Iron Forms Fe(II) and Fe(III) Determination in Pre-Roman Iron Age Archaeological Pottery as a New Tool in Archaeometry
Abstract
:1. Introduction
2. Experimental
2.1. Instrumentation
2.2. Reagents
2.3. Samples
2.4. Methodology
2.5. Total Iron and Selected Elements Determination
2.6. Acid Leachable Fraction Analysis
2.6.1. Sample Extraction by Hydrochloric Acid
2.6.2. Elemental Analysis
2.6.3. Iron Chemical Forms Determination
2.7. Statistical Analysis
3. Results and Discussion
3.1. Analysis of the Pottery Fragments from Four Sites
3.1.1. Chemical Composition of Pottery
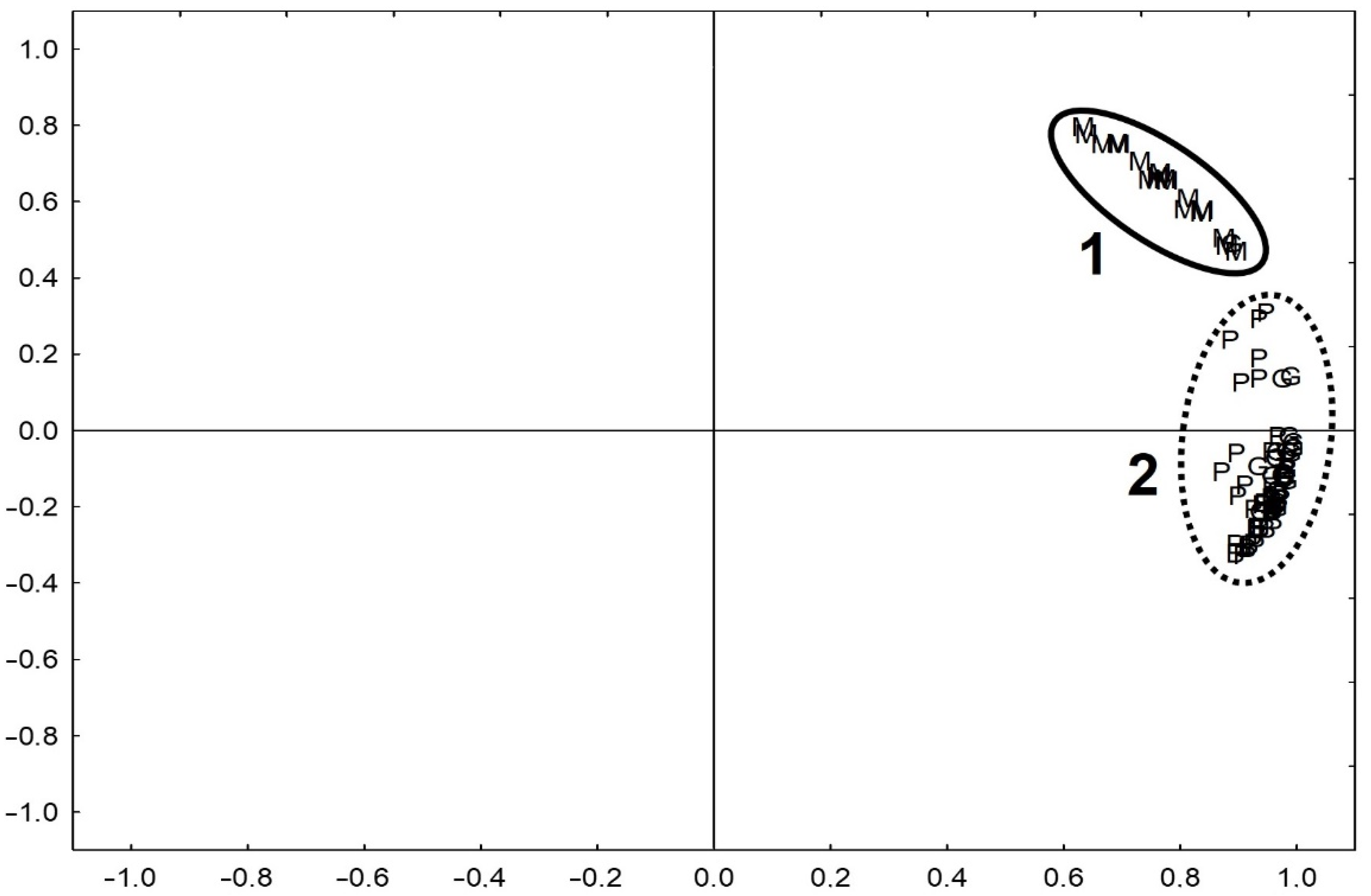
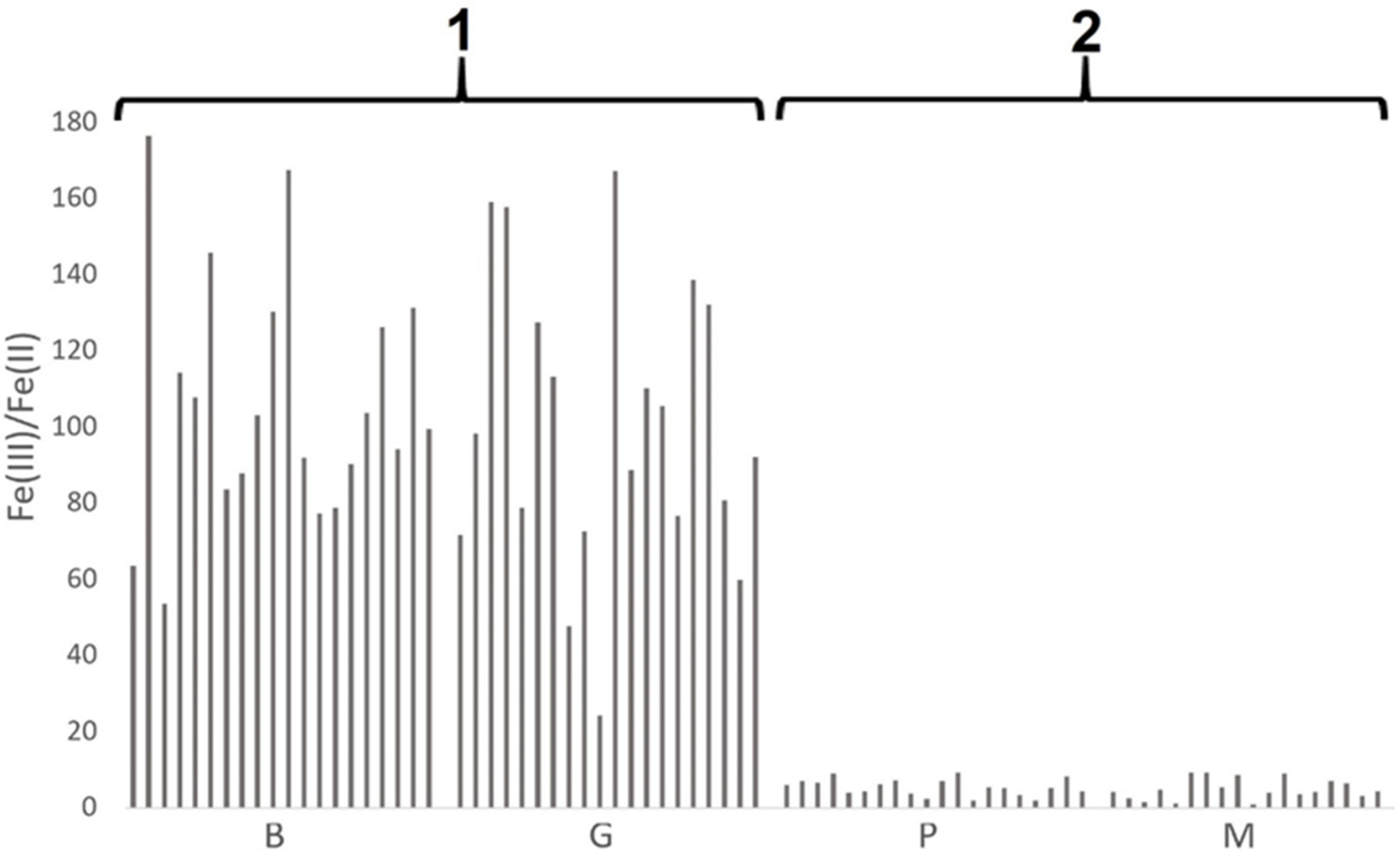
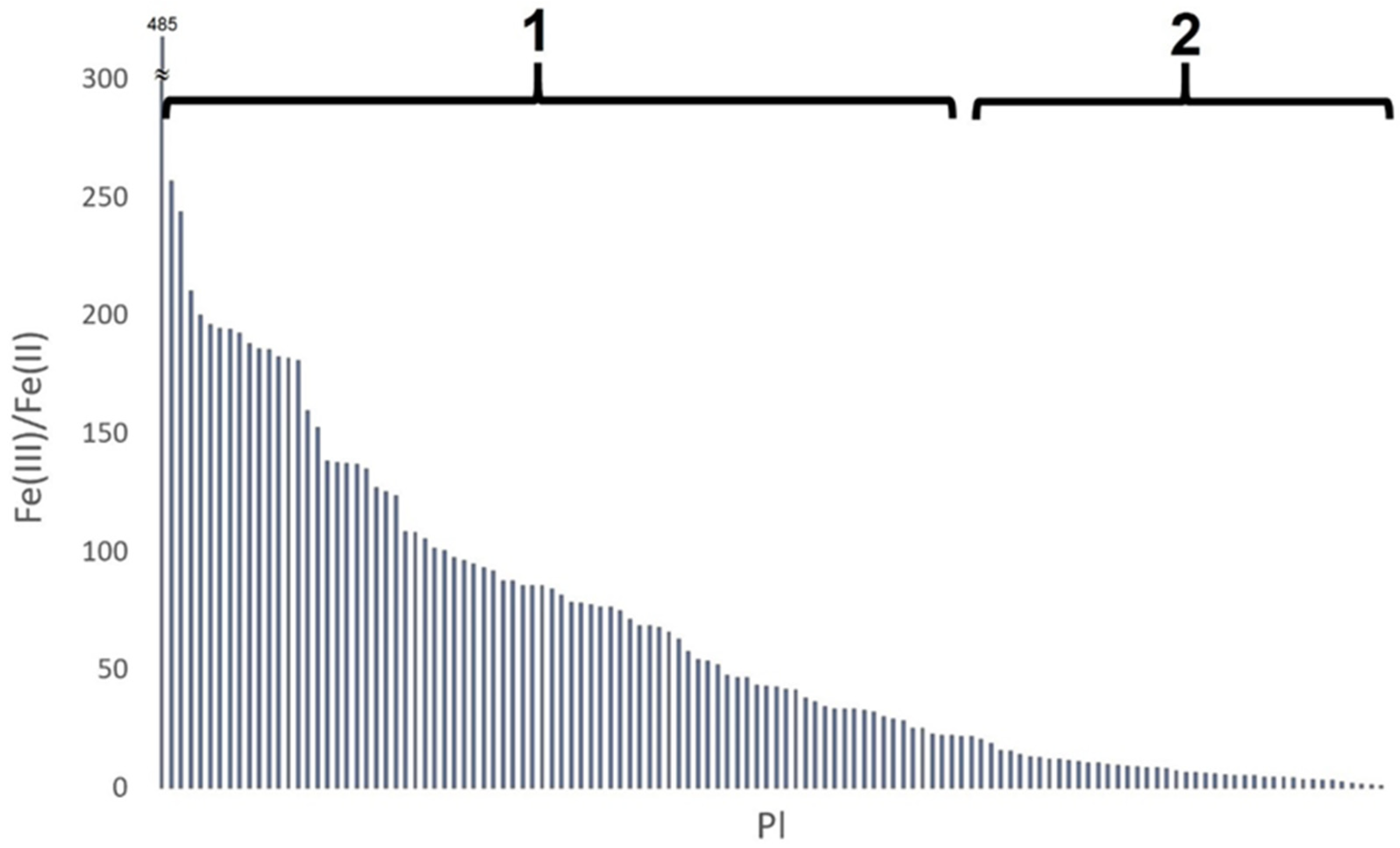
3.1.2. Iron Speciation in Pottery
3.2. Analysis of Pottery Fragments from One Site
3.2.1. Chemical Composition of Pottery
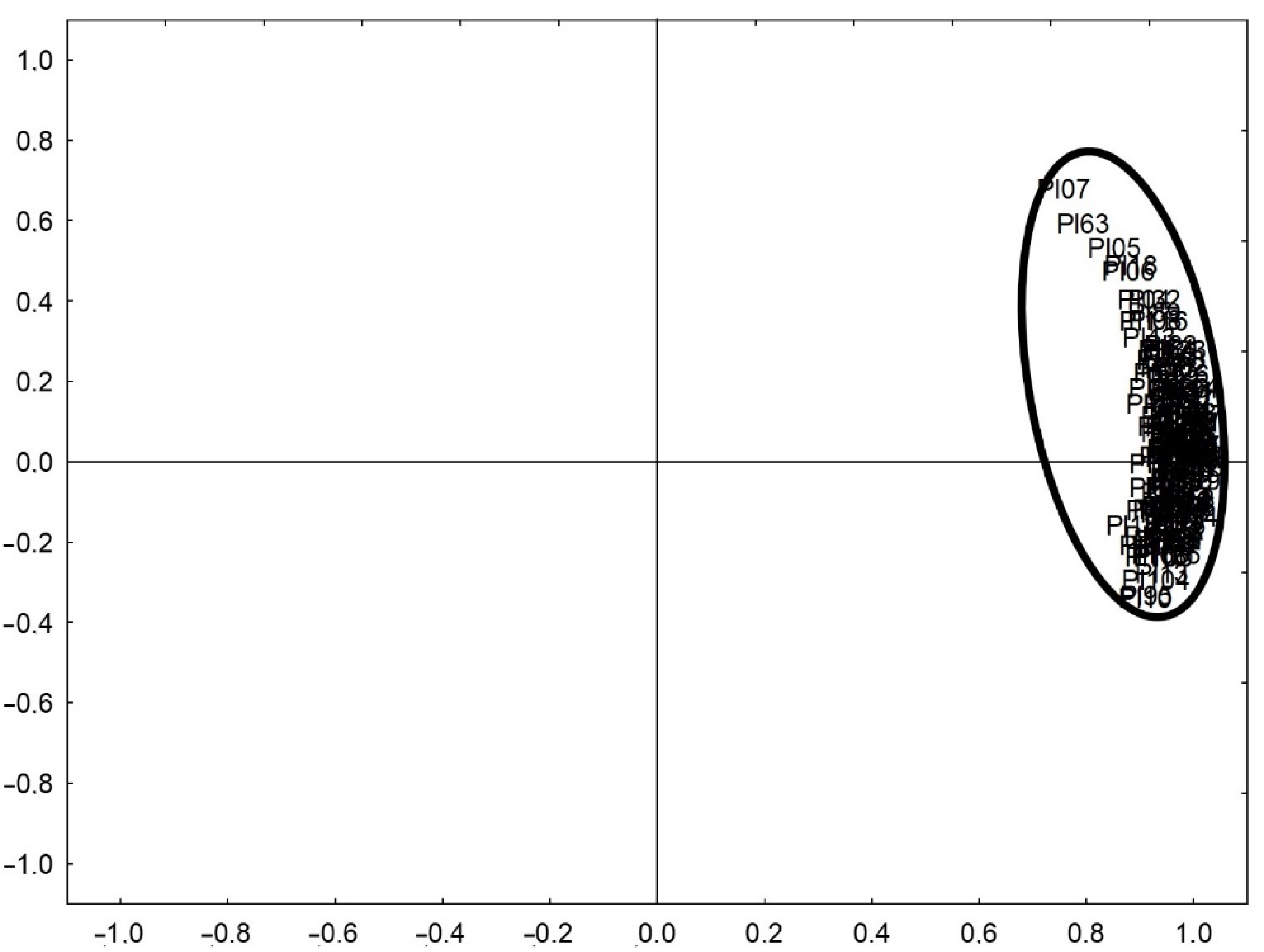
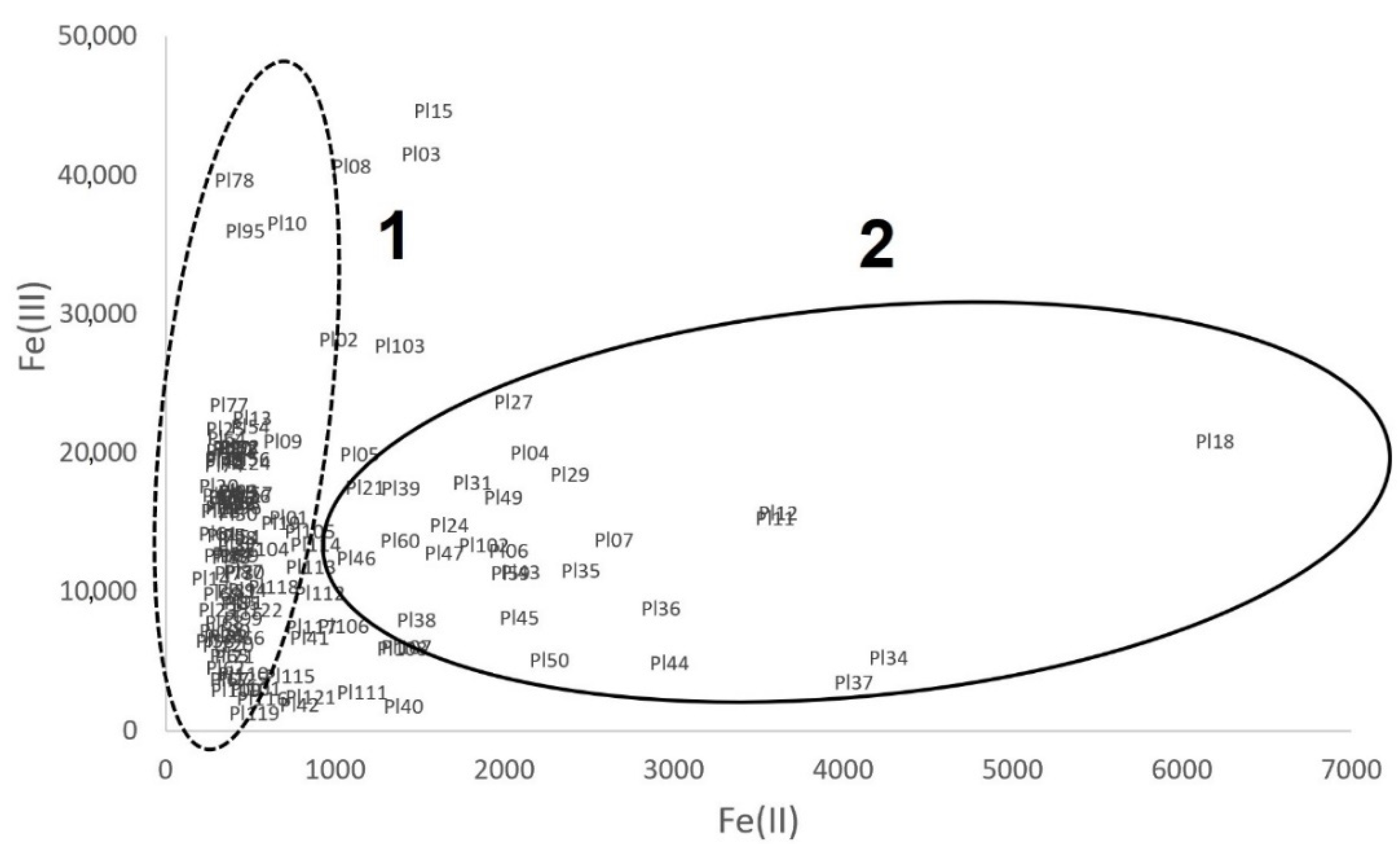
3.2.2. Iron Speciation in Pottery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shelach, G. On the invention of pottery. Science 2012, 336, 1644–1645. [Google Scholar] [CrossRef] [PubMed]
- Wagner, U.; Gebhard, R.; Grosse, G.; Hutzelmann, T.; Murad, E.; Riederer, J.; Shimada, I.; Wagner, F. Clay: An important raw material for prehistoric man. Hyperfine Interact. 1998, 117, 323–335. [Google Scholar] [CrossRef]
- Foster, W. Chemistry and Grecian archaeology. J. Chem. Educ. 1933, 10, 270. [Google Scholar] [CrossRef]
- Quarta, G.; Giorgia, A.; Ingravallo, E.; Tiberi, I.; Calcagnile, L. Radiocarbon dates and XRF analyses from two prehistoric contexts in the Badisco area (Otranto–Le). Measurement 2018, 125, 279–283. [Google Scholar] [CrossRef]
- Gójska, A.; Miśta-Jakubowska, E.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I.; Miśta, E. Archaeological applications of spectroscopic measurements. Compatibility of analytical methods in comparative measurements of historical Polish coins. Measurement 2018, 135, 869–874. [Google Scholar] [CrossRef]
- Ergenç, D.; Fort, R. Multi-technical characterization of Roman mortars from Complutum, Spain. Measurement 2019, 147, 106876. [Google Scholar] [CrossRef]
- Lezzerini, M.; Raneri, S.; Pagnotta, S.; Columbu, S.; Gallello, G. Archaeometric study of mortars from the Pisa’s Cathedral Square (Italy). Measurement 2018, 126, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Rosado, L.; Van Pevenage, J.; Vandenabeele, P.; Candeias, A.; Lopes, M.D.C.; Tavares, D.; Alfenim, R.; Schiavon, N.; Mirão, J. Multi-analytical study of ceramic pigments application in the study of Iron Age decorated pottery from SW Iberia. Measurement 2018, 118, 262–274. [Google Scholar] [CrossRef]
- Cano, N.F.; Ribeiro, R.B.; Munita, C.S.; Watanabe, S.; Neves, E.G.; Tamanaha, E.K. Dating and determination of firing temperature of ancient potteries from São Paulo II archaeological site, Brazil by TL and EPR techniques. J. Cult. Herit. 2015, 16, 361–364. [Google Scholar] [CrossRef]
- Ricciardi, P.; Nodari, L.; Gualtieri, S.; De Simone, D.; Fabbri, B.; Russo, U. Firing techniques of black slipped pottery from Nepal (12th–3rd century B.C.): The role of Mossbauer spectroscopy. J. Cult. Herit. 2008, 9, 261–268. [Google Scholar] [CrossRef]
- Venkatachalapathy, R.; Sridharan, T.; Dhanapandian, S.; Manoharan, C. Determination of firing temperature of ancient potteries by means of infrared and Mossbauer studies. Spectrosc. Lett. 2002, 35, 769–779. [Google Scholar] [CrossRef]
- Meloni, S.; Oddone, M.; Genova, N.; Cairo, A. The production of ceramic materials in Roman Pavia: An archaeometric NAA investigation of clay sources and archaeological artifacts. J. Radioanal. Nucl. Chem. 2000, 244, 553–558. [Google Scholar] [CrossRef]
- Bardelli, F.; Barone, G.; Crupi, V.; Longo, F.; Maisano, G.; Majolino, D.; Mazzoleni, P.; Venuti, V. Iron speciation in ancient Attic pottery pigments: A non-destructive SR-XAS investigation. J. Synchrotron Radiat. 2012, 19, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Doménech-Carbó, A.; Doménech Carbó, M.; Costa, V. Electrochemical Methods in Archaeometry, Conservation and Restoration; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bardelli, F.; Barone, G.; Crupi, V.; Longo, F.; Majolino, D.; Mazzoleni, P.; Venuti, V. Combined non-destructive XRF and SR-XAS study of archaeological artefacts. Anal. Bioanal. Chem. 2011, 399, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Nakai, I.; Taguchi, I.; Yamasaki, K. Chemical speciation of archaeological objects by XRF/XANES analysis using synchrotron radiation. Anal. Sci. 1991, 7, 365–368. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.; Silva, T.; Veiga, J. A XANES study of cobalt speciation state in blue-and-white glazes from 16th to 17th century Chinese porcelains. J. Electron Spectrosc. Relat. Phenom. 2012, 185, 97–102. [Google Scholar] [CrossRef]
- Floresta, D.L.; Ardisson, J.D.; Fagundes, M.; Fabris, J.D.; Macedo, W.A.A. Oxidation states of iron as an indicator of the techniques used to burn clays and handcraft archaeological Tupiguarani ceramics by ancient human groups in Minas Gerais, Brazil. Hyperfine Interact. 2013, 224, 121–129. [Google Scholar] [CrossRef]
- Stewart, J.D.; Adams, K.R. Evaluating visual criteria for identifying carbon- and iron-based pottery paints from the four corners region using SEM-EDS. Am. Antiq. 1999, 64, 675–696. [Google Scholar] [CrossRef]
- Orecchio, S. Speciation studies of iron in ancient pots from Sicily (Italy). Microchem. J. 2011, 99, 132–137. [Google Scholar] [CrossRef]
- Begheijn, L.T. Determination of iron(II) in rock, soil and clay. Analyst 1979, 104, 1055–1061. [Google Scholar] [CrossRef]
- Frankel, D.; Webb, J.M. Pottery production and distribution in prehistoric Bronze Age Cyprus. An application of pXRF analysis. J. Archaeol. Sci. 2012, 39, 1380–1387. [Google Scholar] [CrossRef]
- Forster, N.; Grave, P.; Vickery, N.; Kealhofer, L. Non-destructive analysis using PXRF: Methodology and application to archaeological ceramics. X-Ray Spectrom. 2011, 40, 389–398. [Google Scholar] [CrossRef]
- Kozak, L.; Niedzielski, P. The evolution of December 2004 tsunami deposits: Temporal and spatial distribution of potentially toxic metalloids. Chemosphere 2013, 93, 1856–1865. [Google Scholar] [CrossRef] [PubMed]
- Niedzielski, P.; Kozak, L. Iron’s fingerprint of deposits-iron speciation as a geochemical marker. Environ. Sci. Pollut. Res. 2017, 25, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Niedzielski, P.; Zielińska-Dawidziak, M.; Kozak, L.; Kowalewski, P.; Szlachetka, B.; Zalicka, S.; Wachowiak, W. Determination of iron species in samples of iron-fortified food. Food Anal. Methods 2014, 7, 2023–2032. [Google Scholar] [CrossRef] [Green Version]
- Niedzielski, P.; Kozak, L.; Wachelka, M.; Jakubowski, K.; Wybieralska, J. The microwave induced plasma with optical emission spectrometry (MIP–OES) in 23 elements determination in geological samples. Talanta 2015, 132, 591–599. [Google Scholar] [CrossRef]
- Niedzielski, P.; Michałowski, A.; Teska, M.; Krzyżanowska, M.; Jakubowski, K.; Kozak, L.; Krueger, M.; Żółkiewski, M. The analysis of variability of chemical composition of ceramics for archaeometrical studies. In Kulturkonzepte und Konzipierte Kulturen; Michałowski, A., Schuster, J., Eds.; Dr. Rudolf Habelt GmbH: Bonn, Germany, 2018; pp. 199–211. [Google Scholar]
- Electronic Statistics Textbook. StatSoft 2020. Available online: http://www.statsoft.com/textbook/ (accessed on 20 January 2020).
- Moore, D.S. The Basic Practice of Statistics; Palgrave Macmillan: London, UK, 2010. [Google Scholar]
- Doménech-Carbó, A.; Sánchez-Ramosa, S.; Doménech-Carbó, M.; Gimeno-Adelantado, J.V.; Bosch-Reig, F.; Yusá-Marco, D.J.; Saurí-Peris, M.C. Electrochemical determination of the Fe(III)/Fe(II) ratio in archaeological ceramic materials using carbon paste and composite electrodes. Electroanalysis 2002, 14, 685–696. [Google Scholar] [CrossRef]
- Mangueira, G.; Toledo, R.; Teixeira, S.; Franco, R. Evaluation of archeothermometric methods in pottery using electron paramagnetic resonance spectra of iron. Appl. Clay Sci. 2013, 86, 70–75. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, L.; Michałowski, A.; Proch, J.; Krueger, M.; Munteanu, O.; Niedzielski, P. Iron Forms Fe(II) and Fe(III) Determination in Pre-Roman Iron Age Archaeological Pottery as a New Tool in Archaeometry. Molecules 2021, 26, 5617. https://doi.org/10.3390/molecules26185617
Kozak L, Michałowski A, Proch J, Krueger M, Munteanu O, Niedzielski P. Iron Forms Fe(II) and Fe(III) Determination in Pre-Roman Iron Age Archaeological Pottery as a New Tool in Archaeometry. Molecules. 2021; 26(18):5617. https://doi.org/10.3390/molecules26185617
Chicago/Turabian StyleKozak, Lidia, Andrzej Michałowski, Jedrzej Proch, Michal Krueger, Octavian Munteanu, and Przemyslaw Niedzielski. 2021. "Iron Forms Fe(II) and Fe(III) Determination in Pre-Roman Iron Age Archaeological Pottery as a New Tool in Archaeometry" Molecules 26, no. 18: 5617. https://doi.org/10.3390/molecules26185617
APA StyleKozak, L., Michałowski, A., Proch, J., Krueger, M., Munteanu, O., & Niedzielski, P. (2021). Iron Forms Fe(II) and Fe(III) Determination in Pre-Roman Iron Age Archaeological Pottery as a New Tool in Archaeometry. Molecules, 26(18), 5617. https://doi.org/10.3390/molecules26185617







