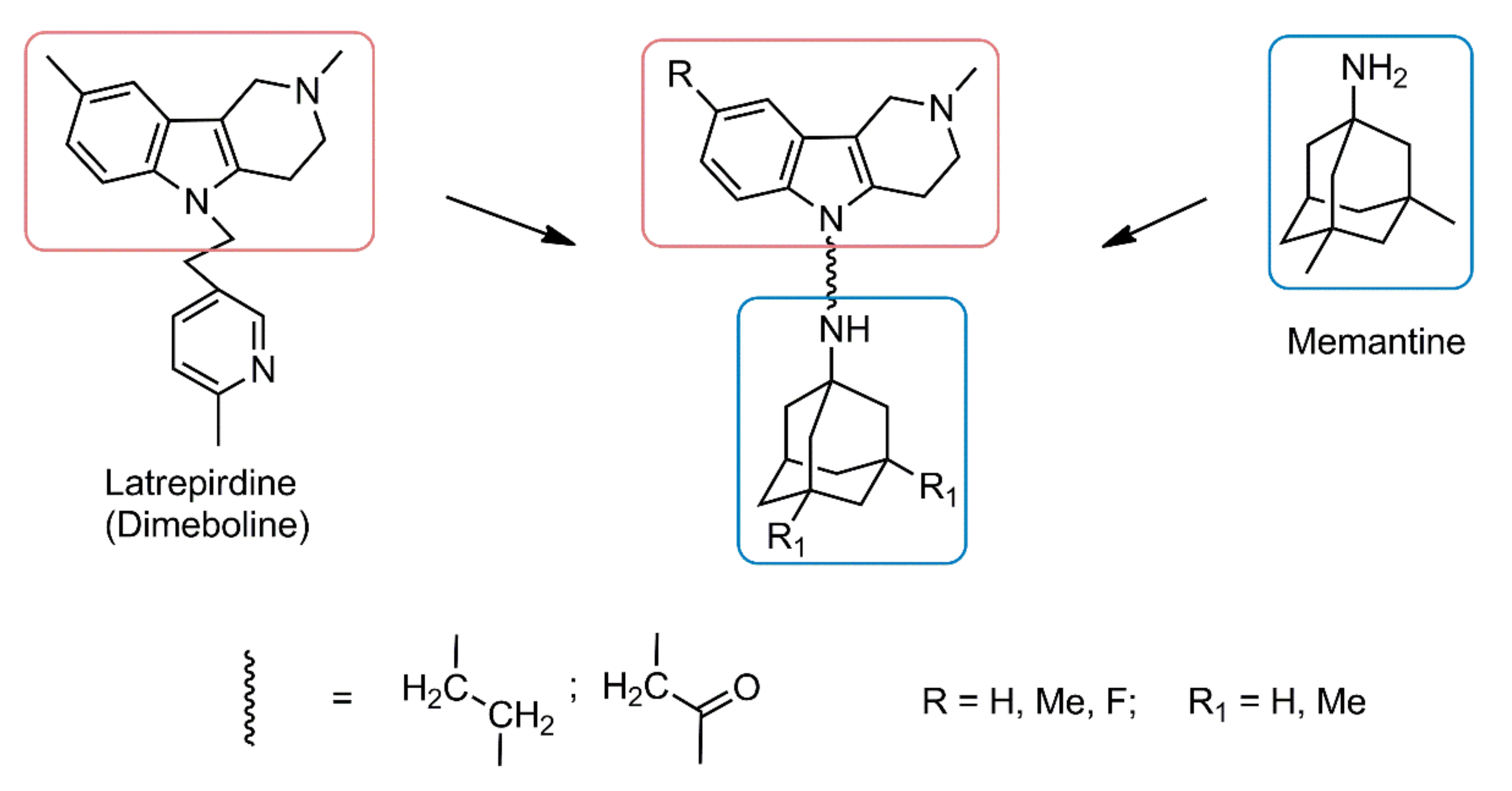
Conjugation of Aminoadamantane and γ-Carboline Pharmacophores Gives Rise to Unexpected Properties of Multifunctional Ligands
Abstract
:1. Introduction
2. Results and Discussion
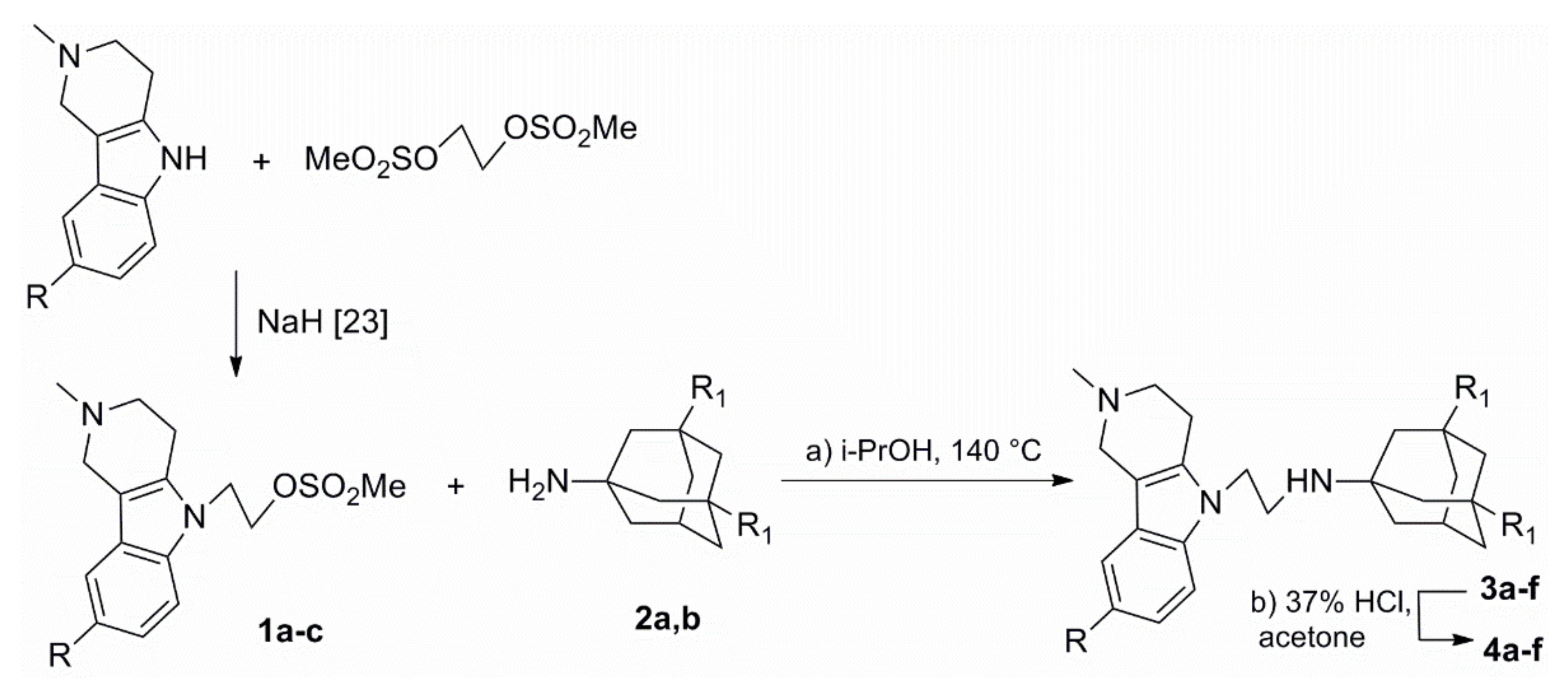
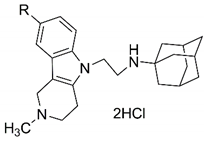
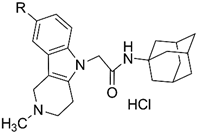
2.1. Chemistry
2.2. Effect of Aminoadamantane–γ-Carboline Conjugates on NMDA Receptors
2.3. Esterase Profiles of Aminoadamantane–γ-Carboline Conjugates and Their Ability to Displace Propidium Iodide from the AChE PAS
2.3.1. Evaluation of the Esterase Profile of Aminoadamantane–γ-Carboline Conjugates
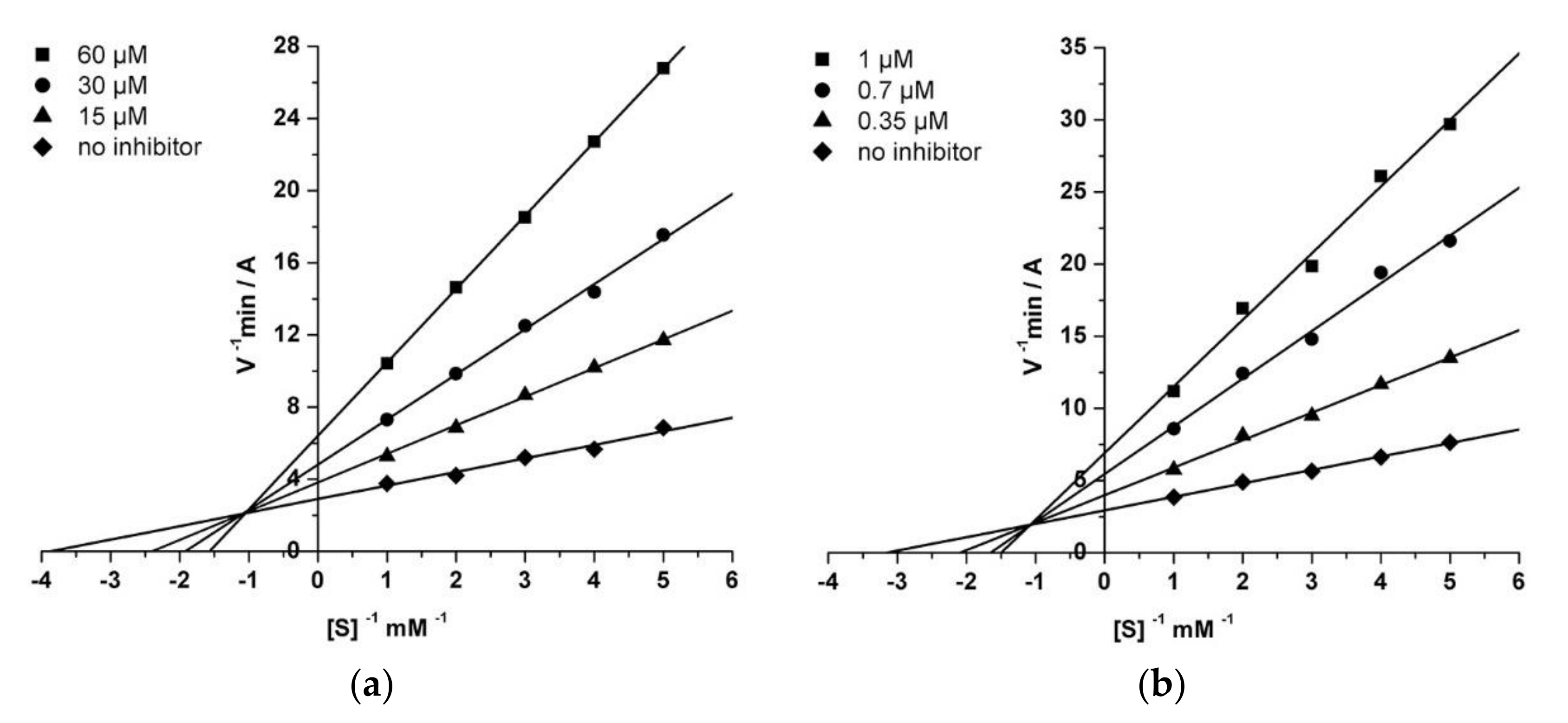
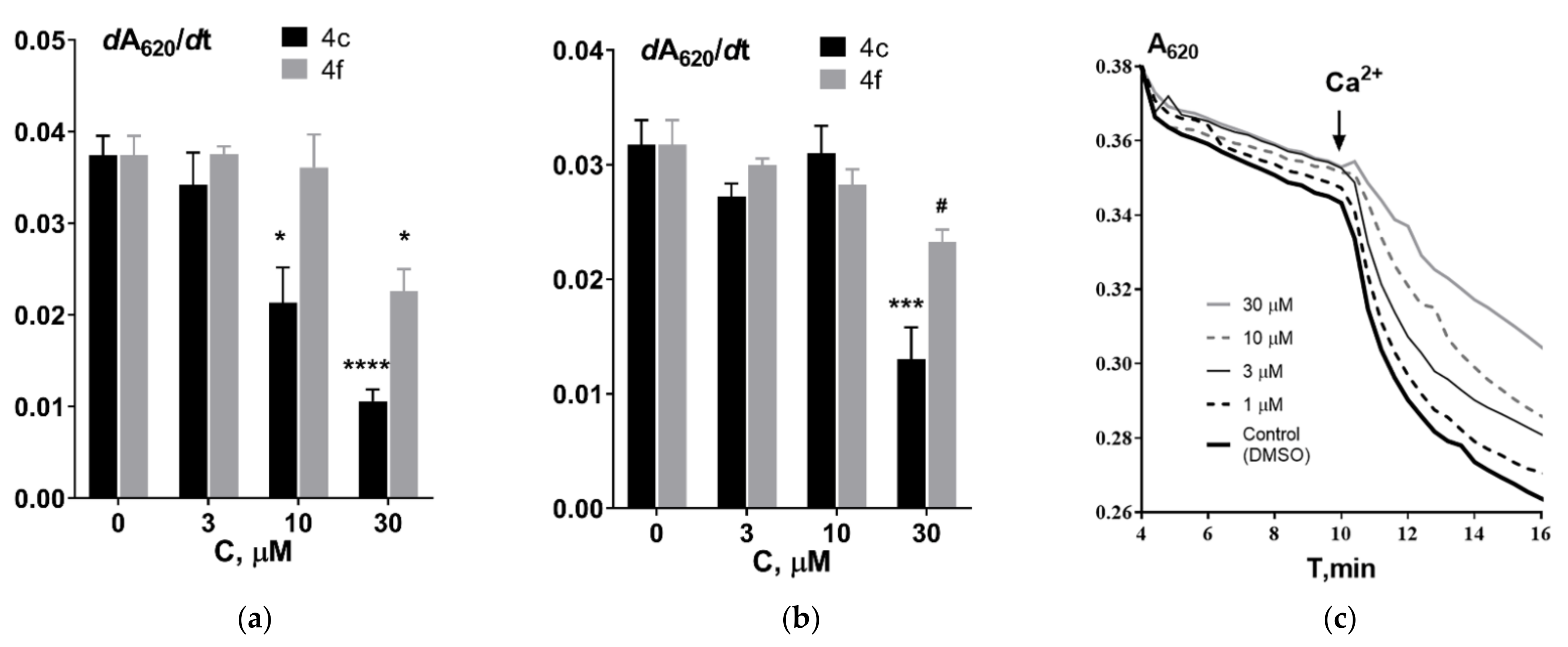
2.3.2. Kinetic Studies of AChE and BChE Inhibition
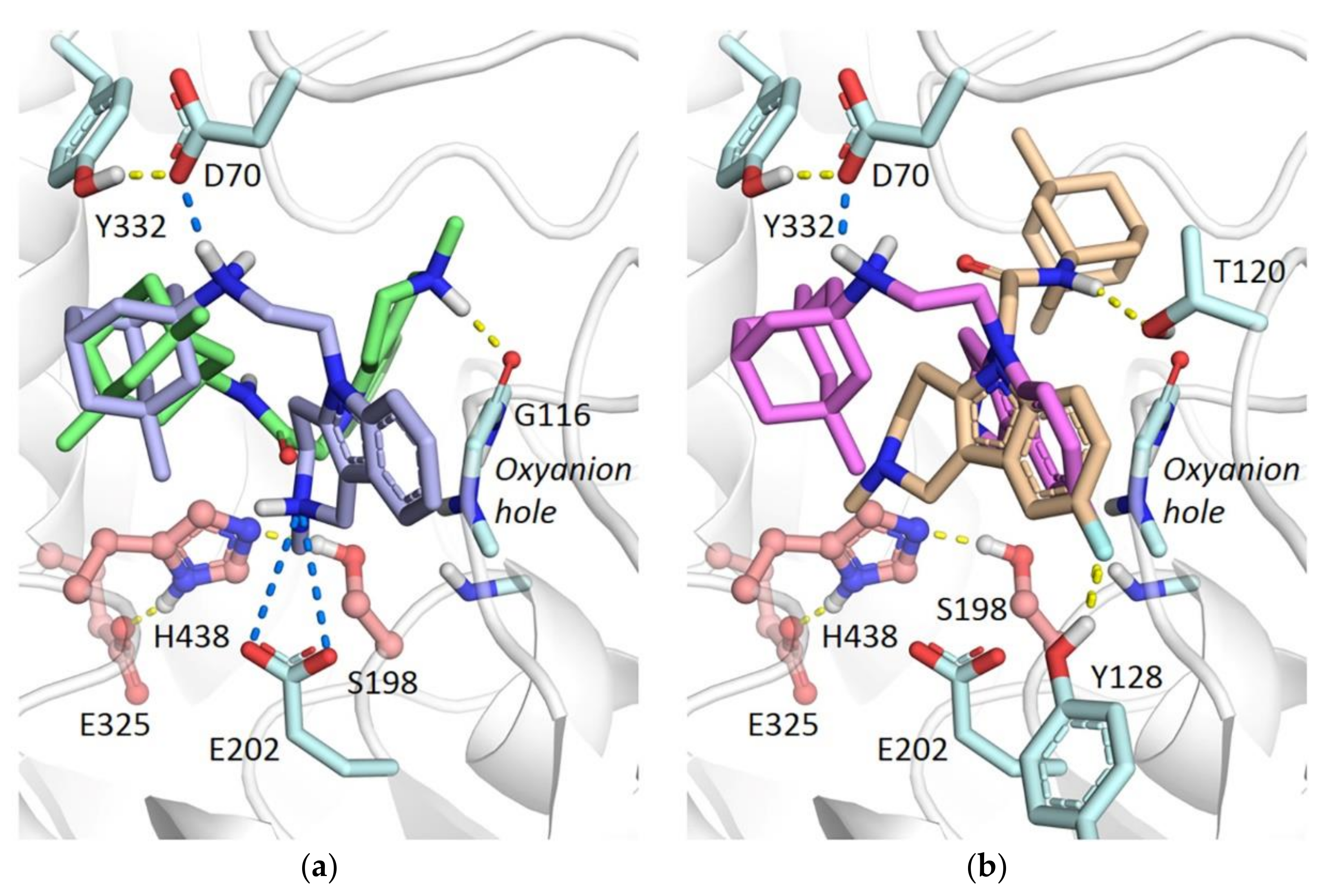
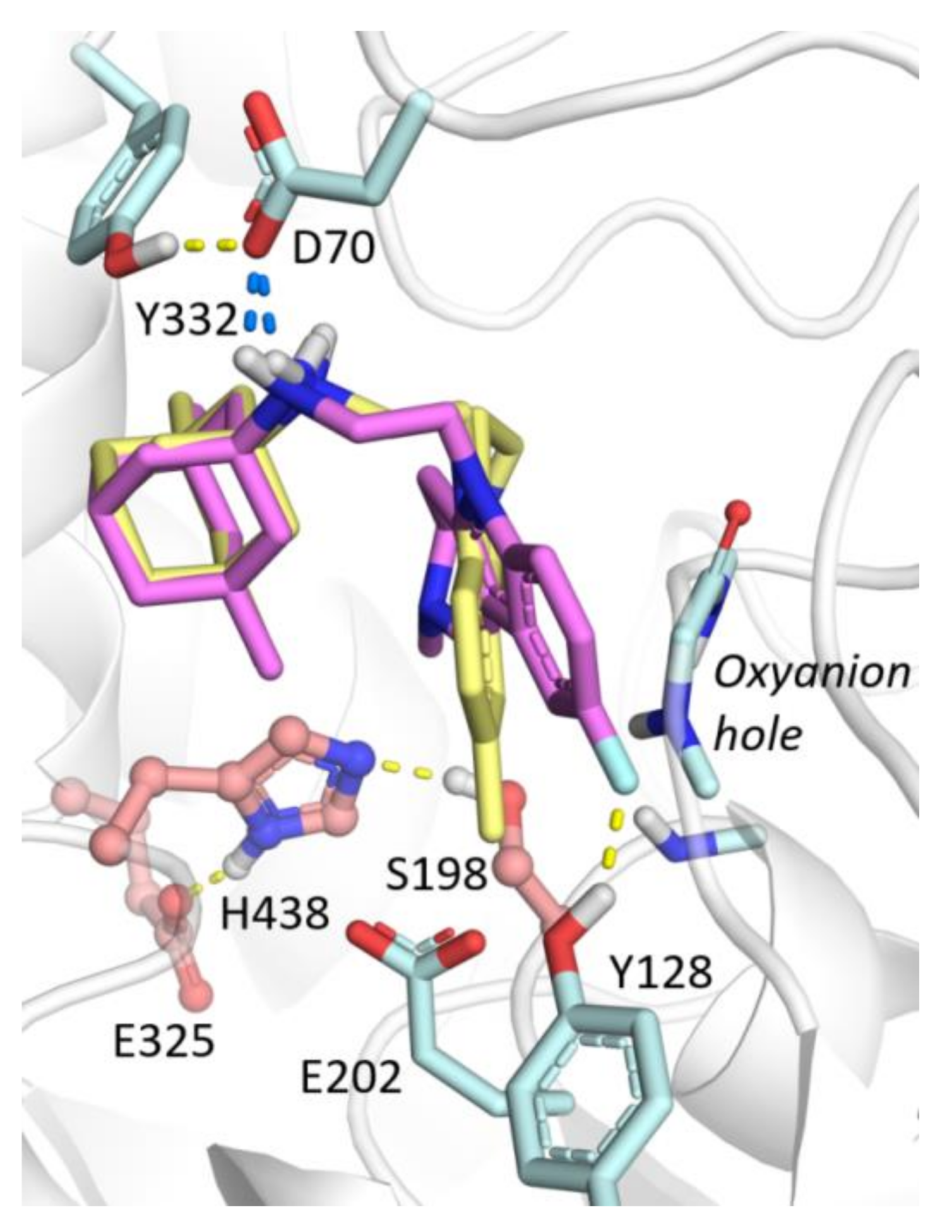
2.3.3. Molecular Docking to BChE
2.3.4. Molecular Docking to AChE
2.3.5. Evaluation of Conjugates Ability to Displace Propidium Iodide from the EeAChE PAS
2.4. Effect of Aminoadamantane–γ-Carboline Conjugates on the Functional Properties of Isolated Rat Liver Mitochondria
2.5. Effect of Aminoadamantane–γ-Carboline Conjugates on Tubulin Polymerization
3. Materials and Methods
3.1. Synthesis Method and Spectral Data
3.1.1. General Procedure for the Preparation of Compounds 4a–f
3.1.2. General Procedure for the Preparation of Compounds 8a–f
3.2. Evaluation of the Effect of New Compounds on NMDA-Receptors by a Radioligand Binding Assay
3.3. Analysis of the Esterase Profile of Compounds and Inhibition Kinetics of AChE and BChE
3.4. Displacement of Propidium Iodide from AChE PAS
3.5. Investigation of the Effect of the Compounds on Mitochondrial Characteristics
3.6. MTT Assay
3.7. Evaluation of the Effect of Compounds on Tubulin Polymerization
3.8. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AChE | acetylcholinesterase; |
| BChE | butyrylcholinesterase; |
| BNPP | bis-4-nitrophenyl phosphate; |
| CAS | catalytic active site; |
| CES | carboxylesterase; |
| DMSO | dimethyl sulfoxide; |
| EeAChE | AChE from Electrophorus electricus; |
| MPT | mitochondrial permeability transition; |
| PAS | peripheral anionic site; |
| QM/MM | quantum mechanics/molecular mechanics; |
| Tb+MAP | tubulin and microtubule-associated proteins. |
References
- World Alzheimer Report 2018 The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International (ADI): London, UK, 2018.
- Bachurin, S.O.; Gavrilova, S.I.; Samsonova, A.; Barreto, G.E.; Aliev, G. Mild cognitive impairment due to Alzheimer disease: Contemporary approaches to diagnostics and pharmacological intervention. Pharmacol. Res. 2018, 129, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Xie, Y.; Meng, X.Y.; Kang, J.S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct.Target Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Calabro, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The biological pathways of Alzheimer disease: A review. AIMS Neurosci. 2021, 8, 86–132. [Google Scholar] [CrossRef] [PubMed]
- Albertini, C.; Salerno, A.; de Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2021, 41, 2606–2633. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Bovina, E.V.; Ustyugov, A.A. Drugs in Clinical Trials for Alzheimer’s Disease: The Major Trends. Med. Res. Rev. 2017, 37, 1186–1225. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Makhaeva, G.F.; Shevtsova, E.F.; Boltneva, N.P.; Kovaleva, N.V.; Lushchekina, S.V.; Rudakova, E.V.; Dubova, L.G.; Vinogradova, D.V.; Sokolov, V.B.; et al. Conjugates of methylene blue with gamma-carboline derivatives as new multifunctional agents for the treatment of neurodegenerative diseases. Sci. Rep. 2019, 9, 4873. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Shevtsova, E.F.; Makhaeva, G.F.; Grigoriev, V.V.; Boltneva, N.P.; Kovaleva, N.V.; Lushchekina, S.V.; Shevtsov, P.N.; Neganova, M.E.; Redkozubova, O.M.; et al. Novel conjugates of aminoadamantanes with carbazole derivatives as potential multitarget agents for AD treatment. Sci. Rep. 2017, 7, 45627. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Sokolov, V.B.; Grigoriev, V.V.; Serebryakova, O.G.; Vikhareva, E.A.; Aksinenko, A.Y.; Barreto, G.E.; Aliev, G.; et al. Conjugates of γ-carbolines and phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer disease. Sci. Rep. 2015, 5, 13164. [Google Scholar] [CrossRef] [Green Version]
- Makhaeva, G.F.; Sokolov, V.B.; Shevtsova, E.F.; Kovaleva, N.V.; Lushchekina, S.V.; Boltneva, N.P.; Rudakova, E.V.; Aksinenko, A.Y.; Shevtsov, P.N.; Neganova, M.E.; et al. Focused design of polypharmacophoric neuroprotective compounds: Conjugates of γ-carbolines with carbazole derivatives and tetrahydrocarbazole. Pure Appl. Chem. 2017, 89, 1167–1184. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Shevtsova, E.F.; Boltneva, N.P.; Lushchekina, S.V.; Kovaleva, N.V.; Rudakova, E.V.; Bachurin, S.O.; Richardson, R.J. Overview of novel multifunctional agents based on conjugates of γ-carbolines, carbazoles, tetrahydrocarbazoles, phenothiazines, and aminoadamantanes for treatment of Alzheimer’s disease. Chem. Biol. Interact. 2019, 308, 224–234. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Shevtsova, E.F.; Aksinenko, A.Y.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Rudakova, E.V.; Pushkareva, E.A.; Serkova, T.P.; Dubova, L.G.; et al. Bis-γ-carbolines as new potential multitarget agents for Alzheimer’s disease. Pure Appl. Chem. 2020, 92, 1057–1080. [Google Scholar] [CrossRef]
- Lipton, S. The Molecular Basis of Memantine Action in Alzheimers Disease and Other Neurologic Disorders: Low-affinity, Uncompetitive Antagonism. Curr. Alzheimer Res. 2005, 2, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Sufian, M.A.; Uddin, M.S.; Begum, M.M.; Akhter, S.; Islam, A.; Mathew, B.; Islam, M.S.; Amran, M.S.; Md Ashraf, G. NMDA Receptor Antagonists: Repositioning of Memantine as a Multitargeting Agent for Alzheimer’s Therapy. Curr. Pharm. Des. 2019, 25, 3506–3518. [Google Scholar] [CrossRef]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Bharadwaj, P.R.; Bates, K.A.; Porter, T.; Teimouri, E.; Perry, G.; Steele, J.W.; Gandy, S.; Groth, D.; Martins, R.N.; Verdile, G. Latrepirdine: Molecular mechanisms underlying potential therapeutic roles in Alzheimer’s and other neurodegenerative diseases. Transl. Psychiatry 2013, 3, e332. [Google Scholar] [CrossRef]
- Eckert, S.H.; Gaca, J.; Kolesova, N.; Friedland, K.; Eckert, G.P.; Muller, W.E. Mitochondrial Pharmacology of Dimebon (Latrepirdine) Calls for a New Look at its Possible Therapeutic Potential in Alzheimer’s Disease. Aging Dis. 2018, 9, 729–744. [Google Scholar] [CrossRef] [Green Version]
- Ustyugov, A.; Shevtsova, E.; Ashraf, G.M.; Tarasov, V.V.; Bachurin, S.O.; Aliev, G. New Therapeutic Property of Dimebon as a Neuroprotective Agent. Curr. Med. Chem. 2018, 25, 5315–5326. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Vinogradova, D.V.; Kireeva, E.G.; Reddy, V.P.; Aliev, G.; Bachurin, S.O. Dimebon attenuates the Abeta-induced mitochondrial permeabilization. Curr. Alzheimer Res. 2014, 11, 422–429. [Google Scholar] [CrossRef]
- Ustyugov, A.; Shevtsova, E.; Bachurin, S. Novel Sites of Neuroprotective Action of Dimebon (Latrepirdine). Mol. Neurobiol. 2015, 52, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Q.; Bezprozvanny, I. Evaluation of Dimebon in cellular model of Huntington’s disease. Mol. Neurodegener. 2008, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, V.B.; Aksinenko, A.Y.; Epishina, T.A.; Goreva, T.V. Vinylation of Tetrahydro-γ-carbolines. Russ. J. Gen. Chem. 2018, 88, 1931–1933. [Google Scholar] [CrossRef]
- Bukke, V.N.; Archana, M.; Villani, R.; Romano, A.D.; Wawrzyniak, A.; Balawender, K.; Orkisz, S.; Beggiato, S.; Serviddio, G.; Cassano, T. The Dual Role of Glutamatergic Neurotransmission in Alzheimer’s Disease: From Pathophysiology to Pharmacotherapy. Int. J. Mol. Sci. 2020, 21, 7452. [Google Scholar] [CrossRef]
- Volianskis, A.; France, G.; Jensen, M.S.; Bortolotto, Z.A.; Jane, D.E.; Collingridge, G.L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain. Res. 2015, 1621, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Pagano, J.; Giona, F.; Beretta, S.; Verpelli, C.; Sala, C. N-methyl-d-aspartate receptor function in neuronal and synaptic development and signaling. Curr. Opin. Pharmacol. 2021, 56, 93–101. [Google Scholar] [CrossRef]
- Tikhonova, I.G.; Baskin, I.I.; Palyulin, V.A.; Zefirov, N.S.; Bachurin, S.O. Structural basis for understanding structure-activity relationships for the glutamate binding site of the NMDA receptor. J. Med. Chem. 2002, 45, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Lappi, S. Interaction of fluorescence probes with acetylcholinesterase. Site and specificity of propidium binding. Biochemistry 1975, 14, 1989–1997. [Google Scholar] [CrossRef]
- Ahmed, H.; Haider, A.; Ametamey, S.M. N-Methyl-D-Aspartate (NMDA) receptor modulators: A patent review (2015-present). Expert Opin. Ther. Pat. 2020, 30, 743–767. [Google Scholar] [CrossRef]
- Companys-Alemany, J.; Turcu, A.L.; Bellver-Sanchis, A.; Loza, M.I.; Brea, J.M.; Canudas, A.M.; Leiva, R.; Vazquez, S.; Pallas, M.; Grinan-Ferre, C. A Novel NMDA Receptor Antagonist Protects against Cognitive Decline Presented by Senescent Mice. Pharmaceutics 2020, 12, 284. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.; Lingenfelter, K.S.; Bender, A.M.; Lindsley, C.W. Classics in Chemical Neuroscience: Memantine. ACS Chem. Neurosci. 2017, 8, 1823–1829. [Google Scholar] [CrossRef] [PubMed]
- Limapichat, W.; Yu, W.Y.; Branigan, E.; Lester, H.A.; Dougherty, D.A. Key binding interactions for memantine in the NMDA receptor. ACS Chem. Neurosci. 2013, 4, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.P.; Raymond, L.A. Extrasynaptic NMDA receptor involvement in central nervous system disorders. Neuron 2014, 82, 279–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, M.; Yong, X.L.H.; Roche, K.W.; Anggono, V. Regulation of NMDA glutamate receptor functions by the GluN2 subunits. J. Neurochem. 2020, 154, 121–143. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Jiang, X.; Zu, Y.; Yang, Y.; Liu, Y.; Sun, X.; Xu, Z.; Ding, H.; Zhao, Q. A comprehensive description of GluN2B-selective N-methyl-D-aspartate (NMDA) receptor antagonists. Eur. J. Med. Chem. 2020, 200, 112447. [Google Scholar] [CrossRef]
- Schreiber, J.A.; Schepmann, D.; Frehland, B.; Thum, S.; Datunashvili, M.; Budde, T.; Hollmann, M.; Strutz-Seebohm, N.; Wunsch, B.; Seebohm, G. A common mechanism allows selective targeting of GluN2B subunit-containing N-methyl-D-aspartate receptors. Commun. Biol. 2019, 2, 420. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2006, 15, 1–12. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agatonovic-Kustrin, S.; Kettle, C.; Morton, D.W. A molecular approach in drug development for Alzheimer’s disease. Biomed. Pharmacother. 2018, 106, 553–565. [Google Scholar] [CrossRef] [PubMed]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.S.; Greig, N.H.; Nagai, T.; et al. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-beta peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Nordberg, A.; Ballard, C.; Bullock, R.; Darreh-Shori, T.; Somogyi, M. A review of butyrylcholinesterase as a therapeutic target in the treatment of Alzheimer’s disease. Prim. Care Companion CNS Disord. 2013, 15, PCC.12r01412. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [Green Version]
- Košak, U.; Brus, B.; Knez, D.; Šink, R.; Žakelj, S.; Trontelj, J.; Pišlar, A.; Šlenc, J.; Gobec, M.; Živin, M.; et al. Development of an in-vivo active reversible butyrylcholinesterase inhibitor. Sci. Rep. 2016, 6, 39495. [Google Scholar] [CrossRef] [Green Version]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Andrisano, V.; Naldi, M.; De Simone, A.; Bartolini, M. A patent review of butyrylcholinesterase inhibitors and reactivators 2010–2017. Expert Opin. Ther. Pat. 2018, 28, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Laizure, S.C.; Herring, V.; Hu, Z.; Witbrodt, K.; Parker, R.B. The role of human carboxylesterases in drug metabolism: Have we overlooked their importance? Pharmacotherapy 2013, 33, 210–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makhaeva, G.F.; Rudakova, E.V.; Kovaleva, N.V.; Lushchekina, S.V.; Boltneva, N.P.; Proshin, A.N.; Shchegolkov, E.V.; Burgart, Y.V.; Saloutin, V.I. Cholinesterase and carboxylesterase inhibitors as pharmacological agents. Russ. Chem. Bull. 2019, 68, 967–984. [Google Scholar] [CrossRef]
- Hsiao, K.; Chapman, P.; Nilsen, S.; Eckman, C.; Harigaya, Y.; Younkin, S.; Yang, F.; Cole, G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 1996, 274, 99–102. [Google Scholar] [CrossRef]
- Hardy, J.; Bogdanovic, N.; Winblad, B.; Portelius, E.; Andreasen, N.; Cedazo-Minguez, A.; Zetterberg, H. Pathways to Alzheimer’s disease. J. Intern. Med. 2014, 275, 296–303. [Google Scholar] [CrossRef]
- Moran, M.A.; Mufson, E.J.; Gomez-Ramos, P. Cholinesterases colocalize with sites of neurofibrillary degeneration in aged and Alzheimer’s brains. Acta Neuropathol. 1994, 87, 284–292. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-β-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [Green Version]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. β-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Dinamarca, M.C.; Alvarez, A. Amyloid-cholinesterase interactions. Implications for Alzheimer’s disease. FEBS J. 2008, 275, 625–632. [Google Scholar] [CrossRef]
- Arce, M.P.; Rodriguez-Franco, M.I.; Gonzalez-Munoz, G.C.; Perez, C.; Lopez, B.; Villarroya, M.; Lopez, M.G.; Garcia, A.G.; Conde, S. Neuroprotective and cholinergic properties of multifunctional glutamic acid derivatives for the treatment of Alzheimer’s disease. J. Med. Chem. 2009, 52, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; Formosa, X.; Galdeano, C.; Gomez, T.; Munoz-Torrero, D.; Ramirez, L.; Viayna, E.; Gomez, E.; Isambert, N.; Lavilla, R.; et al. Tacrine-based dual binding site acetylcholinesterase inhibitors as potential disease-modifying anti-Alzheimer drug candidates. Chem. Biol. Interact. 2010, 187, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Zueva, I.; Dias, J.; Lushchekina, S.; Semenov, V.; Mukhamedyarov, M.; Pashirova, T.; Babaev, V.; Nachon, F.; Petrova, N.; Nurullin, L.; et al. New evidence for dual binding site inhibitors of acetylcholinesterase as improved drugs for treatment of Alzheimer’s disease. Neuropharmacology 2019, 155, 131–141. [Google Scholar] [CrossRef]
- Mesulam, M.M.; Geula, C. Butyrylcholinesterase reactivity differentiates the amyloid plaques of aging from those of dementia. Ann. Neurol. 1994, 36, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Cash, M.K.; Reid, G.A.; Martin, E.; Mitnitski, A.; Geula, C. Butyrylcholinesterase is associated with beta-amyloid plaques in the transgenic APPSWE/PSEN1dE9 mouse model of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2012, 71, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Reid, G.A.; Darvesh, S. Butyrylcholinesterase-knockout reduces brain deposition of fibrillar beta-amyloid in an Alzheimer mouse model. Neuroscience 2015, 298, 424–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darvesh, S.; Reid, G.A. Reduced fibrillar beta-amyloid in subcortical structures in a butyrylcholinesterase-knockout Alzheimer disease mouse model. Chem. Biol. Interact. 2016, 259, 307–312. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Radchenko, E.V.; Palyulin, V.A.; Rudakova, E.V.; Aksinenko, A.Y.; Sokolov, V.B.; Zefirov, N.S.; Richardson, R.J. Organophosphorus compound esterase profiles as predictors of therapeutic and toxic effects. Chem. Biol. Interact. 2013, 203, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Lushchekina, S.V.; Kovaleva, N.V.; Yu Astakhova, T.; Boltneva, N.P.; Rudakova, E.V.; Serebryakova, O.G.; Proshin, A.N.; Serkov, I.V.; Trofimova, T.P.; et al. Amiridine-piperazine hybrids as cholinesterase inhibitors and potential multitarget agents for Alzheimer’s disease treatment. Bioorg. Chem. 2021, 112, 104974. [Google Scholar] [CrossRef]
- Copeland, R.A. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists; Wiley: New York, NY, USA, 2005; p. 384. [Google Scholar]
- Carosati, E.; Sciabola, S.; Cruciani, G. Hydrogen bonding interactions of covalently bonded fluorine atoms: From crystallographic data to a new angular function in the GRID force field. J. Med. Chem. 2004, 47, 5114–5125. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Kharlamova, A.D.; Petukhova, E.O.; Mikhailov, A.S.; Podyachev, S.N.; Saifina, L.F.; Petrov, K.A.; et al. 6-Methyluracil Derivatives as Bifunctional Acetylcholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. ChemMedChem 2015, 10, 1863–1874. [Google Scholar] [CrossRef]
- Lushchekina, S.V.; Masson, P. Slow-binding inhibitors of acetylcholinesterase of medical interest. Neuropharmacology 2020, 177, 108236. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, B.; Pérez, C.; Morales-García, J.A.; Alonso-Gil, S.; Pérez-Castillo, A.; Romero, A.; López, M.G.; Villarroya, M.; Conde, S.; Rodríguez-Franco, M.I. New Melatonin–N,N-Dibenzyl(N-methyl)amine Hybrids: Potent Neurogenic Agents with Antioxidant, Cholinergic, and Neuroprotective Properties as Innovative Drugs for Alzheimer’s Disease. J. Med. Chem. 2014, 57, 3773–3785. [Google Scholar] [CrossRef]
- Rochais, C.; Lecoutey, C.; Gaven, F.; Giannoni, P.; Hamidouche, K.; Hedou, D.; Dubost, E.; Genest, D.; Yahiaoui, S.; Freret, T.; et al. Novel multitarget-directed ligands (MTDLs) with acetylcholinesterase (AChE) inhibitory and serotonergic subtype 4 receptor (5-HT4R) agonist activities as potential agents against Alzheimer’s disease: The design of donecopride. J. Med. Chem. 2015, 58, 3172–3187. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Rudakova, E.V.; Proshin, A.N.; Serkov, I.V.; Radchenko, E.V.; Palyulin, V.A.; et al. New Hybrids of 4-Amino-2,3-polymethylene-quinoline and p-Tolylsulfonamide as Dual Inhibitors of Acetyl- and Butyrylcholinesterase and Potential Multifunctional Agents for Alzheimer’s Disease Treatment. Molecules 2020, 25, 3915. [Google Scholar] [CrossRef]
- Ghotbi, G.; Mahdavi, M.; Najafi, Z.; Moghadam, F.H.; Hamzeh-Mivehroud, M.; Davaran, S.; Dastmalchi, S. Design, synthesis, biological evaluation, and docking study of novel dual-acting thiazole-pyridiniums inhibiting acetylcholinesterase and β-amyloid aggregation for Alzheimer’s disease. Bioorg. Chem. 2020, 103, 104186. [Google Scholar] [CrossRef]
- Sánchez Montero, J.M.; Agis-Torres, A.; Solano, D.; Söllhuber, M.; Fernandez, M.; Villaro, W.; Gómez-Cañas, M.; García-Arencibia, M.; Fernández-Ruiz, J.; Egea, J.; et al. Analogues of cannabinoids as multitarget drugs in the treatment of Alzheimer’s disease. Eur. J. Pharmacol. 2021, 895, 173875. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Rudakova, E.V.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Radchenko, E.V.; et al. Conjugates of tacrine and 1,2,4-thiadiazole derivatives as new potential multifunctional agents for Alzheimer’s disease treatment: Synthesis, quantum-chemical characterization, molecular docking, and biological evaluation. Bioorg. Chem. 2020, 94, 103387. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, E.F.; Maltsev, A.V.; Vinogradova, D.V.; Shevtsov, P.N.; Bachurin, S.O. Mitochondria as a promising target for developing novel agents for treating Alzheimer’s disease. Med. Res. Rev. 2021, 41, 803–827. [Google Scholar] [CrossRef]
- Shevtzova, E.F.; Kireeva, E.G.; Bachurin, S.O. Effect of beta-amyloid peptide fragment 25-35 on nonselective permeability of mitochondria. Bull. Exp. Biol. Med. 2001, 132, 1173–1176. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Vinogradova, D.V.; Shevtsova, E.F.; Goreva, T.V.; Epishina, T.A.; Aksinenko, A.Y.; Sokolov, V.B. Modification of gamma-carbolines with N-substituted propionamides as a new approach to mitoprotective agents. Russ. Chem. Bull. 2014, 62, 816–819. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Vinogradova, D.V.; Neganova, M.E.; Shevtsov, P.N.; Lednev, B.V.; Bachurin, S.O. Mitochondria are an Important Target in the Search for new Drugs for the Treatment of Alzheimer′s Disease and Senile Dementia. Biomed. Chem. Res. Meth. 2018, 1, e00058. [Google Scholar] [CrossRef]
- Boyman, L.; Coleman, A.K.; Zhao, G.; Wescott, A.P.; Joca, H.C.; Greiser, B.M.; Karbowski, M.; Ward, C.W.; Lederer, W.J. Dynamics of the mitochondrial permeability transition pore: Transient and permanent opening events. Arch. Biochem. Biophys. 2019, 666, 31–39. [Google Scholar] [CrossRef]
- Varidaki, A.; Hong, Y.; Coffey, E.T. Repositioning Microtubule Stabilizing Drugs for Brain Disorders. Front. Cell Neurosci. 2018, 12, 226. [Google Scholar] [CrossRef]
- Cattanach, C.J.; Cohen, A.; Heath-Brown, B. Studies in the indole series. IV. Tetrahydro-1H-pyrido[4,3-b]-indoles as serotonin antagonists. J. Chem Soc. Perkin 1 1968, 10, 1235–1243. [Google Scholar] [CrossRef]
- Toscan, C.E.; Rahimi, M.; Bhadbhade, M.; Pickford, R.; McAlpine, S.R.; Lock, R.B. Thioimidazoline based compounds reverse glucocorticoid resistance in human acute lymphoblastic leukemia xenografts. Org. Biomol. Chem. 2015, 13, 6299–6312. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.-M.; Gu, Z.-Q.; Costa, A.M.; Yamada, K.A.; Mansson, P.E.; Giordano, T.; Skolnick, P.; Jones, K.A. (2S, 4R)-4-methylglutamic acid (SYM 2081): A selective, high-affinity ligand for kainate receptors. J. Pharmacol. Exp. Ther. 1997, 280, 422–427. [Google Scholar]
- Dana, C.; Benavides, J.; Schoemaker, H.; Scatton, B. Pharmacological characterisation and autoradiographic distribution of polyamine-sensitive [3H]ifenprodil binding sites in the rat brain. Neurosci. Lett. 1991, 125, 45–48. [Google Scholar] [CrossRef]
- Coughenour, L.L.; Barr, B.M. Use of trifluoroperazine isolates a [(3)H]Ifenprodil binding site in rat brain membranes with the pharmacology of the voltage-independent ifenprodil site on N-methyl-D-aspartate receptors containing NR2B subunits. J. Pharmacol. Exp. Ther. 2001, 296, 150–159. [Google Scholar]
- Nowak, G.; Trullas, R.; Layer, R.T.; Skolnick, P.; Paul, I.A. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J. Pharmacol. Exp. Ther. 1993, 265, 1380–1386. [Google Scholar] [PubMed]
- Bachurin, S.; Tkachenko, S.; Baskin, I.; Lermontova, N.; Mukhina, T.; Petrova, L.; Ustinov, A.; Proshin, A.; Grigoriev, V.; Lukoyanov, N.; et al. Neuroprotective and cognition-enhancing properties of MK-801 flexible analogs. Structure-activity relationships. Ann. N. Y. Acad. Sci. 2001, 939, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Newton, P.; Harrison, P.; Clulow, S. A novel method for determination of the affinity of protein: Protein interactions in homogeneous assays. J. Biomol. Screen. 2008, 13, 674–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sterri, S.H.; Johnsen, B.A.; Fonnum, F. A radiochemical assay method for carboxylesterase, and comparison of enzyme activity towards the substrates methyl [1-14C] butyrate and 4-nitrophenyl butyrate. Biochem. Pharmacol. 1985, 34, 2779–2785. [Google Scholar] [CrossRef]
- Bachurin, S.O.; Shevtzova, E.P.; Lermontova, N.N.; Serkova, T.P.; Ramsay, R.R. The effect of dithiocarbamates on neurotoxic action of 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP) and on mitochondrial respiration chain. Neurotoxicology 1996, 17, 897–903. [Google Scholar]
- Åkerman, K.E.O.; Wikström, M.K.F. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Niks, M.; Otto, M. Towards an Optimized Mtt Assay. J. Immunol. Methods 1990, 130, 149–151. [Google Scholar] [CrossRef]
- Shelanski, M.L.; Gaskin, F.; Cantor, C.R. Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA 1973, 70, 765–768. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, P.; Lushchekina, S.; Schopfer, L.M.; Lockridge, O. Effects of viscosity and osmotic stress on the reaction of human butyrylcholinesterase with cresyl saligenin phosphate, a toxicant related to aerotoxic syndrome: Kinetic and molecular dynamics studies. Biochem. J. 2013, 454, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Voevodin, V.; Antonov, A.; Nikitenko, D.; Shvets, P.; Sobolev, S.; Sidorov, I.; Stefanov, K.; Voevodin, V.; Zhumatiy, S. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6. [Google Scholar] [CrossRef] [Green Version]



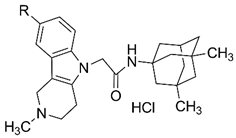
| Compound | R | Ki, µM, MK801-Binding Site | Ki, µM, Ifenprodil-Binding Site |
|---|---|---|---|
 | |||
| 4a | H | 1.9 ± 0.2 | 1.7 ± 0.2 |
| 4b | Me | 7.9 ± 0.8 | 8.8 ± 1.0 |
| 4c | F | 4.7 ± 0.5 | 3.1 ± 0.3 |
 | |||
| 8a | H | 63.5 ± 5.8 | 112.7 ± 10.4 |
| 8b | Me | 55.1 ± 5.1 | 89.7 ± 8.7 |
| 8c | F | 80.6 ± 8.5 | 110.2 ± 10.8 |
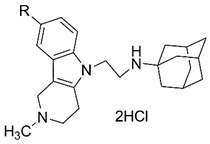 | |||
| 4d | H | 3.3 ± 0.3 | 4.4 ± 0.3 |
| 4e | Me | 10.3 ± 1.6 | 8.2 ± 0.9 |
| 4f | F | 5.4 ± 0.6 | 5.1 ± 0.5 |
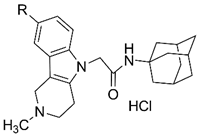 | |||
| 8d | H | 49.5 ± 4.5 | 79.1 ± 7.1 |
| 8e | Me | 72.7 ± 6.6 | 104.5 ± 10.0 |
| 8f | F | 47.3 ± 4.7 | 73.8 ± 7.0 |
| Reference Agents | |||
| dimebon | 50.4 ± 4.3 | 77.2 ± 5.7 | |
| memantine | 0.8 ± 0.1 | 115.1 ± 6.5 | |
| No | R | IC50 (µM) or % of Inhibition of the Enzyme with 20 µM Concentration of the Compound | % of Displacement of Propidium Iodide from the PAS of EeAChE with 20 µM Concentration of the Compound | ||
|---|---|---|---|---|---|
| AChE | BChE | CES | |||
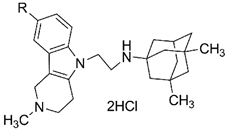 | |||||
| 4a | H | 19.5 ± 0.3% | 0.666 ± 0.037 | 15.6 ± 0.3% | 12.5 ± 1.1% |
| 4b | Me | 50.3 ± 3.4 | 5.07 ± 0.09 | 10.4 ± 0.5% | 14.7 ± 0.9% |
| 4c | F | 39.7 ± 3.2 | 0.729 ± 0.011 | 6.9 ± 0.4% | 13.1 ± 1.0% |
 | |||||
| 8a | H | 7.1 ± 0.5% | 9.66 ± 0.52 | 5.2 ± 1.2% | n.a. |
| 8b | Me | 17.7 ± 1.4% | 33.7 ± 3.5 | 6.4 ± 0.5% | 2.8 ± 0.2% |
| 8c | F | 17.3 ± 0.8% | 6.00 ± 0.06 | 8.4 ± 0.9% | n.a. |
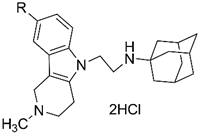 | |||||
| 4d | H | 112 ± 5 | 2.48 ± 0.22 | 2.9 ± 0.8% | 10.1 ± 0.9% |
| 4e | Me | 63.3 ± 3.3 | 9.05 ± 0.80 | 2.5 ± 0.5% | 15.1 ± 1.2% |
| 4f | F | 13.6 ± 1.3 | 2.53 ± 0.10 | 8.6 ± 1.7% | 17.8 ± 1.5% |
 | |||||
| 8d | H | 5.4 ± 1.0% | 26.5 ± 0.4 | 8.8 ± 1.2% | 1.4 ± 0.2% |
| 8e | Me | 13.5 ± 0.9% | 43.2 ± 2.9 | 9.9 ± 1.5% | 1.2 ± 0.1% |
| 8f | F | 22.1 ± 1.8% | 17.1 ± 1.3 | 6.2 ± 0.6% | n.a. |
| Reference Agents | |||||
| dimebon | 36.3 ± 3.6 | 5.76 ± 0.51 | n.a. | 4.1 ± 0.3% | |
| memantine | 1.3 ± 0.2% | 13.7 ± 1.5% | 3.0 ± 0.4% | n.a. | |
| donepezil | 0.040 ± 0.004 | 19.2 ± 2.0 | >100 | 11.9 ± 0.9% | |
| tacrine | 0.60 ± 0.05 | 0.029 ± 0.002 | n.a. | 4.2 ± 0.3% | |
| BNPP | n.a. | n.a. | 1.80 ± 0.11 | n.e. | |
| Compound | R | Rate of Ca2+-Induced Mitochondrial Swelling (dA620/dt), % * | Rate of Tb+MAP Polymerization (dA355/dt), % ** |
|---|---|---|---|
 | |||
| 4a | H | 33 ± 4 | 126 ± 9 |
| 4b | Me | 39 ± 7 | 153 ± 33 |
| 4c | F | 31 ± 10 | 166 ± 11 |
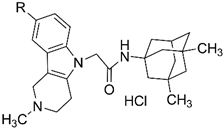 | |||
| 8a | H | 42 ± 21 | 237 ± 55 |
| 8b | Me | 87 ± 33 | 314 ± 46 |
| 8c | F | 63 ± 25 | 359 ± 25 |
 | |||
| 4d | H | 35 ± 13 | 162 ± 19 |
| 4e | Me | 42 ± 9 | 239 ± 21 |
| 4f | F | 42 ± 8 | 356 ± 14 |
 | |||
| 8d | H | 45 ± 17 | 128 ± 15 |
| 8e | Me | 49 ± 12 | 133 ± 28 |
| 8f | F | 61 ± 24 | 154 ± 14 |
| Reference Agents | |||
| dimebon | 36 ± 11 | 162 ± 23 | |
| memantine | 70 ± 9 | 89 ± 7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachurin, S.O.; Makhaeva, G.F.; Shevtsova, E.F.; Aksinenko, A.Y.; Grigoriev, V.V.; Shevtsov, P.N.; Goreva, T.V.; Epishina, T.A.; Kovaleva, N.V.; Pushkareva, E.A.; et al. Conjugation of Aminoadamantane and γ-Carboline Pharmacophores Gives Rise to Unexpected Properties of Multifunctional Ligands. Molecules 2021, 26, 5527. https://doi.org/10.3390/molecules26185527
Bachurin SO, Makhaeva GF, Shevtsova EF, Aksinenko AY, Grigoriev VV, Shevtsov PN, Goreva TV, Epishina TA, Kovaleva NV, Pushkareva EA, et al. Conjugation of Aminoadamantane and γ-Carboline Pharmacophores Gives Rise to Unexpected Properties of Multifunctional Ligands. Molecules. 2021; 26(18):5527. https://doi.org/10.3390/molecules26185527
Chicago/Turabian StyleBachurin, Sergey O., Galina F. Makhaeva, Elena F. Shevtsova, Alexey Yu. Aksinenko, Vladimir V. Grigoriev, Pavel N. Shevtsov, Tatiana V. Goreva, Tatiana A. Epishina, Nadezhda V. Kovaleva, Elena A. Pushkareva, and et al. 2021. "Conjugation of Aminoadamantane and γ-Carboline Pharmacophores Gives Rise to Unexpected Properties of Multifunctional Ligands" Molecules 26, no. 18: 5527. https://doi.org/10.3390/molecules26185527
APA StyleBachurin, S. O., Makhaeva, G. F., Shevtsova, E. F., Aksinenko, A. Y., Grigoriev, V. V., Shevtsov, P. N., Goreva, T. V., Epishina, T. A., Kovaleva, N. V., Pushkareva, E. A., Boltneva, N. P., Lushchekina, S. V., Gabrelyan, A. V., Zamoyski, V. L., Dubova, L. G., Rudakova, E. V., Fisenko, V. P., Bovina, E. V., & Richardson, R. J. (2021). Conjugation of Aminoadamantane and γ-Carboline Pharmacophores Gives Rise to Unexpected Properties of Multifunctional Ligands. Molecules, 26(18), 5527. https://doi.org/10.3390/molecules26185527









