Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results
2.1. Chemical Identification of the Wheat Grain Metabolites
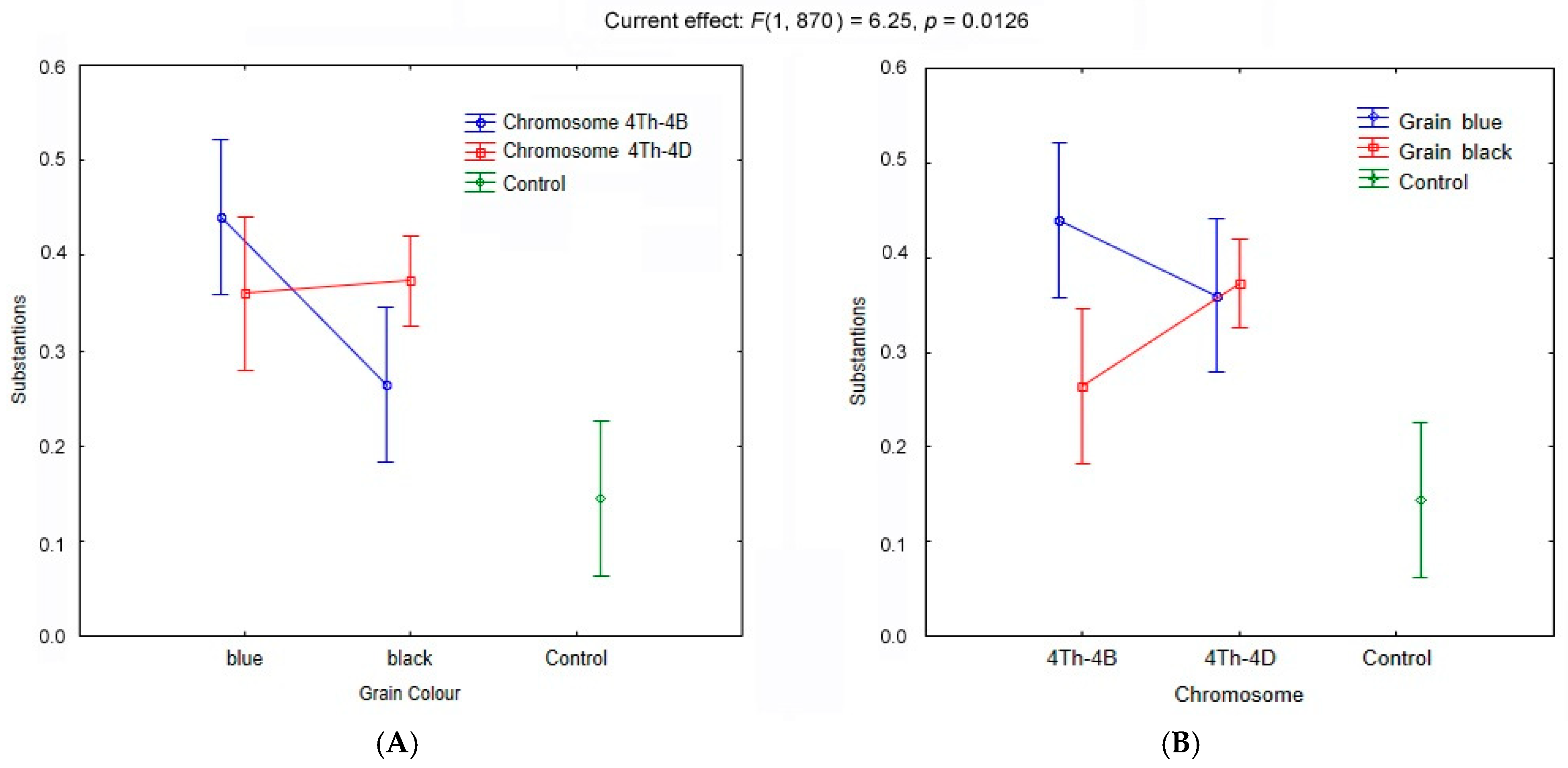
2.2. Similarities and Differences in Metabolites among the Lines
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemicals and Reagents
4.3. Fractional Maceration
4.4. Liquid Chromatography
4.5. MS
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Identified Compound | Molecular Formula | Calculated Mass | Observed Mass | Fragment Ions, m/z | References | |
|---|---|---|---|---|---|---|---|
| [M − H]– | [M + H]+ | ||||||
| Anthocyanin | |||||||
| 1 | Cyanidin 3-(2″-galloylglucoside) | C28H25O15+ | 601.4891 | 602 | 592; 556; 429; 349; 287; 231 | [59] | |
| 2 | Cyanidin-3-O-3″,6″-O-Dimalonylglucoside | C27H25O17 | 621.4772 | 622 | 612,395, 328, 287, 221 | [31] | |
| 3 | Cyanidin-3-O-glucoside [Cyanidin 3-O-β-d-Glucoside; Kuromarin] | C21H21O11+ | 449.3848 | 450 | 287; 213; 169; 115 | [31,54,60] | |
| 4 | Malvidin 3-O-rutinoside | C29H35O16 | 639.5786 | 640 | 493; 331; 315 | [31,61] | |
| 5 | Malvidin 3-O-rutinoside-5-O-glucoside | C35H45O21 | 801.7192 | 802 | 639; 493; 331 | [31,61] | |
| 6 | Peonidin 3-O-rutinoside | C28H33O15 | 609.5526 | 610 | 463; 343; 301; 285; 258 | [31,59,62] | |
| 7 | Peonidin 3-rutinoside-5-glucoside | C34H43O20 | 771.6932 | 772 | 705; 463; 367; 301 | [31] | |
| 8 | Peonidin-3-O-glucoside | C22H23O11 + | 463.4114 | 464 | 301; 445; 286; 258 | [31,59,63] | |
| 9 | Petunidin | C16H13O7 | 317.2702 | 318 | 300; 256; 238; 198 | [31,61] | |
| 10 | Petunidin 3-O-rutinoside-5-O-glucoside | C34H43O21 | 787.6926 | 788 | 625; 479; 317 | [31,61] | |
| Cinnamic Acid Derivative | |||||||
| 11 | Ferulic acid methyl ester | C11H12O4 | 208.2106 | 207 | 179; 135 | [64] | |
| Hydroxycinnamic Acid | |||||||
| 12 | 1-Caffeoyl-β-d-glucose [Caffeic acid-glucoside] | C15H18O9 | 342.298 | 341 | 161; 143 | [34,63] | |
| 13 | 1-O-Sinapoyl-β-d-glucose | C17H22O10 | 386.3576 | 387 | 205 | [63] | |
| 14 | Caffeic acid derivative | C16H18O9Na | 377.2985 | 377 | 341; 178; 143 | [65] | |
| 15 | Caftaric acid [Cis-Caftaric acid; 2-Caffeoyl-l-Tartaric acid] | C13H12O9 | 312.23 | 311 | 267; 293; 249; 193; 183; 167 | [37,49,63,66] | |
| 16 | Chlorogenic acid [3-O-Caffeoylquinic acid] | C16H18O9 | 354.3087 | 355 | 337; 319; 301; 239; 222; 227 | [54,67] | |
| 17 | Ferulic acid | C10H10O4 | 194.184 | 195 | 137 | [54,68] | |
| Coumarin | |||||||
| 18 | Fraxetin | C10H8O5 | 208.1675 | 207 | 179; 161; 135; 117 | [69,70,71] | |
| 19 | Fraxetin-7-O-sulfate | C10H8O8S | 288.2307 | 287 | 207; 163; 119 | [69] | |
| Dihydrochalcone | |||||||
| 20 | Phlorizin [Phloridzin; Phlorizoside; Floridzin; Phloretin 2′-Glucoside] | C21H24O10 | 436.4093 | 437 | 275; 257; 203; 173; 150 | [37,53,55,72] | |
| Flavan-3-ol | |||||||
| 21 | Catechin [d-Catechol] | C15H14O6 | 290.2681 | 291 | 245; 203; 145 | [53,55,56,66,68,73,74,75,76] | |
| 22 | Epicatechin | C15H14O6 | 290.2681 | 291 | 273; 255; 213; 147; 127 | [37,53,63,68,75,76,77] | |
| 23 | Gallocatechin [+(−) Gallocatechin] | C15H14O7 | 306.2675 | 305 | 287; 249; 205; 151; 138; 125 | [37,66,73] | |
| Flavanone | |||||||
| 24 | Naringenin [Naringetol] | C15H12O5 | 272.5228 | 273 | 156; 191; 112 | [53,57,63,73,78] | |
| Flavone | |||||||
| 25 | 6-C-hexosyl-chrysoeriol O-rhamnoside-O-hexoside | C33H38O21 | 770.6422 | 771 | 753; 704; 687; 585; 529; 499; 427; 422; 385; 337; 207 | [27] | |
| 26 | Acacetin C-glucoside methylmalonylated | C26H26O13 | 546.4758 | 547 | 529; 511; 427; 301; 253; 172 | [79] | |
| 27 | Apigenin | C15H10O5 | 270.2369 | 271 | 252; 239; 226; 211 | [34] | |
| 28 | Apigenin 2″-O-sinapoyl, C-hexosyl, C-pentosyl | C37H38O18 | 770.6868 | 768 | 545; 425; 365; 335; 185 | [29] | |
| 29 | Apigenin 6,8-di-C-pentoside | C25H26O13 | 534.4661 | 535 | 499; 481; 415; 409; 307; 291 | [34,49,80] | |
| 30 | Apigenin 6-C-deoxyhexoside-8-C-pentoside | C26H28O13 | 548.4927 | 549 | 531; 465; 369; 319; 248 | [34] | |
| 31 | Apigenin-6-C-β-galactosyl-8-C-β-glycosyl-O-glycuronopyranoside | C33H38O21 | 770.6422 | 771 | 679; 651; 561; 511; 457; 367; 313; 297; 267; 249; 215; 207; 177; 121 | [28] | |
| 32 | Apigenin 8-C-hexoside-6-C-pentoside | C26H28O14 | 564.4921 | 565 | 547; 529; 511; 481; 427; 349; 325; 313 | [28,29,35] | |
| 33 | Apigenin 8-C-pentoside-6-C-hexoside | C26H28O14 | 564.4921 | 565 | 547; 529; 511; 427; 391; 325; 291; | [28,29,35] | |
| 34 | Chrysoeriol | C16H12O6 | 300.2629 | 301 | 286; 258; 229 | [33,60,81] | |
| 35 | Chrysoeriol C-hexoside-C-pentoside | C27H30O15 | 594.5181 | 595 | 577; 558; 499; 481; 427; 379; 327; 287 | [27,29,32,35] | |
| 36 | Cirsiliol | C17H14O7 | 330.2889 | 329 | 229; 211; 171; 155 | [52] | |
| 37 | Dihydroxy tetramethoxyflavanone | C19H20O8 | 376.3573 | 377 | 361; 323; 265; 179 | [37] | |
| 38 | Diosmetin [Luteolin 4′-Methyl Ether; Salinigricoflavonol] | C16H12O6 | 300.2629 | 301 | 286; 258; 177; 138 | [81,82,83] | |
| 39 | Genistein C-glucosylglucoside | C27H30O15 | 594.5181 | 595 | 577; 529; 457; 427; 302 | [79] | |
| 40 | Hydroxy dimethoxyflavone hexoside | C23H24O10 | 460.4307 | 461 | 301; 286; 258; 243 | [37] | |
| 41 | Luteolin | C15H10O6 | 286.2363 | 285 | 199; 151 | [33,36,50,52,73,84] | |
| 42 | Luteolin 8-C-Glucoside [Orientin; Orientin (Flavone); Lutexin] | C21H20O11 | 448.3769 | 449 | 431; 413; 356; 333; 290; 267; 233; 227 | [28,36,49,55] | |
| 43 | Luteolin 8-C-hexoside-6-C-pentoside | C26H28O15 | 580.4915 | 581 | 563; 515; 485; 413; 377; 342; 205 | [27,29,32,35] | |
| 44 | Luteolin 8-C-pentoside-6-C-hexoside | C26H28O15 | 580.4915 | 581 | 563; 528; 515; 496; 443; 413; 341; 335; 323; 181 | [28,29,33] | |
| 45 | Myricetin | C15H10O8 | 318.2351 | 317 | 273; 260; 238 | [37,38,63,73,85] | |
| 46 | Orientin 7-O-deoxyhexoside [Luteolin 8-C-glucoside 7-O-deoxyhexoside] | C27H30O15 | 594.5181 | 595 | 577; 528; 510; 438; 427; 325 | [34] | |
| 47 | Pentahydroxy dimethoxyflavone | C17H14O9 | 362.2877 | 363 | 345; 326; 247; 201; 155 | [37] | |
| 48 | Pentahydroxy dimethoxyflavone hexoside | C23H24O14 | 524.4283 | 525 | 463; 363; 257 | [37] | |
| 49 | Pentahydroxy trimethoxy flavone | C18H16O10 | 392.3136 | 393 | 375; 357; 328; 269; 230; 218 | [37] | |
| 50 | Tricin | C17H14O7 | 330.2889 | 331 | 315; 287; 285; 270; 229 | [28,33,36,54] | |
| 51 | Tetrahydroxy-dimethoxyflavone-hexoside [Syringetin-hexoside; dimethyl-myricetin-hexoside] | C23H24O13 | 508.4289 | 509 | 347; 329; 316; 265; 185; 181 | [38,49,80] | |
| 52 | Trihydroxy methoxyflavone triacetate | C18H18O9 | 378.3301 | 379 | 361; 321; 287; 234; 223; 167 | [37] | |
| 53 | Vicenin-2 [Apigenin-6,8-Di-C-Glucoside] | C27H30O15 | 594.5181 | 595 | 577; 559; 541; 529; 523; 499; 469; 439; 427; 391 | [28,29,30,34,35,50,55] | |
| 54 | Vitexin 2″-O-glucoside [Apigenin 8-C-glucoside 2″-O-glucoside] | C27H30O15 | 594.5181 | 595 | 577; 541; 457; 288 | [34] | |
| 55 | Vitexin 6″-O-glucoside [Apigenin 8-C-glucoside 6″-O-glucoside] | C27H30O15 | 594.5181 | 595 | 577; 559; 528; 511; 499; 493; 487; 445; 427 | [34] | |
| 56 | Wighteone-O-glucoside | C26H28O10 | 500.4945 | 501 | 339; 262; 185; 167 | [79] | |
| Flavonol | |||||||
| 57 | Ampelopsin [Dihydromyricetin; Ampeloptin] | C15H12O8 | 320.251 | 321 | 303; 285; 163 | [86,87] | |
| 58 | Isorhamnetin [Isorhamnetol; Quercetin 3′-Methyl ether; 3-Methylquercetin] | C16H12O7 | 316.2623 | 315 | 300; 272; 243; 145 | [38,53] | |
| 59 | Kaempferide | C16H12O6 | 300.2629 | 301 | 286; 258; 229; 174; 153 | [52,88] | |
| 60 | Kaempferol | C15H10O6 | 286.2363 | 287 | 268; 214; 196; 160; 123 | [52,63,73,89] | |
| 61 | Quercetin | C15H10O7 | 302.2357 | 301 | 283; 255; 227 | [38,53,54,57,63,68,73,76] | |
| 62 | Rhamnetin I [β-Rhamnocitrin; Quercetin 7-Methyl ether] | C16H12O7 | 316.2623 | 317 | 255; 197; 139; 122 | [86] | |
| 63 | Rhamnetin II | C16H12O7 | 316.2623 | 317 | 256; 121; 228; 111 | [86] | |
| 64 | Selgin [Selagin; 3′-O-Methyltricetin; Tricetin ′3-O-methyl ether] | C16H12O7 | 316.2623 | 317 | 315; 256; 161 | [90] | |
| 65 | Taxifolin-3-O-glucoside | C21H22O12 | 466.3922 | 467 | 449; 373; 258; 199; 177 | [63] | |
| 66 | Taxifolin-O-pentoside [Dihydroquercetin pentoside] | C20H20O11 | 436.371 | 437 | 303; 259; 177; 169 | [37] | |
| Gallotannin | |||||||
| 67 | β-Glucogallin [1-O-Galloyl-β-d-Glucose; Galloyl glucose] | C13H16O10 | 332.2601 | 331 | 313; 295; 277; 171; 140; 127 | [53,91] | |
| Hydroxybenzoic Acid | |||||||
| 68 | 4-Hydroxybenzoic acid [PHBA; Benzoic acid] | C7H6O3 | 138.1207 | 139 | 137; 121 | [35,49,63,74] | |
| 69 | Cis-salvianolic acid J | C27H22O12 | 538.4564 | 539 | 523; 481; 393; 360; 319; 247; 204; 191; 120 | [81] | |
| 70 | Hydroxy methoxy dimethylbenzoic acid | C10H12O4 | 196.1999 | 197 | 179; 160; 133 | [37] | |
| 71 | Salvianolic acid D | C20H18O10 | 418.3509 | 417 | 373; 329; 287 | [49,92] | |
| 72 | Salvianolic acid F | C17H14O6 | 314.2895 | 313 | 295; 277; 223; 171; 155 | [49,92] | |
| 73 | Salvianolic acid G | C18H12O7 | 340.2837 | 341 | 323; 260; 199; 168 | [81,92] | |
| Lignan | |||||||
| 74 | Dimethyl-secoisolariciresinol | C22H30O6 | 390.4700 | 391 | 355; 336; 308; 218; 149 | [93] | |
| 75 | Hinokinin | C20H18O6 | 354.3533 | 355 | 336; 318; 300; 207; 181; 177 | [28,30,54] | |
| 76 | Pinoresinol | C20H22O6 | 358.3851 | 357 | 339; 311; 267; 213; 197; 171; 155; 139 | [28,30,34] | |
| 77 | Podophyllotoxin [Podofilox; Condylox; Condyline; Podophyllinic acid lactone] | C22H22O8 | 414.4053 | 415 | 397; 379; 310; 275; 250; 182 | [93] | |
| 78 | Syringaresinol | C22H26O8 | 418.4436 | 419 | 357; 327; 275; 185; 158 | [54] | |
| Phenolic Acid | |||||||
| 79 | 1-O-caffeoyl-5-O-feruloylquinic acid | C26H26O12 | 530.4774 | 531 | 513; 415; 337; 195; 176; 115 | [52] | |
| 80 | 4-O-Caffeoyl-5-O-p-coumaroylquinic acid | C25H24O11 | 500.4515 | 501 | 339; 244; 189; 140 | [55,84] | |
| 81 | Feruloyl sulfate | C10H10O7S | 274.2472 | 273 | 193; 192; 149 | [94] | |
| 82 | Gallic acid hexoside | C13H16O10 | 332.2601 | 333 | 242; 212; 182; 159 | [95] | |
| Stilbene | |||||||
| 83 | Pinosylvin [3,5-Stilbenediol; Trans-3,5-Dihydroxystilbene] | C14H12O2 | 212.2439 | 213 | 197; 183; 166; 124 | [96] | |
| 84 | Polydatin [Piceid; trans-Piceid] | C20H22O8 | 390.3839 | 391 | 355; 333; 265; 227; 209; 145 | [73,77] | |
| 85 | Resveratrol [trans-Resveratrol; 3,4′,5-Trihydroxystilbene; Stilbentriol] | C14H12O3 | 228.2433 | 229 | 228; 142; 114 | [37,73] | |
| Other Compounds | |||||||
| 86 | Undecanedioic acid | C11H20O4 | 216.2741 | 217 | 173; 157; 142; 118; 115 | [34] | |
| 87 | Myristoleic acid [Cis-9-Tetradecanoic acid] | C14H26O2 | 226.3550 | 227 | 209; 138; 127; 110 | [34] | |
| 88 | 11-Hydroperoxy-octadecatrienoic acid | C18H30O4 | 310.4284 | 309 | 291; 209; 207; 125 | [97] | |
| 89 | 9,10-Dihydroxy-8-oxooctadec-12-enoic acid [oxo-DHODE; oxo-Dihydroxy-octadecenoic acid] | C18H32O5 | 328.4437 | 327 | 229; 211; 171; 135; 125 | [35,36] | |
| 90 | Dihydroxy docosanoic acid | C22H44O4 | 372.5824 | 371 | 327; 297; 282; 251; 187; 125 | [34] | |
| 91 | Docosenoic acid [2-Docosenoic acid]) | C22H42O2 | 338.5677 | 339 | 322; 295; 256; 215; 163 | [34] | |
| 92 | Hydroxy methoxy dimethylbenzoic acid | C10H12O4 | 196.1999 | 195 | 177; 129 | [34] | |
| 93 | Pentacosenoic acid | C25H48O2 | 380.6474 | 381 | 363; 293; 173; 135 | [34] | |
| 94 | Salvianic acid C | C18H18O9 | 378.3301 | 379 | 361; 343; 335; 326; 247; 237; 205; 151; 129 | [92] | |
| 95 | Vebonol | C30H44O3 | 452.6686 | 453 | 435; 336; 209 | [86] | |
| 96 | Cyclopassifloic acid glucoside | C37H62O12 | 698.8810 | 699 | 537; 421; 348; 203 | [31] | |
| 97 | (3S, 3′S, all-E)-zeaxanthin [Zeaxanthin; (3S,3′S)-Zeaxanthin] | C40H56O2 | 568.8714 | 569 | 551; 375; 329; 279; 235; 210; 153 | [51] | |
| 98 | Cryptoxanthin [β-cryptoxanthin] | C40H56O | 552.872 | 553 | 461; 337; 199 | [51] | |
| 99 | Isocryptotanshinone II | C19H20O3 | 296.3603 | 297 | 279; 149; 146 | [98] | |
| 100 | Tanshinone IIB [(S)-6-(Hydroxymethyl)-1,6-Dimethyl-6,7,8,9-Tetrahydrophenanthro [1,2-B]Furan-10,11-Dione] | C19H18O4 | 310.3438 | 309 | 291; 273; 251; 235; 209; 207; 122 | [98] | |
| 101 | β-Amyrin [β-Amyrenol; Amyrin; Olean-12-en-3β-ol] | C30H50O | 426.7174 | 427 | 409; 391; 373; 292; 269; 240; 190; 145; 137 | [34] | |
| 102 | Gibberellic acid | C19H22O6 | 346.3744 | 347 | 301; 282; 263; 242; 201; 185; 139 | [99] | |
| 103 | Betunolic acid | C30H46O3 | 454.3446 | 455 | 436; 355; 236; 226 | [86] | |
| 104 | Ursolic acid | C30H48O3 | 456.7003 | 457 | 439; 263; 177; 145 | [52,56,81,100] | |
| 105 | Squalene (Trans-Squalene; Spinacene; Supraene) | C30H50 | 410.718 | 411 | 235; 218; 177; 147 | [101,102] | |
| 106 | Uvaol | C30H50O2 | 442.7168 | 443 | 425; 407; 315; 304; 287; 230; 154; 137 | [34] | |
| 107 | l-Histidine | C6H9N3O2 | 155.1546 | 156 | … | [103] | |
| 108 | l-Tryptophan [Tryptophan; (S)-Tryptophan] | C11H12N2O2 | 204.2252 | 205 | 188; 146; 118 | [34,91,104] | |
| 109 | l-Valine | C5H11NO2 | 117.1463 | 118 | … | [103] | |
| 110 | Tyrosine [(2S)-2-Amino-3-(4-Hydroxyphnyl) Propanoic acid] | C9H11NO3 | 181.19 | 182 | 155; 127; 116 | [104] | |
| 111 | Sespendole | C33H45NO4 | 519.7147 | 520 | 184; 125 | [86] | |
| 112 | Berberine [Berberin; Umbelletine; Berbericine] | C20H18NO4 | 336.3612 | 337 | 320; 303; 207; 206; 115 | [105] | |
| 113 | GA8-hexose gibberellin | C25H34O12 | 526.5303 | 527 | 365; 305; 275; 245; 203; 143 | [91] | |
| 114 | Abscisic acid [Dormin; Abscisin II; (S)-(+)-Abscisic acid] | C15H20O4 | 264.3169 | 265 | 247; 122 | [99] | |
| 115 | Ketoprofen [Orudis; 2-(3-Benzoylphenyl) Propionic acid; Profenid] | C16H14O3 | 254.2806 | 253 | 209; 191; 165; 121 | [106] | |
| 116 | Adenosine | C10H13N5O4 | 267.2413 | 268 | 136 | [103] | |
| 117 | Ergosterol [Provitamin D2; Ergosterin] | C28H44O | 396.6484 | 397 | 379; 361; 309; 282; 239; 189; 125 | [34] | |
| 118 | Avenasterol [Delta7-Avenasterol; 7-Dehydroavenasterol] | C29H48O | 412.6908 | 413 | 395; 376; 358; 336; 325; 271; 269; 251; 225; 224; 201; 165; 159; 124 | [34] | |
| 119 | β-Sitostenone [Stigmast-4-En-3-One; Sitostenone] | C29H48O | 412.6908 | 413 | 493; 375; 358; 269; 261; 235; 152; 147 | [34] | |
| 120 | β-Sitosterin [β-Sitosterol] | C29H50O | 414.7067 | 415 | 395; 377; 297; 268; 213; 163 133; | [37,100] | |
| 121 | Campestenone | C28H46O | 398.6642 | 399 | 337; 319; 311; 266; 239; 189; 182; 127 | [34] | |
| 122 | Fucosterol [Fucostein; Trans-24-Ethylidenecholesterol] | C29H48O | 412.6908 | 413 | 395; 375; 355; 340; 303; 267; 201; 195; 167; 121 | [34] | |
| 123 | Oxo-hydroxy sitosterol | C29H48O3 | 444.6896 | 445 | 427; 385; 319; 205; 165; 164; 137 | [34] | |
| 124 | Vapiprost | C30H39NO4 | 477.6350 | 478 | 337; 121; 263 | [86] | |
| 125 | Hexadecatrienoic acid [Hexadeca-2,4,6-trienoic acid] | C16H26O2 | 250.3764 | 251 | 233; 204; 147 | [34] | |
References
- Loskutov, I.G.; Khlestkina, E.K. Wheat, Barley, and Oat Breeding for Health Benefit Components in Grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I. Anthocyanin composition in black, blue, pink, purple, and red cereal grains. J. Agr. Food Chem. 2006, 54, 4696–4704. [Google Scholar] [CrossRef] [PubMed]
- Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Tikhonova, M.A.; Shoeva, O.Y.; Tenditnik, M.V.; Ovsyukova, M.V.; Akopyan, A.A.; Dubrovina, N.I.; Amstislavskaya, T.G.; Khlestkina, E.K. Evaluating the Effects of Grain of Isogenic Wheat Lines Differing in the Content of Anthocyanins in Mouse Models of Neurodegenerative Disorders. Nutrients 2020, 12, 3877. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Žilić, S.; Serpen, A.; Akıllıoǧlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Zeven, A. Wheats with purple and blue grains: A review. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Khlestkina, E. Genes determining the coloration of different organs in wheat. Russ. J. Genet. Appl. Res. 2013, 3, 54–65. [Google Scholar] [CrossRef]
- Khlestkina, E.; Shoeva, O.Y.; Gordeeva, E. Flavonoid biosynthesis genes in wheat. Russ. J. Genet. Appl. Res. 2015, 5, 268–278. [Google Scholar] [CrossRef]
- Himi, E.; Maekawa, M.; Miura, H.; Noda, K. Development of PCR markers for Tamyb10 related to R-1, red grain color gene in wheat. Theoret. Appl. Genet. 2011, 122, 1561–1576. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, J.; Zhuang, L.; Wang, Y.; Pu, J.; Feng, Y.; Chu, C.; Wang, X.; Qi, Z. Physical localization of a novel blue-grained gene derived from Thinopyrum bessarabicum. Mol. Breed. 2013, 31, 195–204. [Google Scholar] [CrossRef]
- Dubcovsky, J.; Luo, M.-C.; Zhong, G.-Y.; Bransteitter, R.; Desai, A.; Kilian, A.; Kleinhofs, A.; Dvořák, J. Genetic map of diploid wheat, Triticum monococcum L. and its comparison with maps of Hordeum vulgare L. Genetics 1996, 143, 983–999. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, B.; Mu, S.; Zhou, H.; Li, Z. Physical mapping of the blue-grained gene(s) from Thinopyrum ponticum by GISH and FISH in a set of translocation lines with different seed colors in wheat. Genome 2006, 49, 1109–1114. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Hart, G.E.; Devos, K.M.; Gale, M.D.; Rogers, W.J. Catalogue of gene symbols for wheat. In Proceedings of the 9th International Wheat Genetics Symposium; Slinkard, A.E., Ed.; University Extension Press, University of Saskatchewan: Saskatoon, SK, Canada, 1998; pp. 1–235. [Google Scholar]
- Dobrovolskaya, O.; Arbuzova, V.; Lohwasser, U.; Röder, M.; Börner, A. Microsatellite mapping of complementary genes for purple grain colour in bread wheat (Triticum aestivum) L. Euphytica 2006, 150, 355–364. [Google Scholar] [CrossRef]
- Usenko, N.I.; Khlestkina, E.K.; Asavasanti, S.; Gordeeva, E.I.; Yudina, R.S.; Otmakhova, Y.S. Possibilities of enriching food products with anthocyanins by using new forms of cereals. Foods Raw Mater. 2018, 6, 128–135. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilov J. Genet. Breed. 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlová, B.; Ošťádalová, M.; Šmejkal, K.; Vovk, I.; Ulrih, N.P. Anthocyanins in purple and blue wheat grains and in resulting bread: Quantity, composition, and thermal stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gambacorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovanniello, V.; Del Nobile, M.A.; Padalino, L.; Lecce, L.; Borrelli, G.M.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamel, T.H.; Wright, A.J.; Pickard, M.; Abdel-Aal, E.S.M. Characterization of anthocyanin-containing purple wheat prototype products as functional foods with potential health benefits. Cereal Chem. 2020, 97, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.-S.M.; Hucl, P. Composition and stability of anthocyanins in blue-grained wheat. J. Agric. Food Chem. 2003, 51, 2174–2180. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Devanand, L.L.; Lu, Y.; John, K.M.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar]
- Gordeeva, E.; Badaeva, E.; Yudina, R.; Shchukina, L.; Shoeva, O.; Khlestkina, E. Marker-assisted development of a blue-grained substitution line carrying the Thinopyrum ponticum chromosome 4Th(4D) in the spring bread wheat Saratovskaya 29 background. Agronomy 2019, 9, 723. [Google Scholar] [CrossRef] [Green Version]
- Gordeeva, E.; Badaeva, E.; Adonina, I.; Khlestkina, E.; Shoeva, O.Y. Marker-based development of wheat near-isogenic and substitution lines with high anthocyanin content in grains. In Current Challenges in Plant Genetics, Genomics, Bioinformatics, and Biotechnology: Proceedings of the Fifth International Scientific Conference PlantGen2019; Kochetov, A., Salina, E., Eds.; Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2019; p. 82. [Google Scholar]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Laganà, A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 3143–3158. [Google Scholar] [CrossRef]
- Dinelli, G.; Segura-Carretero, A.; Di Silvestro, R.; Marotti, I.; Arráez-Román, D.; Benedettelli, S.; Ghiselli, L.; Fernadez-Gutierrez, A. Profiles of phenolic compounds in modern and old common wheat varieties determined by liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2011, 1218, 7670–7681. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef] [Green Version]
- Leoncini, E.; Prata, C.; Malaguti, M.; Marotti, I.; Segura-Carretero, A.; Catizone, P.; Dinelli, G.; Hrelia, S. Phytochemical profile and nutraceutical value of old and modern common wheat cultivars. PLoS ONE 2012, 7, e45997. [Google Scholar] [CrossRef] [Green Version]
- Garg, M.; Chawla, M.; Chunduri, V.; Kumar, R.; Sharma, S.; Sharma, N.K.; Kaur, N.; Kumar, A.; Mundey, J.K.; Saini, M.K. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Stallmann, J.; Schweiger, R.; Pons, C.A.; Müller, C. Wheat growth, applied water use efficiency and flag leaf metabolome under continuous and pulsed deficit irrigation. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef]
- Ozarowski, M.; Piasecka, A.; Paszel-Jaworska, A.; de Chaves, D.S.A.; Romaniuk, A.; Rybczynska, M.; Gryszczynska, A.; Sawikowska, A.; Kachlicki, P.; Mikolajczak, P.L. Comparison of bioactive compounds content in leaf extracts of Passiflora incarnata, P. caerulea and P. alata and in vitro cytotoxic potential on leukemia cell lines. Rev. Bras. Farmacogn. 2018, 28, 179–191. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop. Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Van Hoyweghen, L.; De Bosscher, K.; Haegeman, G.; Deforce, D.; Heyerick, A. In vitro inhibition of the transcription factor NF-κB and cyclooxygenase by Bamboo extracts. Phytother. Res. 2014, 28, 224–230. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of chemopreventive components from halophytes belonging to Aizoaceae and Cactaceae through LC/MS—Bioassay guided approach. J. Chromatogr. Sci. 2020, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Rafsanjany, N.; Senker, J.; Brandt, S.; Dobrindt, U.; Hensel, A. In vivo consumption of cranberry exerts ex vivo antiadhesive activity against FimH-dominated uropathogenic Escherichia coli: A combined in vivo, ex vivo, and in vitro study of an extract from Vaccinium macrocarpon. J. Agric. Food Chem. 2015, 63, 8804–8818. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yang, M.; I Koo, S.; O Song, W.; K Chun, O. Food matrix affecting anthocyanin bioavailability. Curr. Med. Chem. 2011, 18, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Lucioli, S. Chapter 3: Anthocyanins: Mechanism of action and therapeutic efficacy. In Medicinal Plants as Antioxidant Agents: Understanding Their Mechanism of Action and Therapeutic Efficacy; Capasso, A., Ed.; Research Signpost: Kerala, India, 2012; pp. 27–57. ISBN 97881-308-0509-2. [Google Scholar]
- Matsumoto, H.; Nakamura, Y.; Tachibanaki, S.; Kawamura, S.; Hirayama, M. Stimulatory effect of cyanidin 3-glycosides on the regeneration of rhodopsin. J. Agric. Food Chem. 2003, 51, 3560–3563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef]
- Cvorovic, J.; Tramer, F.; Granzotto, M.; Candussio, L.; Decorti, G.; Passamonti, S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch. Biochem. Biophys. 2010, 501, 151–157. [Google Scholar] [CrossRef]
- Hou, D.-X. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 2003, 3, 149–159. [Google Scholar] [CrossRef]
- Gordeeva, E.; Shamanin, V.; Shoeva, O.; Kukoeva, T.; Morgounov, A.; Khlestkina, E. The strategy for marker-assisted breeding of anthocyanin-rich spring bread wheat (Triticum aestivum L.) cultivars in Western Siberia. Agronomy 2020, 10, 1603. [Google Scholar] [CrossRef]
- Arbuzova, V.; Maystrenko, O.; Popova, O. Development of near-isogenic lines of the common wheat cultivar ‘Saratovskaya 29′. Cereal Res. Commun. 1998, 26, 39–46. [Google Scholar] [CrossRef]
- Tereshchenko, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Börner, A.; Khlestkina, E.K. The D Genome Carries a Gene Determining Purple Grain Colour in Wheat. Cereal Res. Commun. 2012, 40, 334–341. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [Green Version]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.-S.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar]
- Mercadante, A.Z.; Rodrigues, D.B.; Petry, F.C.; Mariutti, L.R.B. Carotenoid esters in foods-A review and practical directions on analysis and occurrence. Food Res. Int. 2017, 99, 830–850. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Kumar, B. HPLC–QTOF–MS/MS-based rapid screening of phenolics and triterpenic acids in leaf extracts of Ocimum species and their interspecies variation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 225–238. [Google Scholar] [CrossRef]
- Santos, S.A.; Vilela, C.; Freire, C.S.; Neto, C.P.; Silvestre, A.J. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from Eucalyptus wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sandhir, R.; Singh, A.; Kumar, P.; Mishra, A.; Jachak, S.; Singh, S.P.; Singh, J.; Roy, J. Comparative analysis of phenolic compound characterization and their biosynthesis genes between two diverse bread wheat (Triticum aestivum) varieties differing for chapatti (unleavened flat bread) quality. Front. Plant Sci. 2016, 7, 1870. [Google Scholar] [CrossRef] [Green Version]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Tao, S.; Zhang, S. Characterization and quantification of polyphenols and triterpenoids in thinned young fruits of ten pear varieties by UPLC-Q TRAP-MS/MS. Molecules 2019, 24, 159. [Google Scholar] [CrossRef] [Green Version]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remon, A.; Lamuela-Raventós, R.M. Evaluation of a method to characterize the phenolic profile of organic and conventional tomatoes. J. Agric. Food Chem. 2012, 60, 3373–3380. [Google Scholar] [CrossRef] [PubMed]
- StatSoft, Inc. STATISTICA (Data Analysis Software System), Version 10: New Features and Enhancements; StatSoft: Tulsa, OK, USA, 2011. [Google Scholar]
- Ruiz, A.; Hermosín-Gutiérrez, I.; Vergara, C.; von Baer, D.; Zapata, M.; Hitschfeld, A.; Obando, L.; Mardones, C. Anthocyanin profiles in south Patagonian wild berries by HPLC-DAD-ESI-MS/MS. Food Res. Int. 2013, 51, 706–713. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, A.; Hermosin-Gutierrez, I.; Mardones, C.; Vergara, C.; Herlitz, E.; Vega, M.; Dorau, C.; Winterhalter, P.; von Baer, D. Polyphenols and antioxidant activity of calafate (Berberis microphylla) fruits and other native berries from Southern Chile. J. Agric. Food Chem. 2010, 58, 6081–6089. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, P.C.; Saha, S. Anthocyanin profiling of Berberis lycium Royle berry and its bioactivity evaluation for its nutraceutical potential. J. Food Sci. Technol. 2016, 53, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Thomford, N.E.; Dzobo, K.; Chopera, D.; Wonkam, A.; Maroyi, A.; Blackhurst, D.; Dandara, C. In vitro reversible and time-dependent CYP450 inhibition profiles of medicinal herbal plant extracts Newbouldia laevis and Cassia abbreviata: Implications for herb-drug interactions. Molecules 2016, 21, 891. [Google Scholar] [CrossRef] [Green Version]
- El-sayed, M.; Abbas, F.A.; Refaat, S.; El-Shafae, A.M.; Fikry, E. UPLC-ESI-MS/MS Profile of The Ethyl Acetate Fraction of Aerial Parts of Bougainvillea’Scarlett O’Hara’Cultivated in Egypt. Egypt. J. Chem. 2021, 64, 6–7. [Google Scholar]
- Yasir, M.; Sultana, B.; Anwar, F. LC–ESI–MS/MS based characterization of phenolic components in fruits of two species of Solanaceae. J. Food Sci. Technol. 2018, 55, 2370–2376. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.; Domingues, M.R.M.; Silvestre, A.J.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Abeywickrama, G.; Debnath, S.C.; Ambigaipalan, P.; Shahidi, F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016, 64, 9342–9351. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukui, M.; Nakazawa, T.; Hoshikawa, A.; Ohsawa, K. Metabolic Fate of Fraxin Administered Orally to Rats. J. Nat. Prod. 2006, 69, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiao, B.; Hao, Z.; Sun, Z. Simultaneous determination of fraxin and its metabolite, fraxetin, in rat plasma by liquid chromatography-tandem mass spectrometry and its application in a pharmacokinetic study. J. Chromatogr. B 2016, 1017, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, W.; Liu, H.; Wu, G.; Song, M.; Yang, B.; Yang, D.; Wang, Q.; Kuang, H. Simultaneous Determination of Aesculin, Aesculetin, Fraxetin, Fraxin and Polydatin in Beagle Dog Plasma by UPLC-ESI-MS/MS and Its Application in a Pharmacokinetic Study after Oral Administration Extracts of Ledum palustre L. Molecules 2018, 23, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Wang, Y.; Yang, M.; Cao, J.; Khan, A.; Cheng, G. UHPLC-ESI-HRMS/MS analysis on phenolic compositions of different E Se tea extracts and their antioxidant and cytoprotective activities. Food Chem. 2020, 318, 126512. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Pandey, A.K.; Singh, K.; Upadhyay, S.K. Molecular characterization and global expression analysis of lectin receptor kinases in bread wheat (Triticum aestivum). PLoS ONE 2016, 11, e0153925. [Google Scholar]
- Schoedl, K.; Forneck, A.; Sulyok, M.; Schuhmacher, R. Optimization, in-house validation, and application of a liquid chromatography–tandem mass spectrometry (LC–MS/MS)-based method for the quantification of selected polyphenolic compounds in leaves of grapevine (Vitis vinifera L.). J. Agric. Food Chem. 2011, 59, 10787–10794. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Vedova, A.D.; Gardiman, M.; Flamini, R. Characterization of Non-Anthocyanic Flavonoids in Some Hybrid Red Grape Extracts Potentially Interesting for Industrial Uses. Molecules 2015, 20, 18095–18106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paudel, L.; Wyzgoski, F.J.; Scheerens, J.C.; Chanon, A.M.; Reese, R.N.; Smiljanic, D.; Wesdemiotis, C.; Blakeslee, J.J.; Riedl, K.M.; Rinaldi, P.L. Nonanthocyanin secondary metabolites of black raspberry (Rubus occidentalis L.) fruits: Identification by HPLC-DAD, NMR, HPLC-ESI-MS, and ESI-MS/MS analyses. J. Agric. Food Chem. 2013, 61, 12032–12043. [Google Scholar] [CrossRef] [PubMed]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2020, 25, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojakowska, A.; Piasecka, A.; García-López, P.M.; Zamora-Natera, F.; Krajewski, P.; Marczak, Ł.; Kachlicki, P.; Stobiecki, M. Structural analysis and profiling of phenolic secondary metabolites of Mexican lupine species using LC–MS techniques. Phytochemistry 2013, 92, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD–ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Xu, L.-L.; Xu, J.-J.; Zhong, K.-R.; Shang, Z.-P.; Wang, F.; Wang, R.-F.; Zhang, L.; Zhang, J.-Y.; Liu, B. Analysis of non-volatile chemical constituents of Menthae Haplocalycis herba by ultra-high performance liquid chromatography-high resolution mass spectrometry. Molecules 2017, 22, 1756. [Google Scholar] [CrossRef] [Green Version]
- Di Loreto, A.; Bosi, S.; Montero, L.; Bregola, V.; Marotti, I.; Sferrazza, R.E.; Dinelli, G.; Herrero, M.; Cifuentes, A. Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis 2018, 39, 2001–2010. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, P.; Zhang, X.; Zhang, Q.; Yang, H.; Shi, H.; Zhang, L. LC–MS/MS determination and pharmacokinetic study of seven flavonoids in rat plasma after oral administration of Cirsium japonicum DC. extract. J. Ethnopharmacol. 2014, 158, 66–75. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Gordon, A.; Schadow, B.; Quijano, C.E.; Marx, F. Chemical characterization and antioxidant capacity of berries from Clidemia rubra (Aubl.) Mart. (Melastomataceae). Food Res. Int. 2011, 44, 2120–2127. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera Royle isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef]
- Xu, X.; Yang, B.; Wang, D.; Zhu, Y.; Miao, X.; Yang, W. The Chemical Composition of Brazilian Green Propolis and Its Protective Effects on Mouse Aortic Endothelial Cells against Inflammatory Injury. Molecules 2020, 25, 4612. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, T.; Li, P.; Liu, R.; Li, Q.; Bi, K. Development of two step liquid–liquid extraction tandem UHPLC–MS/MS method for the simultaneous determination of Ginkgo flavonoids, terpene lactones and nimodipine in rat plasma: Application to the pharmacokinetic study of the combination of Ginkgo biloba dispersible tablets and Nimodipine tablets. J. Chromatogr. B 2016, 1028, 33–41. [Google Scholar]
- Zhou, J.-M.; Gold, N.D.; Martin, V.J.; Wollenweber, E.; Ibrahim, R.K. Sequential O-methylation of tricetin by a single gene product in wheat. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 1115–1124. [Google Scholar] [CrossRef]
- Sun, J.; Liu, X.; Yang, T.; Slovin, J.; Chen, P. Profiling polyphenols of two diploid strawberry (Fragaria vesca) inbred lines using UHPLC-HRMSn. Food Chem. 2014, 146, 289–298. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.-W.; Lau, K.-M.; Hon, P.-M.; Mak, T.C.; Woo, K.-S.; Fung, K.-P. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr. Med. Chem. 2005, 12, 237–246. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.Å.; Smeds, A.I.; Sjöholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Dieminger, N.; Beusch, A.; Lee, Y.-M.; Dunkel, A.; Suess, B.; Skurk, T.; Wahl, A.; Hauner, H.; Hofmann, T. Bioappearance and pharmacokinetics of bioactives upon coffee consumption. Anal. Bioanal. Chem. 2013, 405, 8487–8503. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Volpe, M.G.; Paolucci, M.; Pacifico, S. UHPLC-HR-MS/MS-Guided recovery of bioactive flavonol compounds from greco di tufo vine leaves. Molecules 2019, 24, 3630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simard, F.; Legault, J.; Lavoie, S.; Mshvildadze, V.; Pichette, A. Isolation and identification of cytotoxic compounds from the wood of Pinus resinosa. Phytother. Res. 2008, 22, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; Guerra-Hernández, E.; Cerretani, L.; García-Villanova, B.; Verardo, V. Comprehensive metabolite profiling of Solanum tuberosum L. (potato) leaves by HPLC-ESI-QTOF-MS. Food Res. Int. 2018, 112, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, X.; Rui, W.; Guo, J.; Feng, Y. UPLC/Q-TOF-MS analysis for identification of hydrophilic phenolics and lipophilic diterpenoids from Radix Salviae Miltiorrhizae. Acta Chromatogr. 2015, 27, 711–728. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zhu, J.; Ding, M.; Lv, G. Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography–electrospray tandem mass spectrometry. Talanta 2008, 76, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, P.; Liu, B.; Wei, L.; Xu, Y. Simultaneous determination of fourteen compounds of Hedyotis diffusa Willd extract in rats by UHPLC–MS/MS method: Application to pharmacokinetics and tissue distribution study. J. Pharm. Biomed. Anal. 2018, 159, 490–512. [Google Scholar] [CrossRef] [PubMed]
- Toh, T.; Prior, B.; Van der Merwe, M. Quantification of plasma membrane ergosterol of Saccharomyces cerevisiae by direct-injection atmospheric pressure chemical ionization/tandem mass spectrometry. Anal. Biochem. 2001, 288, 44–51. [Google Scholar] [CrossRef]
- Sun, S.; Gao, Y.; Ling, X.; Lou, H. The combination effects of phenolic compounds and fluconazole on the formation of ergosterol in Candida albicans determined by high-performance liquid chromatography/tandem mass spectrometry. Anal. Mol. 2005, 336, 39–45. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Mei, Y.; Wei, L. Comparison of Multiple Bioactive Constituents in the Flower and the Caulis of Lonicera japonica Based on UFLC-QTRAP-MS/MS Combined with Multivariate Statistical Analysis. Molecules 2019, 24, 1936. [Google Scholar] [CrossRef] [Green Version]
- Perchuk, I.; Shelenga, T.; Gurkina, M.; Miroshnichenko, E.; Burlyaeva, M. Composition of Primary and Secondary Metabolite Compounds in Seeds and Pods of Asparagus Bean (Vigna unguiculata (L.) Walp.) from China. Molecules 2020, 25, 3778. [Google Scholar] [CrossRef]
- Mittal, J.; Sharma, M.M. Enhanced production of berberine in In vitro regenerated cell of Tinospora cordifolia and its analysis through LCMS QToF. 3 Biotech 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Ding, C.; Ge, Q.; Zhou, Z.; Zhi, X. Simultaneous determination of ginkgolides A, B, C and bilobalide in plasma by LC–MS/MS and its application to the pharmacokinetic study of Ginkgo biloba extract in rats. J. Chromatogr. B 2008, 864, 87–94. [Google Scholar] [CrossRef] [PubMed]

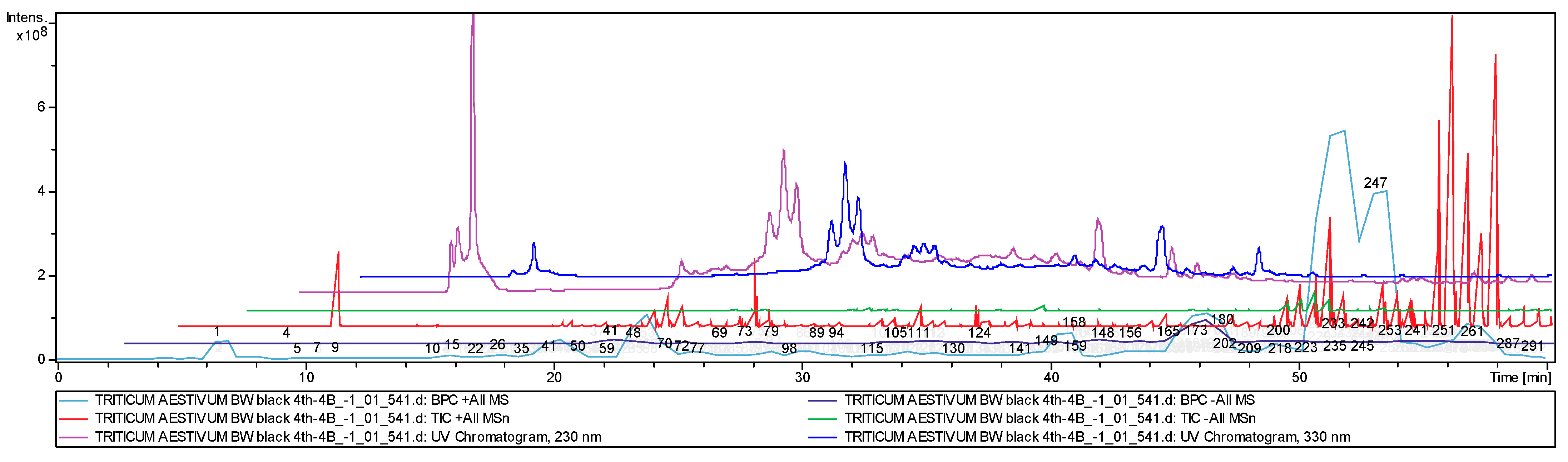
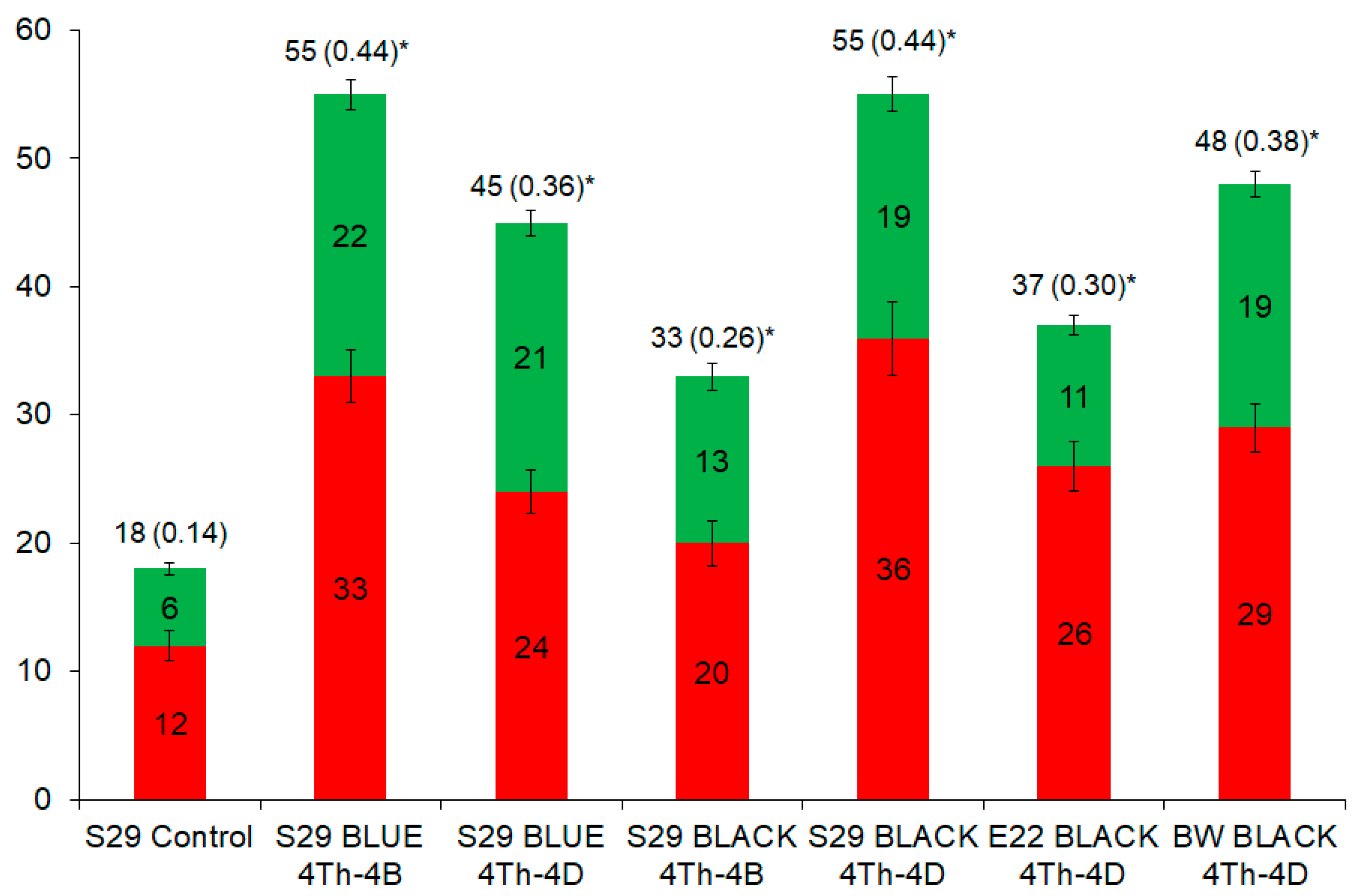
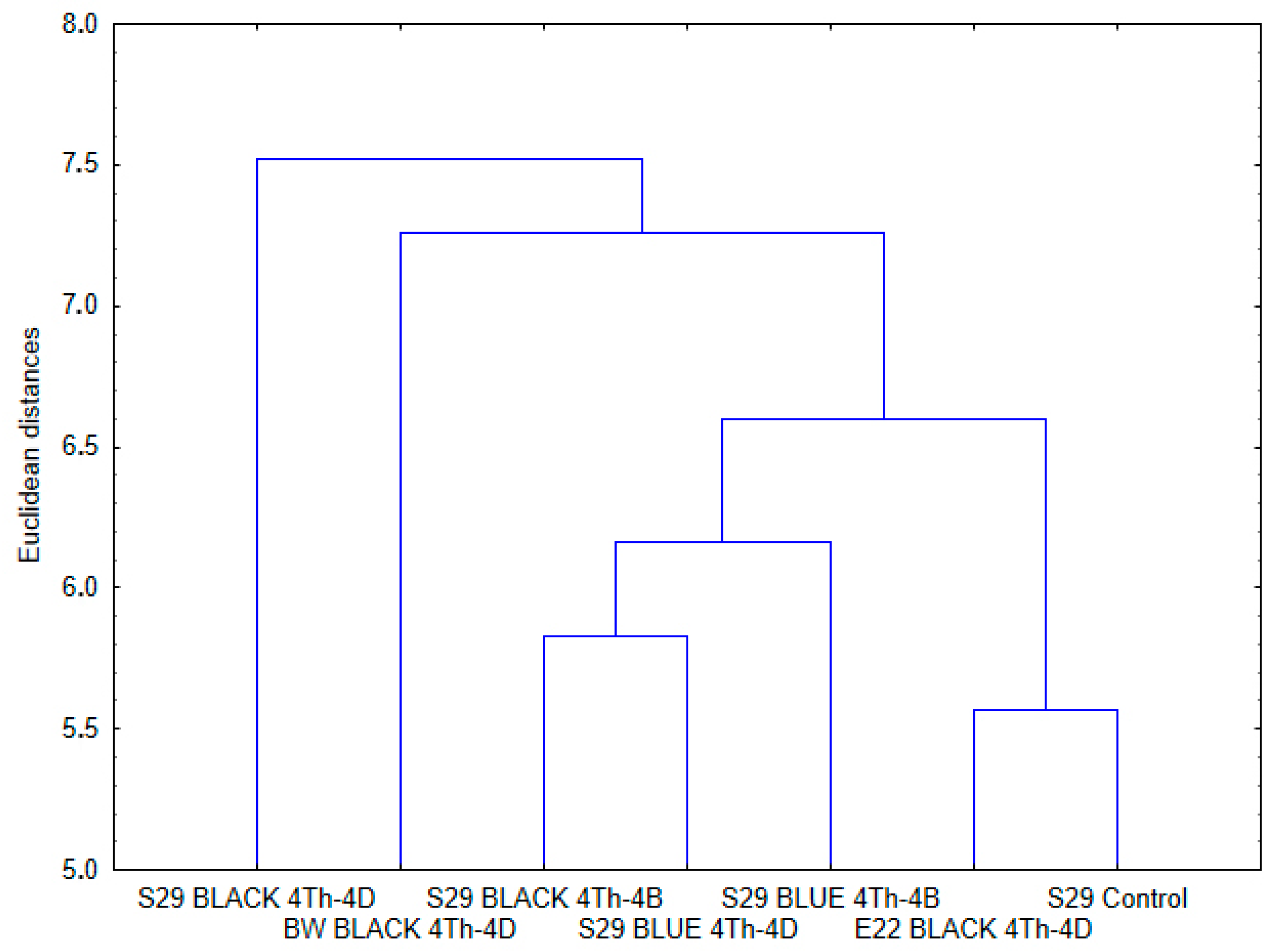
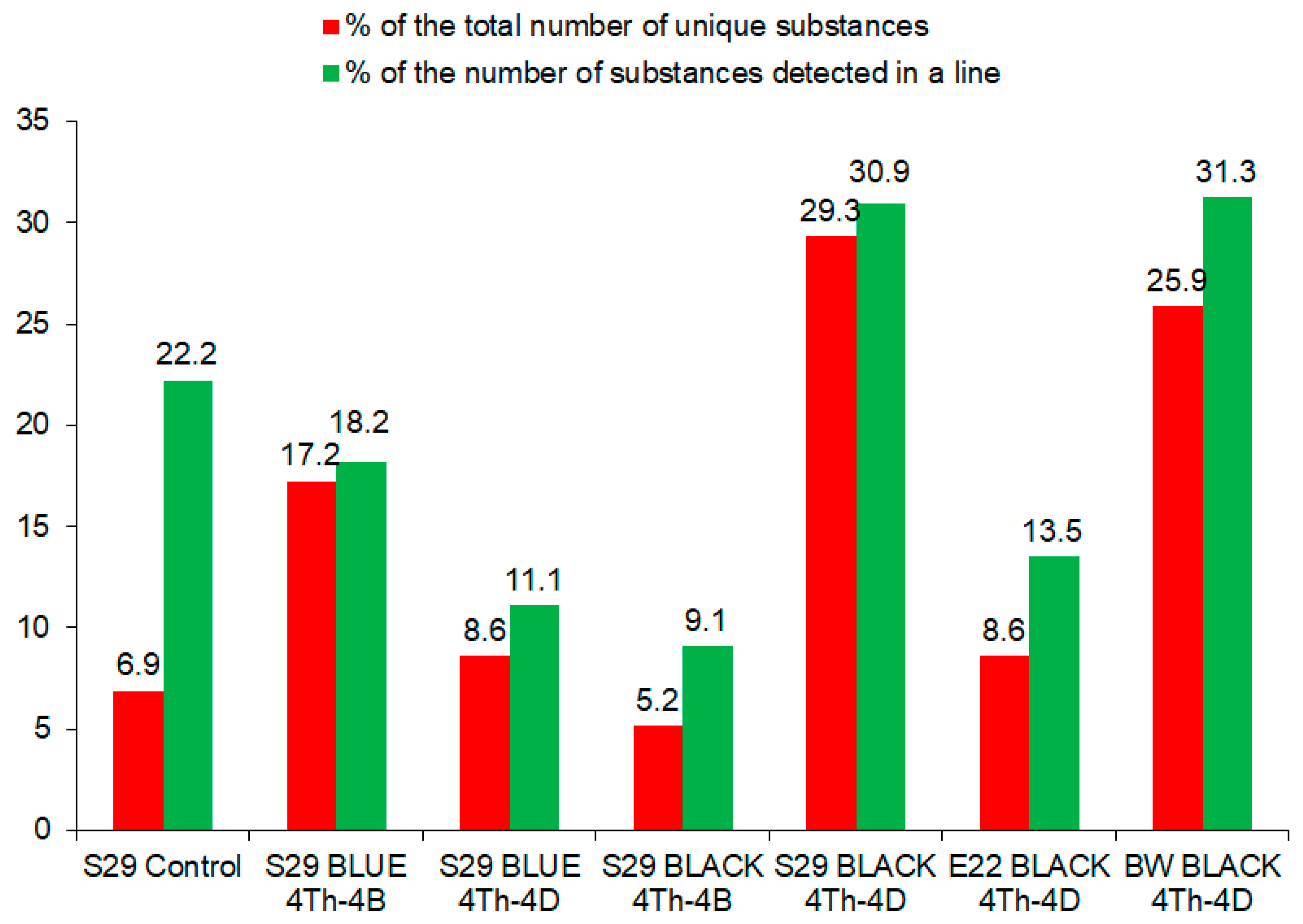
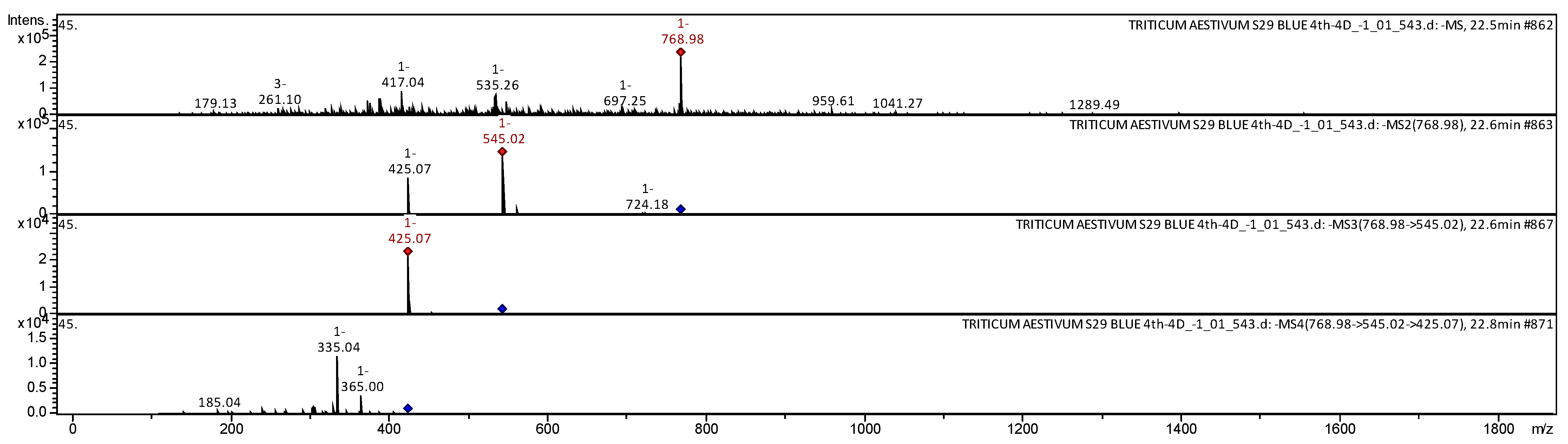
| ID | Classes and Families of Compounds | Name | S29 Control | S29 BLUE 4Th-4B | S29 BLUE 4Th-4D | S29 BLACK 4Th-4B | S29 BLACK 4Th-4D | E22 BLACK 4Th-4D | BW BLACK 4Th-4D |
|---|---|---|---|---|---|---|---|---|---|
| Phenolics | |||||||||
| 1 | Anthocyanin | Cyanidin 3-(2″-galloylglucoside) | yes | ||||||
| 2 | Cyanidin-3-O-3″,6″-O-Dimalonylglucoside | yes | yes | yes | |||||
| 3 | Cyanidin-3-O-glucoside | yes | |||||||
| 4 | Malvidin 3-O-rutinoside | yes | |||||||
| 5 | Malvidin 3-O-rutinoside-5-O-glucoside | yes | yes | ||||||
| 6 | Peonidin 3-O-rutinoside | yes | yes | ||||||
| 7 | Peonidin 3-rutinoside-5-glucoside | yes | |||||||
| 8 | Peonidin-3-O-glucoside | yes | yes | ||||||
| 9 | Petunidin | yes | yes | ||||||
| 10 | Petunidin 3-O-rutinoside-5-O-glucoside | yes | yes | ||||||
| 11 | Cinnamic acid derivative | Ferulic acid methyl ester | yes | ||||||
| 12 | Hydroxycinnamic acid | 1-Caffeoyl-β-d-glucose | yes | ||||||
| 13 | 1-O-Sinapoyl-β-d-glucose | yes | |||||||
| 14 | Caffeic acid derivative | yes | yes | ||||||
| 15 | Caftaric acid | yes | yes | yes | yes | ||||
| 16 | Chlorogenic acid | yes | |||||||
| 17 | Ferulic acid | yes | yes | yes | |||||
| 18 | Coumarin | Fraxetin | yes | ||||||
| 19 | Fraxetin-7-O-sulfate | yes | |||||||
| 20 | Dihydrochalcone | Phlorizin | yes | ||||||
| 21 | Flavan-3-ol | Catechin [d-Catechol] | yes | yes | |||||
| 22 | Epicatechin | yes | |||||||
| 23 | Gallocatechin [+(-)Gallocatechin] | yes | yes | ||||||
| 24 | Flavanone | Naringenin [Naringetol; Naringenine] | yes | yes | yes | yes | |||
| 25 | Flavone | 6-C-hexosyl-chrysoeriol O-rhamnoside-O-hexoside | yes | yes | yes | yes | yes | ||
| 26 | Acacetin C-glucoside methyl malonylated | yes | |||||||
| 27 | Apigenin | yes | yes | yes | |||||
| 28 | Apigenin 2″-O-sinapoyl, C-hexosyl, C-pentosyl | yes | yes | ||||||
| 29 | Apigenin 6,8-di-C-pentoside | yes | yes | yes | yes | ||||
| 30 | Apigenin 6-C-deoxyhexoside-8-C-pentoside | yes | yes | ||||||
| 31 | Apigenin-6-C-β-galactosyl-8-C-β-glycosyl-O-glycuronopyranoside | yes | |||||||
| 32 | Apigenin 8-C-hexoside-6-C-pentoside | yes | yes | yes | yes | yes | yes | yes | |
| 33 | Apigenin 8-C-pentoside-6-C-hexoside | yes | yes | yes | yes | yes | |||
| 34 | Chrysoeriol [Chryseriol] | yes | yes | yes | yes | yes | yes | ||
| 35 | Chrysoeriol C-hexoside-C-pentoside | yes | yes | ||||||
| 36 | Cirsiliol | yes | |||||||
| 37 | Dihydroxy tetramethoxyflavone | yes | |||||||
| 38 | Diosmetin | yes | |||||||
| 39 | Genistein C-glucosyl glucoside | yes | |||||||
| 40 | Hydroxy dimethoxyflavone hexoside | yes | |||||||
| 41 | Luteolin | yes | |||||||
| 42 | Luteolin 8-C-Glucoside | yes | |||||||
| 43 | Luteolin 8-C-hexoside-6-C-pentoside | yes | |||||||
| 44 | Luteolin 8-C-pentoside-6-C-hexoside | yes | yes | yes | yes | ||||
| 45 | Myricetin | yes | |||||||
| 46 | Orientin 7-O-deoxyhexoside [Luteolin 8-C-glucoside 7-O-deoxyhexoside] | yes | |||||||
| 47 | Pentahydroxy dimethoxyflavone | yes | |||||||
| 48 | Pentahydroxy dimethoxyflavone hexoside | yes | yes | ||||||
| 49 | Pentahydroxy trimethoxy flavone | yes | yes | yes | yes | yes | |||
| 50 | Tricin | yes | yes | yes | yes | yes | yes | ||
| 51 | Tetrahydroxy-dimethoxyflavone-hexoside | yes | |||||||
| 52 | Trihydroxy methoxyflavone triacetate | yes | |||||||
| 53 | Vicenin-2 [Apigenin-6,8-Di-C-Glucoside] | yes | yes | ||||||
| 54 | Vitexin 2″-O-glucoside [Apigenin 8-C-glucoside 2″-O-glucoside] | yes | yes | ||||||
| 55 | Vitexin 6″-O-glucoside [Apigenin 8-C-glucoside 6″-O-glucoside] | yes | yes | ||||||
| 56 | Wighteone-O-glucoside | yes | |||||||
| 57 | Flavonol | Ampelopsin | yes | ||||||
| 58 | Isorhamnetin | yes | yes | yes | |||||
| 59 | Kaempferide | yes | |||||||
| 60 | Kaempferol | yes | yes | ||||||
| 61 | Quercetin | yes | |||||||
| 62 | Rhamnetin I | yes | |||||||
| 63 | Rhamnetin II | yes | yes | ||||||
| 64 | Selgin | yes | yes | ||||||
| 65 | Taxifolin-3-O-glucoside | yes | yes | yes | yes | ||||
| 66 | Taxifolin-O-pentoside | yes | yes | yes | |||||
| 67 | Gallotannin | β-Glucogallin [1-O-Galloyl-β-d-Glucose] | yes | yes | yes | yes | yes | ||
| 68 | Hydroxybenzoic acid | 4-Hydroxybenzoic acid | yes | ||||||
| 69 | Cis-salvianolic acid J | yes | yes | yes | |||||
| 70 | Hydroxy methoxy dimethylbenzoic acid | yes | yes | ||||||
| 71 | Salvianolic acid D | yes | yes | yes | yes | yes | yes | ||
| 72 | Salvianolic acid F | yes | |||||||
| 73 | Salvianolic acid G | yes | yes | yes | |||||
| 74 | Lignan | Dimethyl-secoisolariciresinol | yes | ||||||
| 75 | Hinokinin | yes | yes | yes | yes | ||||
| 76 | Pinoresinol | yes | |||||||
| 77 | Podophyllotoxin [Podofilox; Condylox; Condyline; Podophyllinic acid lactone] | yes | |||||||
| 78 | Syringaresinol | yes | yes | yes | yes | yes | |||
| 79 | Phenolic acid | 1-O-caffeoyl-5-O-feruloylquinic acid | yes | yes | yes | yes | |||
| 80 | 4-O-Caffeoyl-5-O-p-coumaroylquinic acid | yes | |||||||
| 81 | Feruloyl sulfate | yes | |||||||
| 82 | Phenolic glucoside | Gallic acid hexoside | yes | ||||||
| 83 | Stilbene | Pinosylvin | yes | ||||||
| 84 | Polydatin [Piceid; trans-Piceid] | yes | |||||||
| 85 | Resveratrol | yes | |||||||
| Others | |||||||||
| 86 | Alpha, omega-dicarboxylic acid | Undecanedioic acid | yes | yes | |||||
| 87 | Carboxylic acid | Myristoleic acid [Cis-9-Tetradecanoic acid] | yes | yes | yes | yes | yes | ||
| 88 | Higher-molecular-weight carboxylic acid | 11-Hydroperoxy-octadecatrienoic acid | yes | ||||||
| 89 | 9,10-Dihydroxy-8-oxooctadec-12-enoic acid | yes | yes | yes | |||||
| 90 | Dihydroxy docosanoic acid | yes | yes | yes | yes | yes | yes | ||
| 91 | Docosenoic acid [2-Docosenoic acid] | yes | |||||||
| 92 | Hydroxy methoxy dimethylbenzoic acid | yes | |||||||
| 93 | Pentacosenoic acid | yes | yes | yes | yes | yes | yes | yes | |
| 94 | Salvianic acid C | yes | yes | yes | yes | ||||
| 95 | Anabolic steroid | Vebonol | yes | yes | yes | yes | |||
| 96 | Cycloartanol [Steroids] | Cyclopassifloic acid glucoside | yes | yes | |||||
| 97 | Carotenoid | (3S, 3′S, all-E)-zeaxanthin [Zeaxanthin; (3S,3′S)-Zeaxanthin] | yes | yes | yes | yes | |||
| 98 | Cryptoxanthin [β-cryptoxanthin] | yes | yes | ||||||
| 99 | Diterpenoid | Isocryptotanshinone II | yes | yes | |||||
| 100 | Tanshinone IIB | yes | |||||||
| 101 | Pentacyclic diterpenoid | β-Amyrin [β-Amyrenol; Amyrin] | yes | ||||||
| 102 | Gibberellic acid | yes | |||||||
| 103 | Triterpenic acid | Betunolic acid | yes | ||||||
| 104 | Ursolic acid | yes | |||||||
| 105 | Triterpenoid | Squalene | yes | ||||||
| 106 | Uvaol | yes | |||||||
| 107 | Essential amino acid | l-Histidine | yes | yes | |||||
| 108 | l-Tryptophan [Tryptophan; (S)-Tryptophan] | yes | yes | yes | yes | yes | yes | ||
| 109 | l-Valine | yes | |||||||
| 110 | Nonessential amino acid | Tyrosine | yes | ||||||
| 111 | Indole sesquiterpene alkaloid | Sespendole | yes | yes | |||||
| 112 | Isoquinoline alkaloid | Berberine [Berberin; Umbelletine; Berbericine] | yes | yes | |||||
| 113 | Phytohormone | GA8-hexose gibberellin | yes | yes | yes | yes | yes | ||
| 114 | Sesquiterpenoid plant hormone | Abscisic acid [Dormin; Abscisin II; (S)-(+)-Abscisic acid] | yes | yes | |||||
| 115 | Propionic acid | Ketoprofen [Orudis; 2-(3-Benzoylphenyl)Propionic acid] | yes | yes | yes | yes | |||
| 116 | Purine | Adenosine | yes | yes | yes | ||||
| 117 | Phytosterol | Ergosterol [Provitamin D2; Ergosterin] | yes | yes | yes | yes | yes | yes | yes |
| 118 | Sterol | Avenasterol | yes | yes | yes | yes | yes | yes | yes |
| 119 | β-Sitostenone [Stigmast-4-En-3-One; Sitostenone] | yes | yes | yes | |||||
| 120 | β-Sitosterin [β-Sitosterol] | yes | yes | yes | |||||
| 121 | Campestenone | yes | yes | yes | yes | yes | |||
| 122 | Fucosterol | yes | yes | yes | |||||
| 123 | Oxo-hydroxy sitosterol | yes | |||||||
| 124 | Thromboxane receptor antagonist | Vapiprost | yes | yes | |||||
| 125 | Unsaturated fatty acid | Hexadecatrienoic acid [Hexadeca-2,4,6-trienoic acid] | yes |
| Factor | Group | Group Size | df | Sum of Ranks | Mean Rank | H Criterion | p Value | Significant Result |
|---|---|---|---|---|---|---|---|---|
| Chromosome Substitution | 4Th-4B | 2 | 2 | 111,625.0 | 446.5 | 23.58 | 0.00001 | yes |
| 4Th-4D | 4 | 227,187.5 | 454.4 | |||||
| Control | 1 | 44,437.5 | 355.5 | |||||
| Genotype of Parental Line/Cultivar | BW | 1 | 2 | 57,562.5 | 460.5 | 2.26 | 0.322 | no |
| E22 | 1 | 52,750.0 | 422.0 | |||||
| S29 | 5 | 272,937.5 | 436.7 | |||||
| Grain Color | Black grains | 4 | 2 | 443.8750 | 443.8 | 25.52 | 0.00001 | yes |
| Blue grains | 2 | 116,875.0 | 467.5 | |||||
| Control | 1 | 44,437.5 | 355.5 | |||||
| Genotype of Line | S29 BLUE (4Th-4B) | 1 | 6 | 60,625.0 | 485.0 | 38.29 | 0.00001 | yes |
| S29 BLUE (4Th-4D) | 1 | 56,250.0 | 450.0 | |||||
| S29 BLACK (4Th-4B) | 1 | 51,000.0 | 408.0 | |||||
| S29 BLACK (4Th-4D) | 1 | 60,625.0 | 485.0 | |||||
| E22 BLACK (4Th-4D) | 1 | 57,562.5 | 460.5 | |||||
| BW BLACK (4Th-4D) | 1 | 52,750.0 | 422.0 | |||||
| Control | 1 | 44,437.5 | 355.5 |
| Genotype | Recurrent Parent | Grain Color | Ba | Pp-D1 + Pp3 | Substituted Chromosome | References |
|---|---|---|---|---|---|---|
| S29 BLUE(4Th-4D) | S29 | blue | + | - | 4D | [25] |
| S29 BLUE(4Th-4B) | S29 | blue | + | - | 4B | [26] |
| S29 BLACK(4Th-4B) | S29 | black | + | + | 4B | |
| S29 BLACK(4Th-4D) | S29 | black | + | + | 4D | |
| BW BLACK(4Th-4D) | BW49880 | black | + | + | 4D | Figure S2 |
| E22 BLACK(4Th-4D) | Element 22 | black | + | + | 4D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razgonova, M.P.; Zakharenko, A.M.; Gordeeva, E.I.; Shoeva, O.Y.; Antonova, E.V.; Pikula, K.S.; Koval, L.A.; Khlestkina, E.K.; Golokhvast, K.S. Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry. Molecules 2021, 26, 5580. https://doi.org/10.3390/molecules26185580
Razgonova MP, Zakharenko AM, Gordeeva EI, Shoeva OY, Antonova EV, Pikula KS, Koval LA, Khlestkina EK, Golokhvast KS. Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry. Molecules. 2021; 26(18):5580. https://doi.org/10.3390/molecules26185580
Chicago/Turabian StyleRazgonova, Mayya P., Alexander M. Zakharenko, Elena I. Gordeeva, Olesya Yu. Shoeva, Elena V. Antonova, Konstantin S. Pikula, Liudmila A. Koval, Elena K. Khlestkina, and Kirill S. Golokhvast. 2021. "Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry" Molecules 26, no. 18: 5580. https://doi.org/10.3390/molecules26185580
APA StyleRazgonova, M. P., Zakharenko, A. M., Gordeeva, E. I., Shoeva, O. Y., Antonova, E. V., Pikula, K. S., Koval, L. A., Khlestkina, E. K., & Golokhvast, K. S. (2021). Phytochemical Analysis of Phenolics, Sterols, and Terpenes in Colored Wheat Grains by Liquid Chromatography with Tandem Mass Spectrometry. Molecules, 26(18), 5580. https://doi.org/10.3390/molecules26185580










