Production of 6-l-[18F]Fluoro-m-tyrosine in an Automated Synthesis Module for 11C-Labeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Manual 6-l-[18F]FMT Radiosynthesis
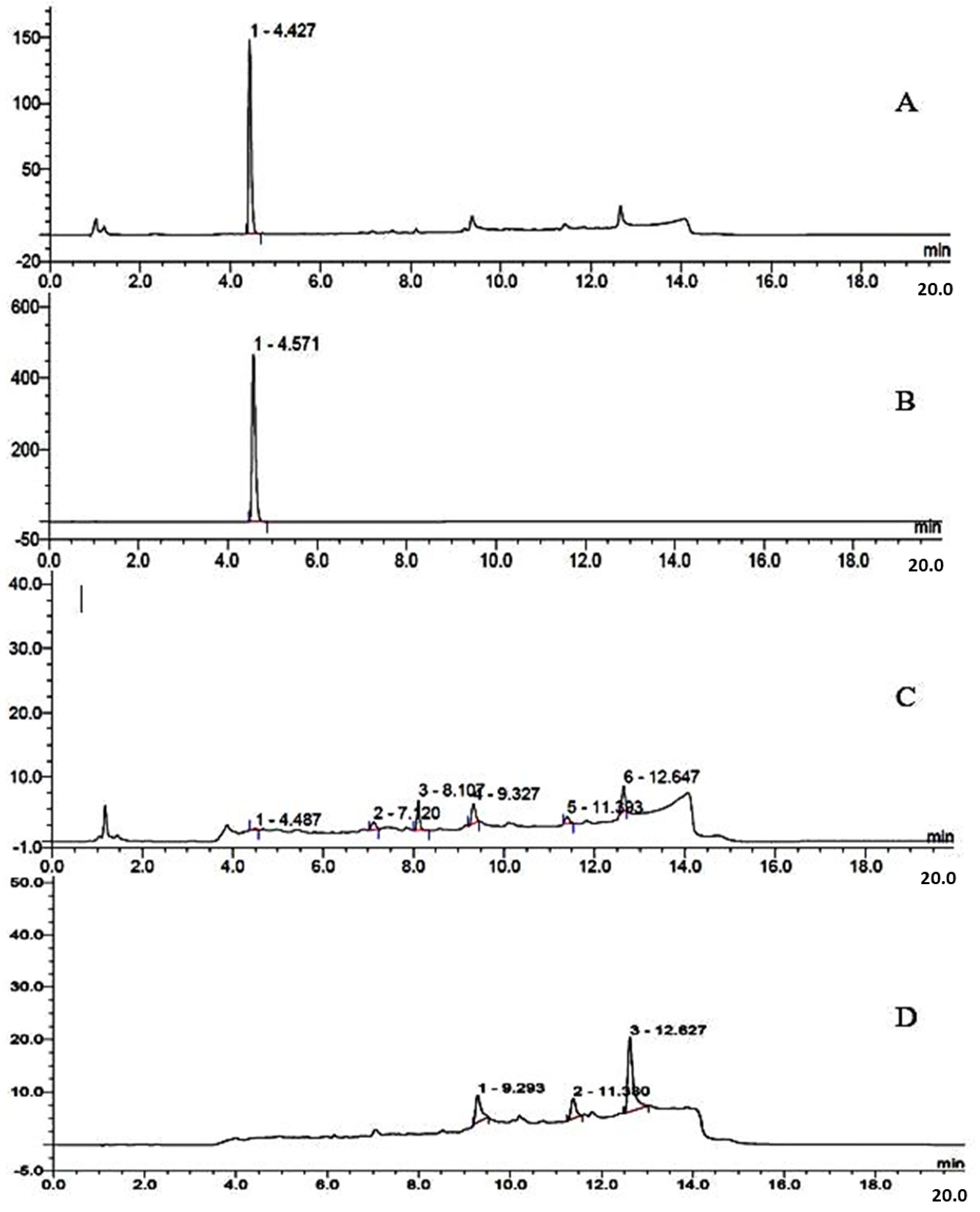
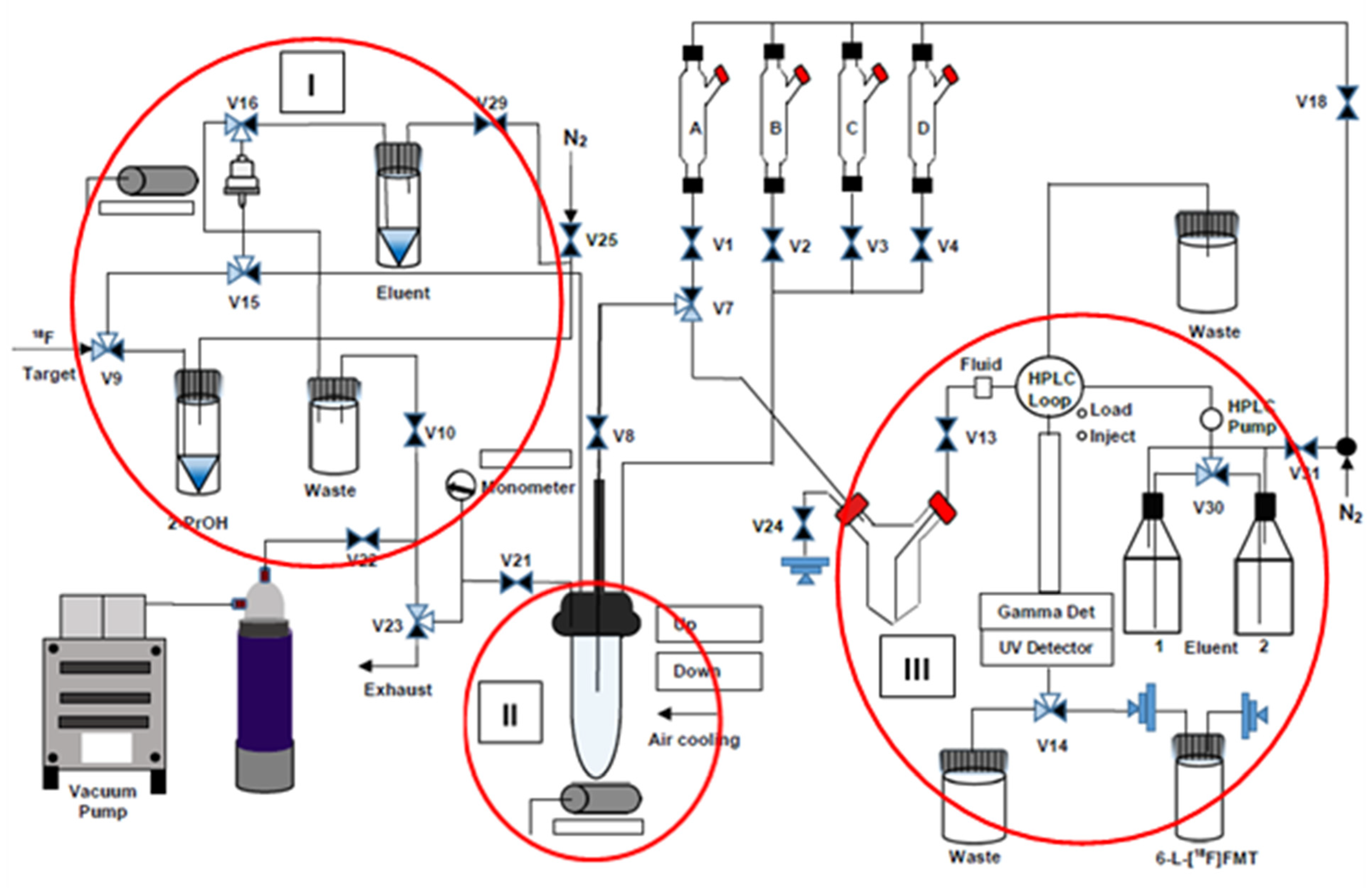
2.2. Automated Production of 6-l-[18F]FMT
3. Experimental
3.1. Materials and Methods
3.2. Production of [18F]Fluoride
3.3. Manual Radiosynthesis of 6-l-[18F]FMT
3.4. Automated Radiosynthesis of 6-l-[18F]FMT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kilbourn, M.R. 11C- and 18F-Radiotracers for In Vivo Imaging of the Dopamine System: Past, Present and Future. Biomedicines 2021, 9, 108. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Biochemistry of post-mortem brains in Parkinson’s disease: Historical overview and future prospects. J. Neural Transm. Suppl. 2007, 72, 113–120. [Google Scholar] [CrossRef]
- DeJesus, O.T.; Sunderland, J.J.; Nickles, J.R.; Mukherjee, J.; Appelman, E.H. Synthesis of radiofluorinated analogs of m-tyrosine as potential l-dopa tracers via direct reaction with acetylhypofluorite. Int. J. Rad. Appl. Instrum. A 1990, 41, 433–437. [Google Scholar] [CrossRef]
- Barrio, J.R.; Huang, S.-C.; Yu, D.-C.; Melega, W.P.; Quintana, J.; Cherry, S.R.; Jacobson, A.; Namavari, M.; Satyamurthy, N.; Phelps, M.E. Radiofluorinated L-m-tyrosines: New in-vivo probes for central dopamine biochemistry. J. Cereb. Blood Flow Metab. 1996, 16, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Nahmias, C.; Wahl, L.; Chirakal, R.; Firnau, G.; Garnett, E.S. A probe for intracerebral aromatic amino-acid decarboxylase activity: Distribution and kinetics of [18F]6-fluoro-L-m-tyrosine in the human brain. Mov. Disord. 1995, 10, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Doudet, D.J.; Chan, G.L.-Y.; Jivan, S.; DeJesus, O.T.; McGeer, E.G.; English, C.; Ruth, T.J.; Holden, J.E. Evaluation of Dopaminergic Presynaptic Integrity: 6-[18F]Fluoro-L-Dopa Versus 6-[18F]Fluoro-L-m-Tyrosine. J. Cereb. Blood Flow Metab. 1999, 19, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Eberling, J.L.; Bankiewicz, K.S.; O’Neil, J.P.; Jagust, W.J. PET 6-[18F]fluoro-L-m-tyrosine Studies of dopaminergic function in human and nonhuman primates. Front. Hum. Neurosci. 2007, 1, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.T.; Palotti, M.; Holden, J.E.; Oh, J.; Okonkwo, O.; Christian, B.T.; Bendlin, B.B.; Buyan-Dent, L.; Harding, S.J.; Stone, C.K.; et al. A dual-tracer study of extrastriatal 6-[18F]fluoro-m-tyrosine and 6-[18F]fluoro-L-dopa uptake in Parkinson’s disease. Synapse 2014, 68, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeJesus, O.; Endres, C.J.; Shelton, S.E.; Nickles, R.J.; Holden, J.E. Evaluation of fluorinated m-tyrosine analogs as PET imaging agents of dopamine nerve terminals: Comparison with 6-fluorodopa. J. Nucl. Med. 1997, 38, 630–636. [Google Scholar] [PubMed]
- Becker, G.; Bahri, M.A.; Michel, A.; Hustadt, F.; Garraux, G.; Luxen, A.; Lemaire, C.; Plenevaux, A. Comparative assessment of 6-[18F]fluoro-L-m-tyrosine and 6-[18F]fluoro-L-dopa to evaluate dopaminergic presynaptic integrity in a Parkinson’s disease rat model. J. Neurochem. 2017, 141, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, C.L.; Christian, B.T.; Holden, J.E.; Dejesus, O.T.; Nickles, R.J.; Buyan-Dent, L.; Bendlin, B.B.; Harding, S.J.; Stone, C.K.; Mueller, B.; et al. A within-subject comparison of 6-[18F]fluoro-m-tyrosine and 6-[18F]fluoro-L-dopa in Parkinson’s disease. Mov. Disord. 2011, 26, 2032–2038. [Google Scholar] [CrossRef]
- Asari, S.; Fujimoto, K.; Miyauchi, A.; Sato, T.; Nakano, I.; Muramatsu, S. Subregional 6-[18F]fluoro-m-tyrosine uptake in the striatum in Parkinson’s disease. BMC Neurol. 2011, 23, 11–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, H.H.; Gee, A.G.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Open letter to journal editors on: International consensus radiochemistry nomenclature guidelines. J. Label. Comp. Radiopharm. 2018, 61, 3, 402–404. [Google Scholar] [CrossRef] [Green Version]
- Namavari, M.; Satyamurthy, N.; Phelps, M.E.; Barrio, J.R. Synthesis of 6-[18F] and 4-[18F]fluoro-L-m-tyrosines via regioselective radio-fluorodestannylation. Appl. Radiat. Isot. 1993, 44, 527–536. [Google Scholar] [CrossRef]
- Van Brocklin, H.F.; Blagoev, M.; Hoepping, A.; O’Neil, J.P.; Klose, M.; Schubiger, P.A.; Ametamey, S. A new precursor for the preparation of 6-[18F]Fluoro-l-m-tyrosine ([18F]FMT): Efficient synthesis and comparison of radiolabeling. Appl. Radiat. Isot. 2004, 61, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.; Wuest, F. The Radiopharmaceutical chemistry of fluorine-18: Electrophilic fluorinations. Radiopharm. Chem. 2019, 285–295. [Google Scholar] [CrossRef]
- Libert, L.C.; Franci, X.; Plenevaux, A.R.; Ooi, T.; Maruoka, K.; Luxen, A.J.; Lemaire, C.F. Production at the Curie level of no carrier-added 6-18F-fluoro-L-dopa. J. Nucl. Med. 2013, 54, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, C.; Libert, L.; Franci, X.; Kuci, S.; Giacomelli, F.; Genon, J.-L.; Luxen, A. Automated production at the curie level of no carrier-added 6-[18F]fuoro-L-dopa and 2-[18F]fuoro-L-tyrosine on a FASTlab synthesizer. J. Label. Comp. Radiopharm. 2015, 58, 281–290. [Google Scholar] [CrossRef]
- Krasikova, R.N.; Zaitsev, V.V.; Ametamey, S.M.; Kuznetsova, O.F.; Fedorova, O.S.; Mosevich, I.K.; Belokon, Y.N.; Vyskočil, Š.; Shatik, S.V.; Nader, M.; et al. Catalytic enantioselective synthesis of 18F-fluorinated α-amino acids under phase-transfer conditions using (S)-NOBIN. Nucl. Med. Biol. 2004, 31, 597–603. [Google Scholar] [CrossRef]
- Pretze, M.; Franck, D.; Kunkel, F.; Foßhag, E.; Wängler, C.; Wängler, B. Evaluation of two nucleophilic syntheses routes for the automated synthesis of 6-[18F]fluoro-l-DOPA. Nucl. Med. Biol. 2017, 45, 35–42. [Google Scholar] [CrossRef]
- Wagner, F.M.; Ermert, J.; Coenen, H.H. Three-Step, ‘‘One-Pot’’ Radiosynthesis of 6-fluoro-3,4-dihydroxy-L-phenylalanine by isotopic exchange. J. Nucl. Med. 2009, 50, 1724–1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meleán, J.C.; Ermert, C.; Coenen., H.H. A three-step radiosynthesis of 6-[18F]fluoro-L-meta-tyrosine starting with [18F]fluoride. J. Label. Comp. Radiopharm. 2015, 58, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of arenes and heteroarenes for applications in positron emission tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Wang, L.; Liu, R.Y.; Patteson, J.; Kwan, E.E.; Vasdev, N.; Liang, S.H. Mechanistic studies and radiofluorination of structurally diverse pharmaceuticals with spirocyclic iodonium(III) ylides. Chem. Sci. 2016, 7, 4407–4417. [Google Scholar] [CrossRef] [Green Version]
- Zlatopolskiy, B.; Zischler, J.; Urusova, E.A.; Endepols, H.; Kordys, E.; Frauendorf, H.; Mottaghy, F.M.; Neumaier, B. A Practical One-Pot Synthesis of Positron Emission Tomography (PET) Tracers via Nickel-Mediated Radiofluorination. Chem. Open 2015, 4, 457–462. [Google Scholar] [CrossRef]
- Tredwell, M.; Preshlock, S.M.; Taylor, N.J.; Gruber, S.; Huiban, M.; Passchier, J.; Mercier, J.; Genicot, C.; Gouverneur, V. A general copper-mediated nucleophilic 18F-luorination of arenes. Angew. Chem. Int. Ed. 2014, 53, 7751–7755. [Google Scholar] [CrossRef]
- Wright, J.S.; Kaur, T.; Preshlock, S.; Tanzeyl, S.S.; Winton, W.P.; Sharninghausen, L.S.; Wiesner, N.; Brooks, A.F.; Sanford, M.S.; Scott, P.J.H. Copper-mediated late-stage radiofuorination: Five years of impact on preclinical and clinical PET imaging. Clin. Transl. Imaging 2020, 8, 167–206. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Krapf, P.; Zarrad, F.; Urusova, E.A.; Kordys, E.; Endepols, H.; Neumaier, B. Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem. Eur. J. 2015, 21, 5972–5979. [Google Scholar] [CrossRef]
- Preshlock, S.; Calderwood, S.; Verhoog, S.; Hienzsch, A.; Cailly, T.; Schedler, M.; Mollitor, J.; Hoepping, A.; Genicot, C.; Tredwell, M.; et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem. Commun. 2016, 52, 8361–8364. [Google Scholar] [CrossRef]
- Zischler, J.; Kolks, N.; Modemann, D.; Neumaier, B.; Zlatopolskiy, B.D. Alcohol-Enhanced Cu-Mediated Radiofluorination. Chem. Eur. J. 2017, 23, 3251–3256. [Google Scholar] [CrossRef]
- Antuganov, D.; Zykov, M.; Timofeev, V.; Timofeeva, K.; Antuganova, Y.; Fedorova, O.; Orlovskaya, V.; Krasikova, R. Copper-mediated radiofluorination of aryl pinacolboronate esters: A straightforward protocol using pyridinium sulfonates. Eur. J. Org. Chem. 2019, 918–922. [Google Scholar] [CrossRef]
- Orlovskaya, V.; Fedorova, O.; Kuznetsova, O.; Krasikova, R. Cu-Mediated Radiofluorination of Aryl Pinacolboronate Esters: Alcohols as Solvents with Application to 6-L-[18F]FDOPA Synthesis. Eur. J. Org. Chem. 2020, 7079–7086. [Google Scholar] [CrossRef]
- Zlatopolskiy, B.D.; Zischler, J.; Schäfer, D.; Urusova, E.A.; Guliyev, M.; Bannykh, O.; Endepols, H.; Neumaier, B. Discovery of 7-[18F]Fluorotryptophan as a Novel Positron Emission Tomography (PET) Probe for the Visualization of Tryptophan Metabolism in Vivo. Med. Chem. 2018, 61, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Kolks, N.; Urusova, E.A.; Zischler, J.; Brugger, M.; Endepols, H.; Neumaier, B.; Zlatopolskiy, B.D. Preparation of labeled aromatic amino acids via late-stage 18F-fluorination of chiral nickel and copper complexes. Chem. Commun. 2020, 56, 9505–9508. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline of Elemental Impurities Q3D. Available online: https://database.ich.org/sites/default/files/Q3D-R1EWG_Document_Step4_Guideline_2019_0322.pdf (accessed on 22 March 2021).
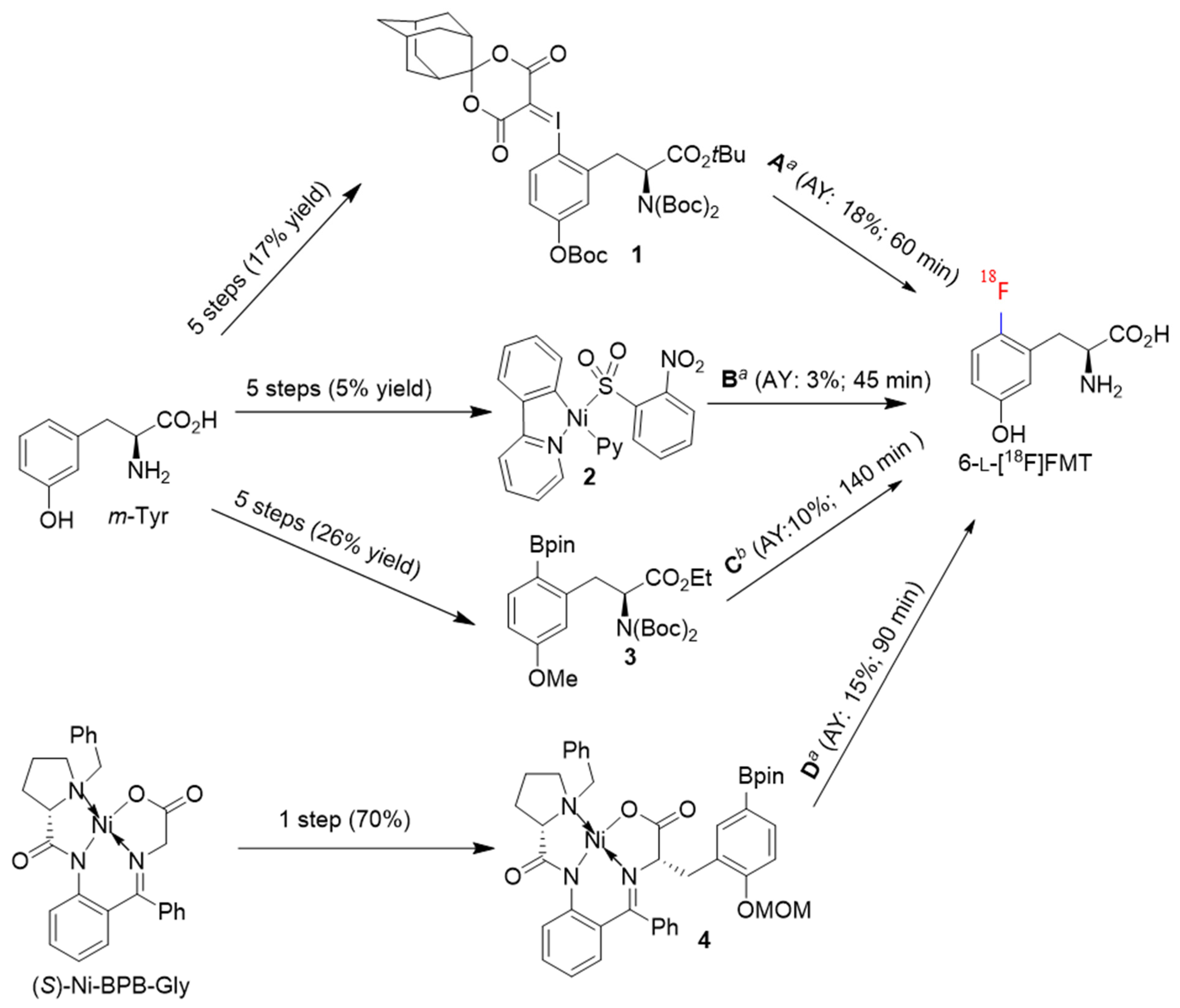
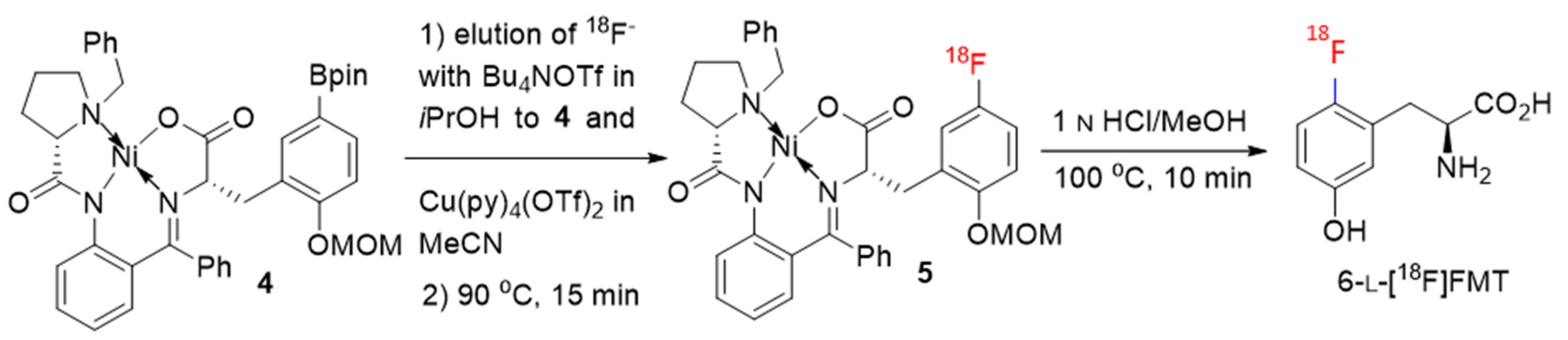


| Nr. | Precursor | Fluorination Conditions | Hydrolysis/Deprotection Conditions | Precursor/Cu-Catalyst (µmol) | AY (%) | Synthesis Time (min);Automation Mode | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 3 | K222/K2C2O4/ K2CO3 Cu(py)4(OTf)2, DMF,120 °C, 20 min, air | 57% HI, 150 °C, 10 min | 20/22 | 10 | 140; Synthra | [29] |
| 2 | 4 | Et4NHCO3, Cu(py)4(OTf)2, nBuOH/DMA 110 °C, 15 min, air | 12 n HCl 110 °C, 10 min | 10/20 | 6 ± 5 | 90; Semi-automated | [34] |
| 3 | 4 | Bu4NOTf, Cu(py)4(OTf)2, iPrOH/MeCN 90 °C, 15 min, N2 | 1 n HCl/MeOH 100 °C, 10 min | 10/16 | 20 ± 3 | 70; TracerLab FX C Pro (modified) | This work |
| Entry | Process | Activated Path/Function |
|---|---|---|
| Block I | ||
| 1 | Loading of [18F]fluoride onto the QMA cartridge | V9-V15-QMA-V16-waste bottle-V10-V22-Vac |
| 2 | Washing of QMA cartridge with iPrOH (3 mL) | V25-V29-V9-V15-QMA-V16-waste bottle-V10-V22-Vac |
| 3 | Elution of [18F]fluoride from the cartridge into the RV. | V25-V29-eluent vial-V16-QMA-V15-RV-V21-V23 |
| Block II | ||
| 4 | Radiofluorination, RV, 90 °C, 15 min stirring | - |
| 5 | Removal of volatiles at 80 °C under N2 flow | V18-V1-V7-V8-RV-V21-V23 |
| 6 | Transfer of 0.5 n HCl in 50% MeOH from B to RV | V18-V2 -RV-V21-V23 |
| 7 | Hydrolysis, RV, 100 °C, 10 min | - |
| 8 | Removal of volatiles at 120 °C under N2 flow | V18-V1-V7-V8-RV-V21-V23 |
| 9 | Transfer of MeOH from C to RV, stirring | V18-V3-RV-V21-V23 |
| 10 | Transfer of 0.1% AcOH from D to RV, stirring | V18-V4-RV-V21-V23 |
| Block III | ||
| 11 | Solution transfer from RV into the V-shaped vessel | V2-RV-V8-V7-V24-V-shaped vessel |
| 12 | Loading the content of V-shaped vessel into HPLC loop | V2-RV-V8-V7-V24-V-shaped vessel-V13, Load/Inject |
| 13 | Collection of the radioactive product in the product vial | V14, manual operation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlovskaya, V.V.; Craig, A.S.; Fedorova, O.S.; Kuznetsova, O.F.; Neumaier, B.; Krasikova, R.N.; Zlatopolskiy, B.D. Production of 6-l-[18F]Fluoro-m-tyrosine in an Automated Synthesis Module for 11C-Labeling. Molecules 2021, 26, 5550. https://doi.org/10.3390/molecules26185550
Orlovskaya VV, Craig AS, Fedorova OS, Kuznetsova OF, Neumaier B, Krasikova RN, Zlatopolskiy BD. Production of 6-l-[18F]Fluoro-m-tyrosine in an Automated Synthesis Module for 11C-Labeling. Molecules. 2021; 26(18):5550. https://doi.org/10.3390/molecules26185550
Chicago/Turabian StyleOrlovskaya, Viktoriya V., Austin S. Craig, Olga S. Fedorova, Olga F. Kuznetsova, Bernd Neumaier, Raisa N. Krasikova, and Boris D. Zlatopolskiy. 2021. "Production of 6-l-[18F]Fluoro-m-tyrosine in an Automated Synthesis Module for 11C-Labeling" Molecules 26, no. 18: 5550. https://doi.org/10.3390/molecules26185550
APA StyleOrlovskaya, V. V., Craig, A. S., Fedorova, O. S., Kuznetsova, O. F., Neumaier, B., Krasikova, R. N., & Zlatopolskiy, B. D. (2021). Production of 6-l-[18F]Fluoro-m-tyrosine in an Automated Synthesis Module for 11C-Labeling. Molecules, 26(18), 5550. https://doi.org/10.3390/molecules26185550






