Determination of Commercial Animal and Vegetable Milks’ Lipid Profile and Its Correlation with Cell Viability and Antioxidant Activity on Human Intestinal Caco-2 Cells
Abstract
:1. Introduction
2. Results
2.1. Comparison of Two Different Protocols for the Recovery of Total and Free Lipid Fractions from Commercial Animal and Vegetables Milks
2.2. Free Fatty Acids Profile of Commercial Animal and Vegetables Milks Obtained by GC-MS Analysis
2.3. Trolox Equivalent Antioxidant Capacity (TEAC) of Commercial Animal and Vegetables Milks
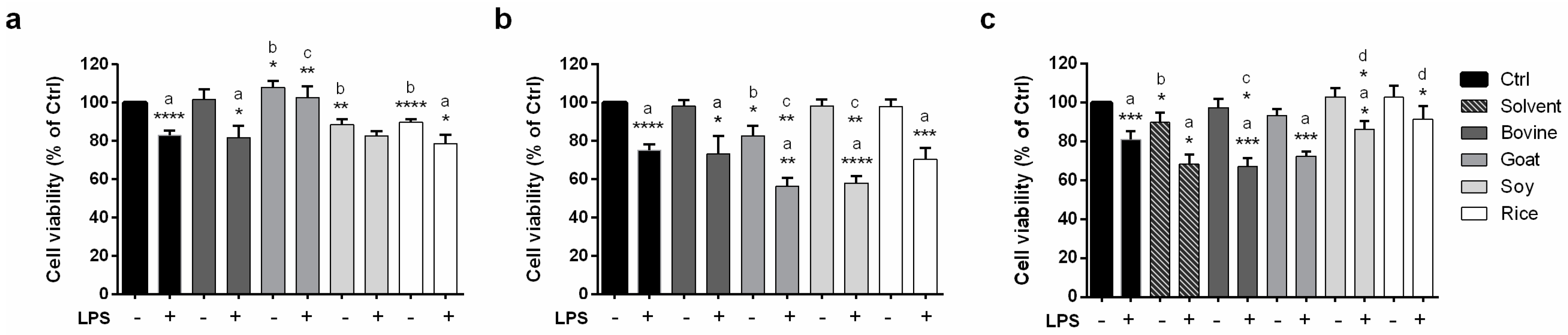
2.4. Modulation of Caco-2 Cell Viability by Animal and Vegetable Milks as Assessed by an MTT Assay
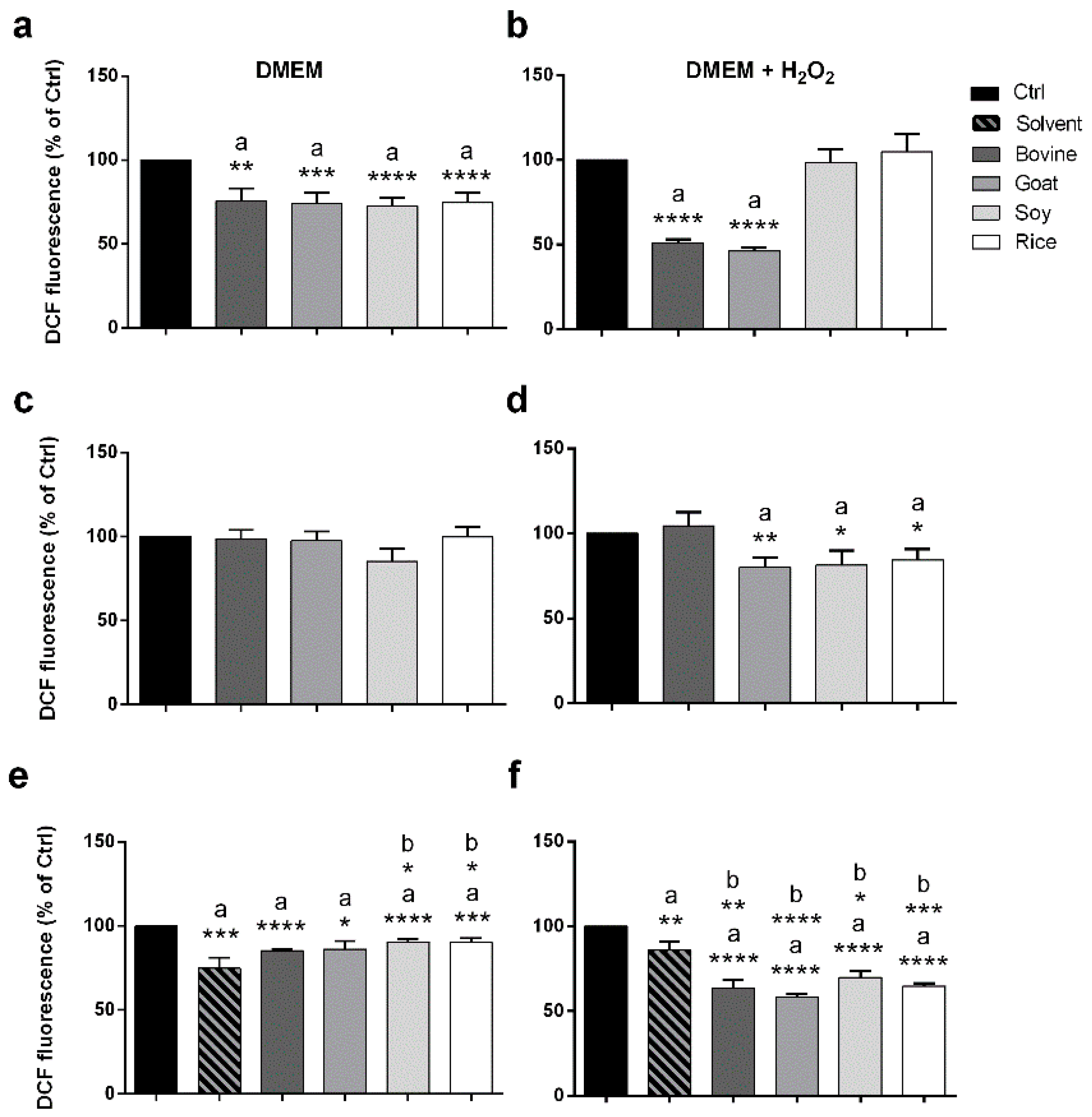
2.5. Antioxidant Potential of Animal and Vegetable Milks Tested on Caco-2 Cells by a DCF Assay
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Milk Lipid Samples
4.2. Total Antioxidant Capacity (TAC) Assay
4.3. Determination of Milk Lipid Fraction by a GC-MS Analysis
Apparatus
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Dichlorofluorescein Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Amores, G.; Virto, M. Total and Free Fatty Acids Analysis in Milk and Dairy Fat. Separations 2019, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 28; United States Department of Agriculture Agricultural Research Service: Beltsville, MD, USA, 2016.
- Gibson, R.A. Milk fat and health consequences. Nestle Nutr. Workshop Ser. Pediatr. Program 2011, 67, 197–207. [Google Scholar] [PubMed] [Green Version]
- Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.A.; Juarez, M. Fatty Acids. In Handbook of Dairy Foods Analysis; Nollet, L.M.L., Toldrá, F., Eds.; CRC Press: Portland, OR, USA, 2009; pp. 211–228. [Google Scholar]
- Markey, O.; Hobbs, D.A.; Givens, D.I. Public health implications of milk fats: The current evidence base and future directions. Clin. Lipidol. 2015, 10, 5–8. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. [Google Scholar] [PubMed]
- Yang, B.; Li, R.; Woo, T.; Browning, J.D.; Song, H.; Gu, Z.; Cui, J.; Lee, J.C.; Fritsche, K.L.; Beversdorf, D.Q.; et al. Maternal dietary docosahexaenoic acid alters lipid peroxidation products and (n-3)/(n-6) fatty acid balance in offspring mice. Metabolites 2019, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Li, R.; Greenlief, C.M.; Fritsche, K.L.; Gu, Z.; Cui, J.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J. Neuroinflamm. 2018, 15, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Fritsche, K.L.; Beversdorf, D.Q.; Gu, Z.; Lee, J.C.; Folk, W.R.; Greenlief, C.M.; Sun, G.Y. Yin-Yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front. Neurol. 2019, 10, 642. [Google Scholar] [CrossRef]
- Geng, X.; Yang, B.; Li, R.; Teng, T.; Ladu, M.J.; Sun, G.Y.; Greenlief, C.M.; Lee, J.C. Effects of Docosahexaenoic Acid and Its Peroxidation Product on Amyloid-β Peptide-Stimulated Microglia. Mol. Neurobiol. 2020, 57, 1085–1098. [Google Scholar] [CrossRef]
- Hanuš, O.; Samková, E.; Krizova, L.; Hasonova, L.; Kala, R. Role of Fatty Acids in Milk Fat and the Influence of Selected Factors on Their Variability—A Review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef] [Green Version]
- German, J.B.; Gibson, R.A.; Krauss, R.M.; Nestel, P.; Lamarche, B.; Van Staveren, W.A.; Steijns, J.M.; de Groot, L.C.P.G.M.; Lock, A.L.; Destaillats, F. A reappraisal of the impact of dairy foods and milk fat on cardiovascular disease risk. Eur. J. Nutr. 2009, 48, 191–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Basson, A.R.; Chen, C.; Sagl, F.; Trotter, A.; Bederman, I.; Gomez-Nguyen, A.; Sundrud, M.S.; Ilic, S.; Cominelli, F.; Rodriguez-Palacios, A. Regulation of intestinal inflammation by dietary fats. Front. Immunol. 2020, 11, 3639. [Google Scholar]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated fatty acids in obesity-associated inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, I.; Kornsteiner, M. Fats and fatty acid requirements for adults. Ann. Nutr. Metab. 2009, 55, 56–75. [Google Scholar] [CrossRef]
- Pilar, G.C.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. Technol. 2018, 81, 1–9. [Google Scholar]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Virsangbhai, C.K.; Goyal, A.; Tanwar, B.; Sihag, M.K. Potential health benefits of conjugated linoleic acid: An important functional dairy ingredient. Eur. J. Nutr. Food Saf. 2020, 11, 200–213. [Google Scholar] [CrossRef]
- Tonolo, F.; Moretto, L.; Ferro, S.; Folda, A.; Scalcon, V.; Sandre, M.; Fiorese, F.; Marin, O.; Bindoli, A.; Rigobello, M.P. Insight into antioxidant properties of milk-derived bioactive peptides in vitro and in a cellular model. J. Pept. Sci. 2019, 25, e3162. [Google Scholar] [CrossRef]
- Rioux, V.; Legrand, P. Saturated fatty acids: Simple molecular structures with complex cellular functions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Verruck, S.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Esmerino, E.A.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; da Cruz, A.G.; Prudencio, E.S. Dairy foods and positive impact on the consumer’s health. Adv. Food Nutr. Res. 2019, 89, 95–164. [Google Scholar]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.; Muskiet, F.A. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Rioux, V. Specific roles of saturated fatty acids: Beyond epidemiological data. Eur. J. Lipid Sci. Technol. 2015, 117, 1489–1499. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef] [Green Version]
- Lad, S.S.; Aparnathi, K.D.; Mehta, B.; Velpula, S. Goat milk in human nutrition and health—A review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1781–1792. [Google Scholar] [CrossRef] [Green Version]
- Bernata, N.; Chafer, M.; Chiralt, A.; Gonzalez-Martınez, C. Vegetable milks and their fermented derivative products. Int. J. Food Stud. 2014, 3, 93–124. [Google Scholar] [CrossRef]
- Silva, A.R.; Silva, M.M.; Ribeiro, B.D. Health issues and technological aspects of plant-based alternative milk. Food Res. Int. 2020, 131, 108972. [Google Scholar] [CrossRef]
- Abou-Dobara, M.I.; Ismail, M.M.; Refaat, N.M. Chemical composition, sensory evaluation and starter activity in cow, soy, peanut and rice milk. J. Nutr. Health Food Eng. 2016, 5, 1–8. [Google Scholar]
- Folch, L.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissue. J. Biol. Chem. 1957, 726, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Bareth, A.; Strohmar, W.; Kitzelmann, E. Gas chromatographic determination of mono- and diglycerides in milk and milk products. Eur. Food Res. Technol. 2003, 216, 365–368. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Milk and Milk Products–Extraction Methods for Lipids and Liposoluble Compounds; ISO: Geneva, Switzerland, 2001; ISO 14156:2001-IDF 172:2001. [Google Scholar]
- Stefanov, I.; Vlaeminck, B.; Fieve, V. A novel procedure for routine milk fat extraction based on dichloromethane. J. Food Compos. Anal. 2010, 23, 852–855. [Google Scholar] [CrossRef]
- Petrovi´c, M.; Kezi´c, N.; Bolanča, V. Optimization of the GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 122, 285–291. [Google Scholar] [CrossRef]
- Aldai, N.; Kramer, J.K.G.; Cruz-Hernandez, C.; Santercole, V.; Delmonte, P.; Mossoba, M.M.; Dugan, M.E.R. Appropriate extraction and methylation techniques for lipid analysis. In Fats and Fatty Acids in Poultry Nutrition and Health; Cherian, G., Poureslami, R., Eds.; Context Products Ltd.: Leicestershire, UK, 2012; pp. 249–290. [Google Scholar]
- Feng, S.; Lock, A.L.; Garnsworthy, P.C. Technical Note: A Rapid Lipid Separation Method for Determining Fatty Acid Composition of Milk. J. Dairy Sci. 2004, 87, 3785–3788. [Google Scholar] [CrossRef] [Green Version]
- Secchiari, P.; Antongiovanni, M.; Mele, M.; Serra, A.; Buccioni, A.; Ferruzzi, G.; Paoletti, F.; Petacchi, F. Effect of kind of dietary fat on the quality of milk fat from Italian Friesian cows. Livest. Prod. Sci. 2003, 83, 43–52. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; Zambonin, C. Solid-phase microextraction and on-fiber derivatization for assessment of mammalian and vegetable milks with emphasis on the content of major phytoestrogens. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bas, P.; Sauvant, D. Variations de la composition des depots lipidiques chez les bovins. INRA Prod. Anim. 2001, 14, 311–322. [Google Scholar] [CrossRef]
- Pakiet, A.; Wilczynski, M.; Rostkowska, O.; Korczynska, J.; Jabłonska, P.; Kaska, L.; Proczko-Stepaniak, M.; Sobczak, E.; Stepnowski, P.; Magkos, F.; et al. The Effect of One Anastomosis Gastric Bypass on Branched-Chain Fatty Acid and Branched-Chain Amino Acid Metabolism in Subjects with Morbid Obesity. Obes. Surg. 2020, 30, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Wang, Z.; Yuan, Z. Characteristics of lipid extraction from Chlorella sp. cultivated in outdoor raceway ponds with mixture of ethyl acetate and ethanol for biodiesel production. Bioresour. Technol. 2015, 191, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Tiwari, S.; Kadyan, S. Applications of emerging processing technologies for quality and safety enhancement of non-bovine milk and milk products. LWT 2021, 149, 111845. [Google Scholar] [CrossRef]
- Dupertuis, Y.M.; Meguid, M.M.; Pichard, C. Colon cancer therapy: New perspectives of nutritional manipulations using polyunsaturated fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 427–432. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Trottier, J.P.; Galvez, J.M.; Angers, P. Analysis of alpha-linolenic acid biohydrogenation intermediates in milk fat with emphasis on conjugated linolenic acids. J. Dairy Sci. 2005, 88, 3231–3239. [Google Scholar] [CrossRef] [Green Version]
- Coakley, M.; Banni, S.; Johnson, M.C.; Mills, S.; Devery, R.; Fitzgerald, G.; Ross, P.; Stanton, C. Inhibitory effect of conjugated alpha-linolenic acid from bifidobacterial of intestinal origin on SW480 cancer cells. Lipids 2008, 44, 249–256. [Google Scholar] [CrossRef]
- Pelczynska, E. Białka mleka jako czynnik alergenny [Milk protein as an allergen]. Med. Wet. 1996, 52, 752–754. [Google Scholar]
- Ducheix, S.; Peres, C.; Härdfeldt, J.; Frau, C.; Mocciaro, G.; Piccinin, E.; Lobaccaro, J.M.; De Santis, S.; Chieppa, M.; Bertrand-Michel, J.; et al. Deletion of Stearoyl-CoA Desaturase-1 From the Intestinal Epithelium Promotes Inflammation and Tumorigenesis, Reversed by Dietary Oleate. Gastroenterology 2018, 155, 1524–1538. [Google Scholar] [CrossRef]
- Petrone, G.; Conte, M.P.; Longhi, C.; di Santo, S.; Superti, F.; Ammendolia, M.G.; Valenti, P.; Seganti, L. Natural milk fatty acids affect survival and invasiveness of Listeria monocytogenes. Lett. Appl. Microbiol. 1998, 27, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Mechanisms of action of (n-3) fatty acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 9, 736–749. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; Chapter 10. [Google Scholar]
- Lindmark Månsson, H. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, M.; Altomare, A.; Cocca, S.; Solida, E.; Ribolsi, M.; Carotti, S.; Rainer, A.; Francesconi, M.; Morini, S.; Cicala, M.; et al. Palmitic acid affects intestinal epithelial barrier integrity and permeability in vitro. Antioxidants 2020, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.; Koetsier, M.A.; Balvers, M.; Beermann, C.; Stahl, B.; van Tol, E.A. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur. J. Nutr. 2008, 47, 183–191. [Google Scholar] [CrossRef]
- De Santis, S.; Cariello, M.; Piccinin, E.; Sabba, C.; Moschetta, A. Extra Virgin Olive Oil: Lesson from Nutrigenomics. Nutrients 2019, 11, 2085. [Google Scholar] [CrossRef] [Green Version]
- De Santis, S.; Clodoveo, M.L.; Cariello, M.; D’Amato, G.; Franchini, C.; Faienza, M.F.; Corbo, F. Polyphenols and obesity prevention: Critical insights on molecular regulation, bioavailability and dose in preclinical and clinical settings. Crit. Rev. Food Sci. Nutr. 2021, 61, 1804–1826. [Google Scholar]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of polyphenols and vitamins in wine-making by-products by supercritical fluid extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Rosano, C.; Franchini, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg. Chem. 2020, 105, 104440. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]

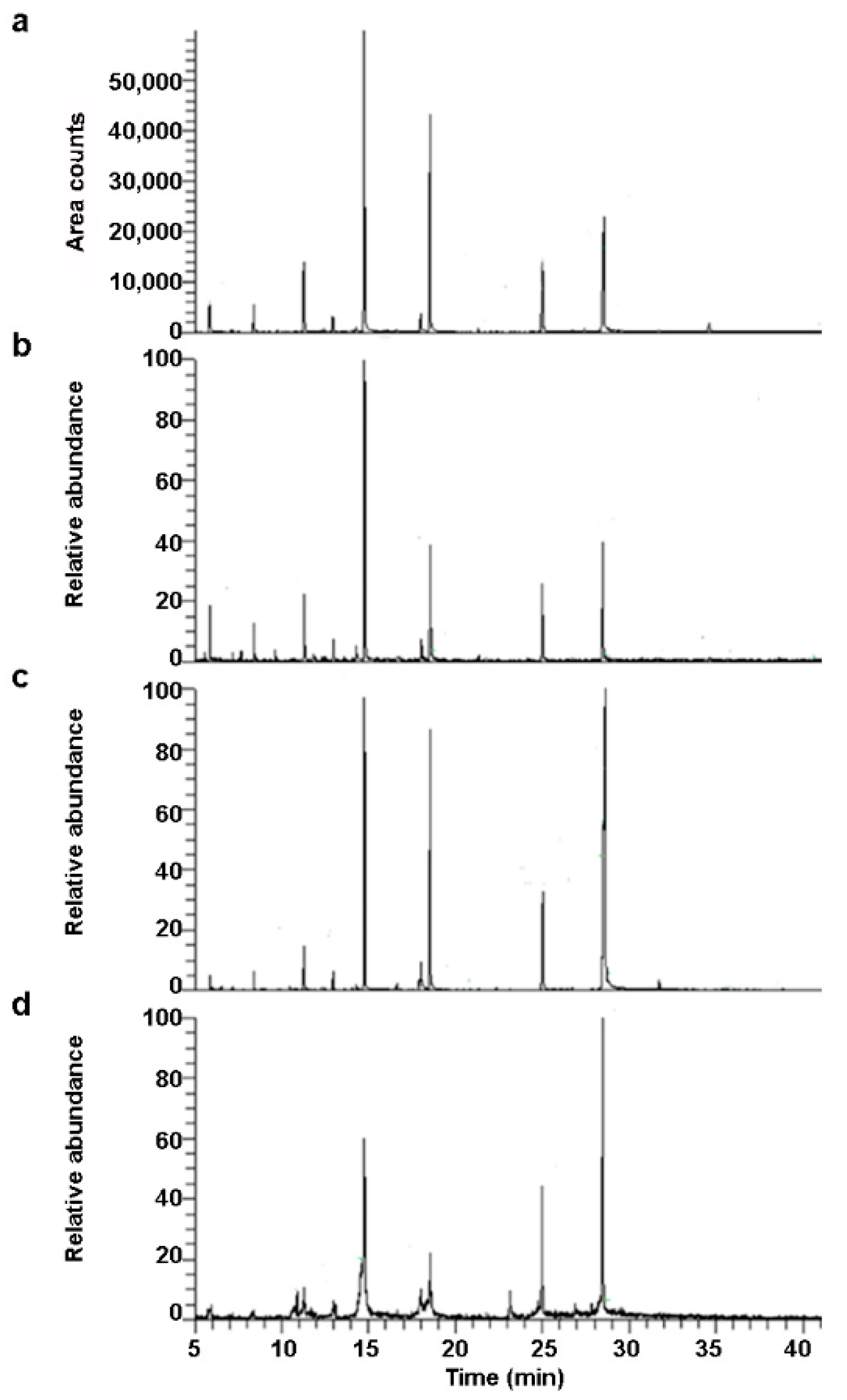

| # | RT * (min) | Compound | Formula | Common Name and Double Bond Position |
|---|---|---|---|---|
| 1 | 5.87 | Heptanoic acid, tert-butyldimethylsilanyl ester | C13H28O2Si | (FFA) C10:0 Capric acid |
| 2 | 8.40 | Dodecanoic acid, trimethylsilyl ester | C15H32O2Si | (FFA) C12:0 Lauric acid |
| 3 | 11.28 | Tetradecanoic acid, trimethylsilyl ester | C17H36O2Si | (FFA) C14:0 Myristic acid |
| 4 | 12.95 | Tetradecanoic acid, 6-methyl, trimethylsilyl ester | C18H38O2Si | (FFA) C15:0 Pentadecanoic acid |
| 5 | 14.29 | Hexadecanoic acid, trimethylsilyl ester | C19H38O2Si | (FFA) C16:1 Sapienic acid (6) Palmitoleic acid (9) |
| 6 | 14.77 | Hexadecanoic acid, trimethylsilyl ester | C19H40O2Si | (FFA) C16:0 Palmitic acid |
| 7 | 16.63 | Heptadecanoic acid, trimethylsilyl ester | C20H42O2Si | (FFA) C17:0 Margaric acid |
| 8 | 17.92 | 17-Octadecynoic acid, trimethylsilyl ester | C21H40O2Si | (FFA) C18:2 Linoleic acid (9,12) Rumenic acid (cis-9, trans-11) |
| 9 | 18.01 | Oleic acid, trimethylsilyl ester | C21H42O2Si | (FFA) C18:1 Oleic acid (9) Eleaidinic acid (trans-9) Vaccenic acid (trans-11) Asclepic acid (11) Petroselaidic acid (trans-6) |
| 10 | 18.56 | Octadecanoic acid, trimethylsilyl ester | C21H44O2Si | (FFA) C18:0 Stearic acid |
| 11 | 21.31 | Myristic acid, 2-(trimethylsiloxy)-1-[(trimethylsiloxy)methyl]ethyl ester | C23H50O4Si2 | (MAG) C14:0 |
| 12 | 23.16 | Pentadecanoic acid, 1,3-bis-(OTMS) propyl ester (β-glyceryl pentadecanoate) | C24H52O4Si2 | (MAG) C15:0 |
| 13 | 24.98 | Hexadecanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester | C25H54O4Si2 | (MAG) C16:0 |
| 14 | 26.70 | Heptadecanoic acid, glycerine-(1)-monoester, bis-O-trimethylsilyl | C26H56O4Si2 | (MAG) C17:0 |
| 15 | 28.58 | Octadecanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester | C27H58O4Si2 | (MAG) C18:0 |
| 16 | 31.70 | Eicosanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester | C29H62O4Si2 | (MAG) C20:0 |
| 17 | 34.57 | Cholesterol trimethylsilyl ether | C30H54OSi | Cholesterol |
| Sample | Milk (n = 3) | Nonsolvent Method Total Lipid Fraction (n = 3) | Solvent Extraction Free Lipid Fraction (n = 3) | ||
|---|---|---|---|---|---|
| (TEAC/mLmilk) | (TEAC/mLmilk) | (TEAC/gextr.fats) | (TEAC/mLmilk) | (TEAC/gtotalfat) | |
| Bovine | 48.04 ± 11.04 | 0.26 ± 0.10 | 16.66 ± 0.02 | 0.35 ± 0.02 | 72.91 ± 0.02 |
| Goat | 40.16 ± 5.96 | 0.28 ± 0.06 | 16.88 ± 0.04 | 0.28 ± 0.07 | 41.18 ± 0.07 |
| Soy | 40.25 ± 5.06 | 0.30 ± 0.08 | 16.11 ± 0.09 | 0.24 ± 0.11 | 38.26 ± 0.09 |
| Rice | 20.11 ± 3.06 | 0.21 ± 0.05 | 35.00 ± 0.06 | 0.25 ± 0.08 | 47.17 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aresta, A.; De Santis, S.; Carocci, A.; Barbarossa, A.; Ragusa, A.; De Vietro, N.; Clodoveo, M.L.; Corbo, F.; Zambonin, C. Determination of Commercial Animal and Vegetable Milks’ Lipid Profile and Its Correlation with Cell Viability and Antioxidant Activity on Human Intestinal Caco-2 Cells. Molecules 2021, 26, 5645. https://doi.org/10.3390/molecules26185645
Aresta A, De Santis S, Carocci A, Barbarossa A, Ragusa A, De Vietro N, Clodoveo ML, Corbo F, Zambonin C. Determination of Commercial Animal and Vegetable Milks’ Lipid Profile and Its Correlation with Cell Viability and Antioxidant Activity on Human Intestinal Caco-2 Cells. Molecules. 2021; 26(18):5645. https://doi.org/10.3390/molecules26185645
Chicago/Turabian StyleAresta, Antonella, Stefania De Santis, Alessia Carocci, Alexia Barbarossa, Andrea Ragusa, Nicoletta De Vietro, Maria Lisa Clodoveo, Filomena Corbo, and Carlo Zambonin. 2021. "Determination of Commercial Animal and Vegetable Milks’ Lipid Profile and Its Correlation with Cell Viability and Antioxidant Activity on Human Intestinal Caco-2 Cells" Molecules 26, no. 18: 5645. https://doi.org/10.3390/molecules26185645
APA StyleAresta, A., De Santis, S., Carocci, A., Barbarossa, A., Ragusa, A., De Vietro, N., Clodoveo, M. L., Corbo, F., & Zambonin, C. (2021). Determination of Commercial Animal and Vegetable Milks’ Lipid Profile and Its Correlation with Cell Viability and Antioxidant Activity on Human Intestinal Caco-2 Cells. Molecules, 26(18), 5645. https://doi.org/10.3390/molecules26185645












