20:5n3 EPA | 5.35 ± 0.53 b | 5.65 ± 0.42 b | 5.78 ± 0.13 b | 6.13 ± 0.24 a | 6.57 ± 0.31 a | 6.64 ± 0.25 a |
| C22:2n6 | 3.42 ± 0.33 b | 3.46 ± 0.47 b | 3.46 ± 0.14 b | 4.07 ± 0.18 a | 4.35 ± 0.05 a | 4.54 ± 0.33 a |
| C22:6n3 DHA | 6.10 ± 0.65 b | 6.38 ± 0.19 b | 6.37 ± 0.35 b | 6.42 ± 0.45 b | 7.66 ± 0.27 a | 7.68 ± 0.25 a |
| ∑PUFA | 32.26 ± 3.21 a | 30.39 ± 2.59 a | 31.07 ± 2.46 a | 32.02 ± 1.98 a | 32.52 ± 2.13 a | 33.51 ± 1.98 a |
| Σn-3 | 14.59 ± 1.17 b | 15.37 ± 2.35 b | 15.50 ± 1.54 b | 15.89 ± 0.87 b | 18.21 ± 1.16 a | 18.43 ± 1.29 a |
| Σn-6 | 15.65 ± 1.54 a | 15.33 ± 1.37 a | 14.77 ± 1.14 a | 15.63 ± 1.66 a | 15.71 ± 1.35 a | 15.79 ± 2.02 a |
| Σn-3/Σn-6 | 0.93 | 0.96 | 1.06 | 1.06 | 1.18 | 1.21 |
| Compound | HHP Treatment Pressure (MPa) | ||||||
|---|---|---|---|---|---|---|---|
| 0.1 | 100 | 200 | 300 | 400 | 500 | ||
| Aldehydes | Hexenoic aldehyde | - | - | - | 0.018 | 0.027 | 0.040 |
| Octanal | 0.011 | - | - | 0.015 | - | 0.015 | |
| 2,4-Heptadienal, (E, E)- | 0.014 | 0.010 | - | 0.017 | 0.017 | 0.038 | |
| Benzaldehyde | 0.019 | - | - | 0.017 | 0.010 | 0.017 | |
| 2-Nonenal, (E)- | 0.025 | 0.019 | 0.0150 | 0.061 | 0.060 | - | |
| 2,6-Nonadienal, (E, E)- | 0.113 | 0.162 | 0.179 | 0.741 | 0.630 | 0.452 | |
| 2,4-Octadiene (E, E) | - | - | - | 0.013 | 0.017 | 0.023 | |
| Alcohols | 2-Penten-1-ol, (Z)-- | - | - | - | - | 0.007 | 0.015 |
| 1-Octen-3-ol | 0.333 | 0.268 | 0.260 | 0.388 | 0.407 | 0.550 | |
| 2-Octen-1-ol, (Z)- | - | - | 0.016 | - | 0.057 | 0.117 | |
| (6Z)-Nonen-1-ol | 0.010 | 0.015 | - | - | - | ||
| 3,6-Nonadien-1-ol, (E, Z)- | 0.183 | - | 0.146 | 0.088 | 0.056 | 0.047 | |
| Esters | 3-Nonenoic acid-ethyl ester | - | - | 0.018 | - | - | - |
| Tetradecanoic acid-ethyl ester | 0.053 | 0.035 | 0.041 | 0.059 | 0.051 | 0.045 | |
| Hexadecanoic acid-ethyl ester | 0.046 | 0.029 | 0.036 | 0.040 | 0.036 | 0.025 | |
| E-11-Hexadecenoic acid-ethyl ester | 0.016 | 0.010 | 0.009 | 0.016 | 0.012 | 0.011 | |
| Ketones | 3-Octanone | 1.461 | 0.663 | 0.644 | 0.639 | 0.616 | 0.648 |
| 2-Undecanone | 0.017 | 0.017 | 0.015 | - | - | - | |
| Hydrocarbon | 2-Octene, (E)- | - | - | - | - | - | 0.087 |
| 3,5-Octadiene, (Z, Z)- | - | - | - | 0.039 | 0.349 | 1.183 | |
| 1,3-trans,5-cis-octatriene | 0.110 | 0.016 | 0.101 | 0.411 | 0.386 | 0.438 | |
| E, Z-4-Ethylidenecyclohexene | - | 0.028 | - | - | - | - | |
| (E, E, E)-2,4,6-Octatriene | - | - | - | 0.033 | 0.046 | 0.111 | |
| 1,3-cyclooctadiene | 0.085 | 0.131 | 0.092 | 0.047 | 0.437 | 0.726 | |
| Cyclooctene-3-ethenyl | - | - | - | - | - | 0.105 | |
| Other class | 1,3-Dimethylbenzene | 0.022 | 0.018 | 0.010 | 0.016 | 0.014 | 0.023 |
| Benzene, 1-Methyl-3-(1-methylethyl)- | 0.006 | 0.012 | - | - | 0.017 | 0.025 | |
| Oxime-, methoxy-phenyl- | 0.019 | - | 0.051 | 0.034 | - | - | |
| Phenol, 4-ethyl- | - | - | - | - | - | 0.014 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Wang, R.; Zhang, T.; Xu, Y.; Jiang, S.; Zhao, Y. High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds. Molecules 2021, 26, 5731. https://doi.org/10.3390/molecules26195731
Ma Y, Wang R, Zhang T, Xu Y, Jiang S, Zhao Y. High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds. Molecules. 2021; 26(19):5731. https://doi.org/10.3390/molecules26195731
Chicago/Turabian StyleMa, Yuyang, Runfang Wang, Tietao Zhang, Yunsheng Xu, Suisui Jiang, and Yuanhui Zhao. 2021. "High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds" Molecules 26, no. 19: 5731. https://doi.org/10.3390/molecules26195731
APA StyleMa, Y., Wang, R., Zhang, T., Xu, Y., Jiang, S., & Zhao, Y. (2021). High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds. Molecules, 26(19), 5731. https://doi.org/10.3390/molecules26195731