Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome
Abstract
1. Background
2. Materials and Methods
2.1. Autopsy Procedure
2.2. Morphometric Analysis of the Ovaries
2.3. Hormone Quantification
2.4. Statistical Analysis
3. Results
3.1. Estrus Cycle
3.2. Ovulatory Response
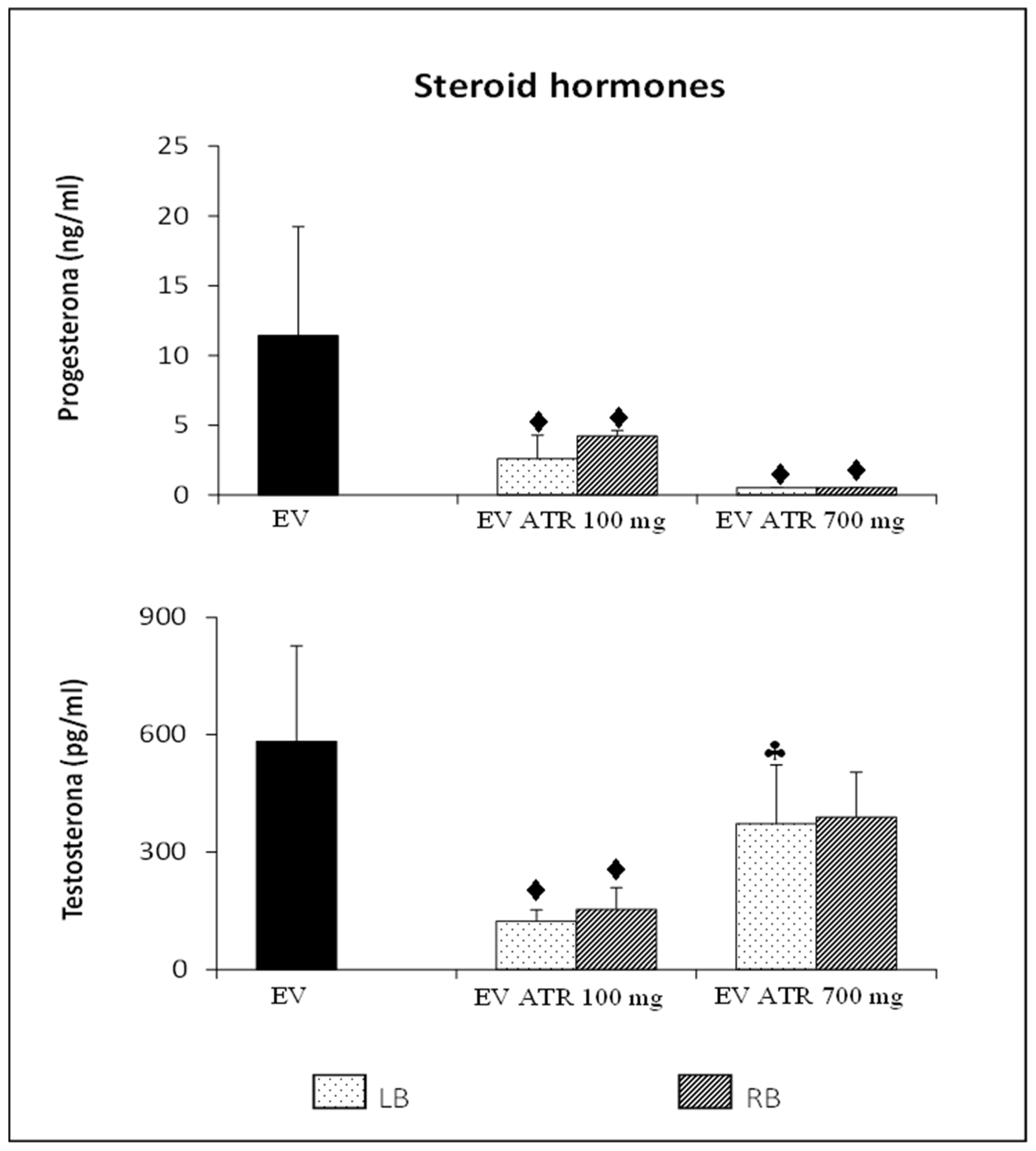
3.3. Steroid Hormone Concentration
3.3.1. Progesterone
3.3.2. Testosterone
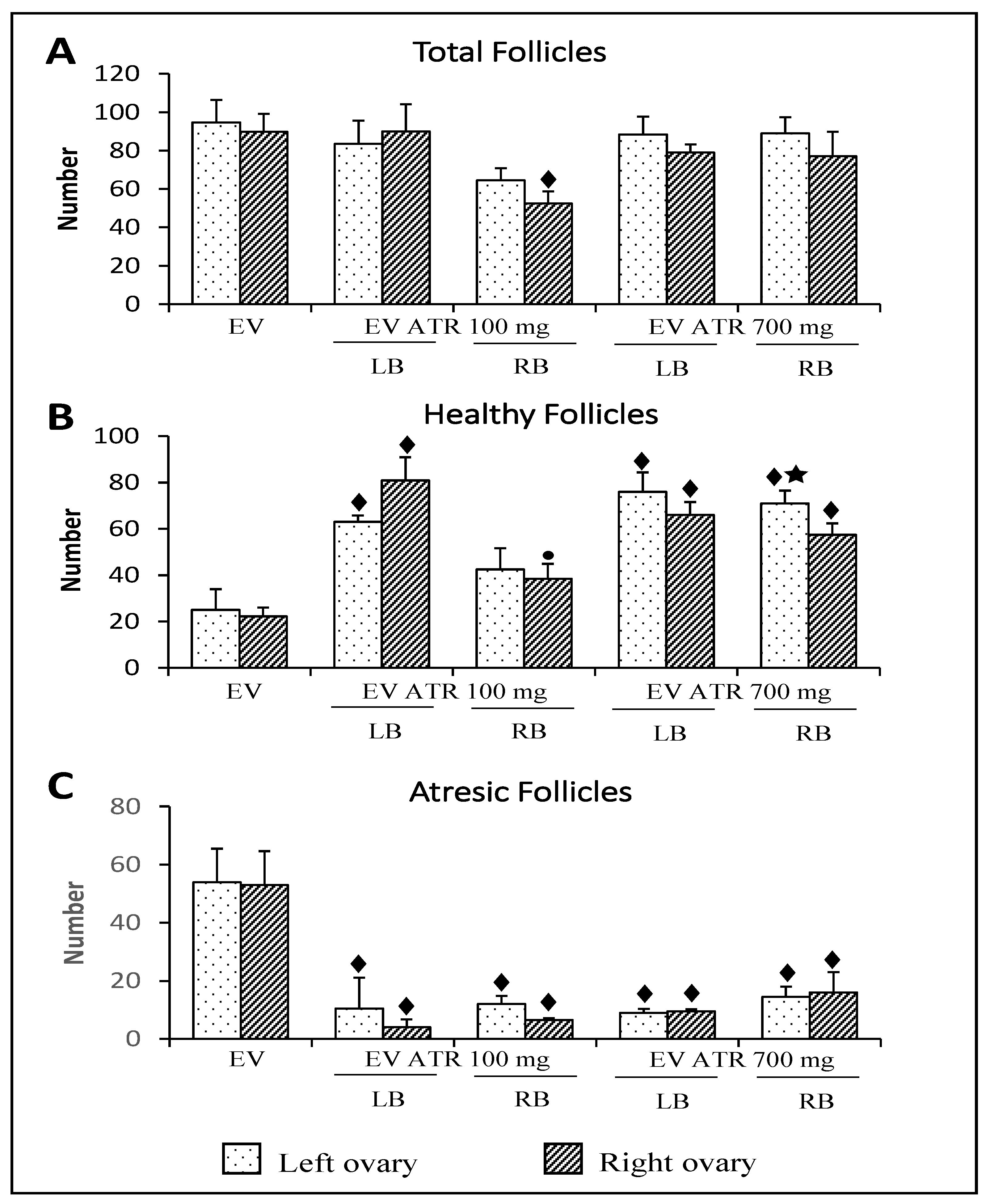
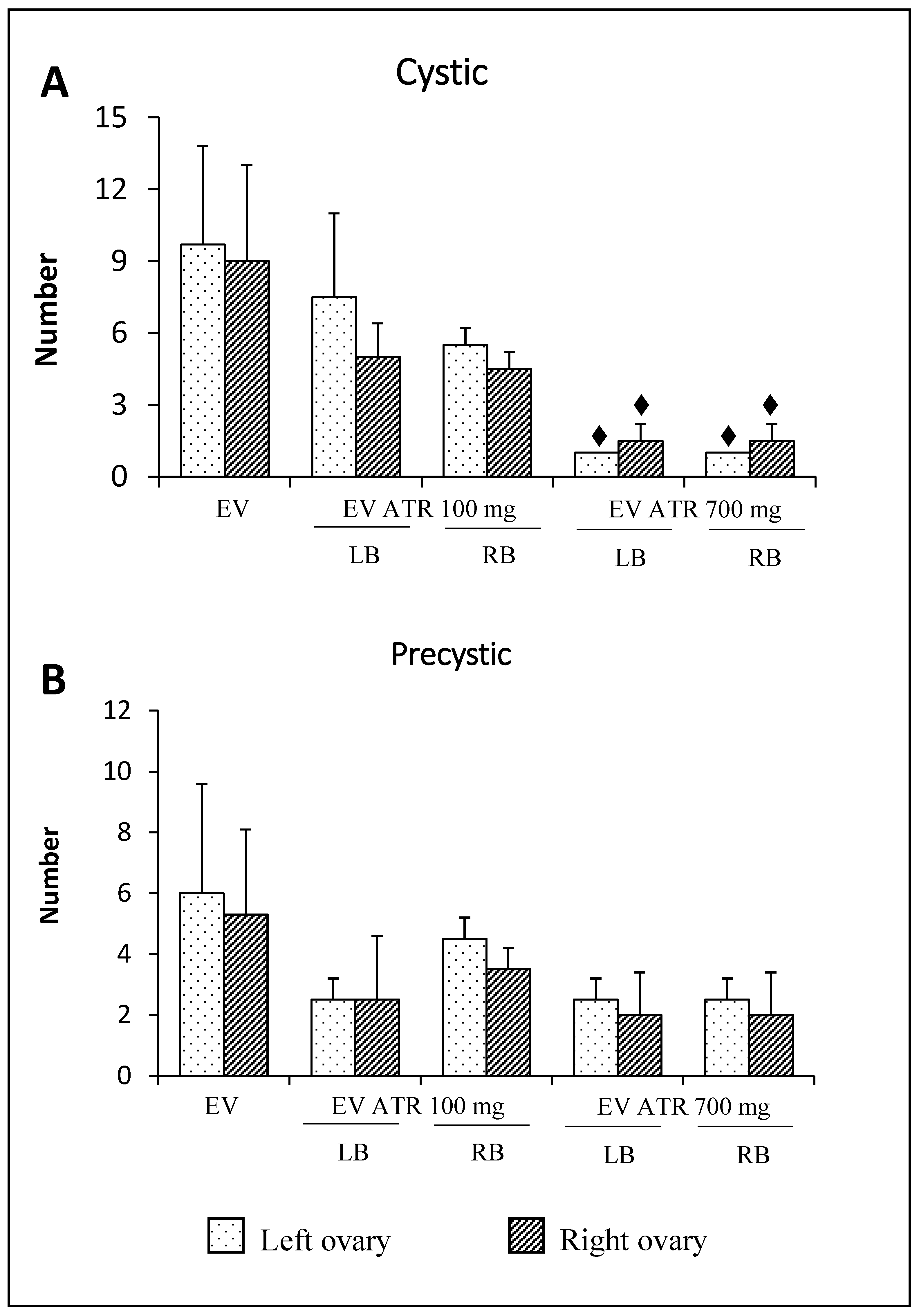
3.4. Follicular Population Analysis
4. Discussion
5. Strengths and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bremer, A.A. Polycystic Ovary Syndrome in the Pediatric Population. Metab. Syndr. Relat. Disord. 2010, 8, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic Ovary Syndrome: Etiology, Pathogenesis and Diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Barria, A.; Leyton, V.; Ojeda, S.R.; Lara, H.E. Ovarian Steroidal Response to Gonadotropins and Beta-Adrenergic Stimulation Is Enhanced in Polycystic Ovary Syndrome: Role of Sympathetic Innervation. Endocrinology 1993, 133, 2696–2703. [Google Scholar] [CrossRef]
- Rosa-e-Silva, A.; Guimaraes, M.A.; Padmanabhan, V.; Lara, H.E. Prepubertal Administration of Estradiol Valerate Disrupts Cyclicity and Leads to Cystic Ovarian Morphology during Adult Life in the Rat: Role of Sympathetic Innervation. Endocrinology 2003, 144, 4289–4297. [Google Scholar] [CrossRef]
- Sotomayor-Zárate, R.; Dorfman, M.; Paredes, A.; Lara, H.E. Neonatal Exposure to Estradiol Valerate Programs Ovarian Sympathetic Innervation and Follicular Development in the Adult Rat1. Biol. Reprod. 2008, 78, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Matalliotakis, I.; Kourtis, A.; Koukoura, O.; Panidis, D. Polycystic Ovary Syndrome: Etiology and Pathogenesis. Arch. Gynecol. Obstet. 2006, 274, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.E.; Ferruz, J.L.; Luza, S.; Bustamante, D.A.; Borges, Y.; Ojeda, S.R. Activation of Ovarian Sympathetic Nerves in Polycystic Ovary Syndrome. Endocrinology 1993, 133, 2690–2695. [Google Scholar] [CrossRef]
- Han, S.Y.; McLennan, T.; Czieselsky, K.; Herbison, A.E. Selective Optogenetic Activation of Arcuate Kisspeptin Neurons Generates Pulsatile Luteinizing Hormone Secretion. Proc. Natl. Acad. Sci. USA 2015, 112, 13109–13114. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A.; Kunz, L.; Krieger, A.; Proskocil, B.; Spindel, E.; Amsterdam, A.; Dissen, G.A.; Ojeda, S.R.; Wessler, I. FSH Regulates Acetycholine Production by Ovarian Granulosa Cells. Reprod. Biol. Endocrinol. 2006, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Urra, J.; Blohberger, J.; Tiszavari, M.; Mayerhofer, A.; Lara, H.E. In Vivo Blockade of Acetylcholinesterase Increases Intraovarian Acetylcholine and Enhances Follicular Development and Fertility in the Rat. Sci. Rep. 2016, 6, 30129. [Google Scholar] [CrossRef] [PubMed]
- Burden, H.W.; Lawrence, I.E. Experimental Studies on the Acetylcholinesterase-Positive Nerves in the Ovary of the Rat. Anat. Rec. 1978, 190, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.E.; Chávez, R.; Domínguez Casalá, R. Ovulation, Follicular Growth and Ovarian Reactivity to Exogenous Gonadotropins in Adult Rats with Unilateral or Bilateral Section of the Vagi Nerves. Rev. Investig. Clín. 1986, 38, 167–171. [Google Scholar]
- Morales-Ledesma, L.; Betanzos-García, R.; Domínguez-Casalá, R. Unilateral or Bilateral Vagotomy Performed on Prepubertal Rats at Puberty Onset of Female Rat Deregulates Ovarian Function. Arch. Med. Res. 2004, 35, 279–283. [Google Scholar] [CrossRef]
- Morales, L.; Ricardo, B.; Bolaños, A.; Chavira, R.; Domínguez, R. Ipsilateral Vagotomy to Unilaterally Ovariectomized Pre-Pubertal Rats Modifies Compensatory Ovarian Responses. Reprod. Biol. Endocrinol. 2007, 5, 24. [Google Scholar] [CrossRef]
- Linares, R.; Hernández, D.; Morán, C.; Chavira, R.; Cárdenas, M.; Domínguez, R.; Morales-Ledesma, L. Unilateral or Bilateral Vagotomy Induces Ovulation in Both Ovaries of Rats with Polycystic Ovarian Syndrome. Reprod. Biol. Endocrinol. 2013, 11, 68. [Google Scholar] [CrossRef]
- Linares, R.; Rosas, G.; Vieyra, E.; Ramírez, D.A.; Velázquez, D.R.; Espinoza, J.A.; Morán, C.; Domínguez, R.; Morales-Ledesma, L. In Adult Rats With Polycystic Ovarian Syndrome, Unilateral or Bilateral Vagotomy Modifies the Noradrenergic Concentration in the Ovaries and the Celiac Superior Mesenteric Ganglia in Different Ways. Front. Physiol. 2019, 10, 1309. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Fritz, S. Ovarian Acetylcholine and Muscarinic Receptors: Hints of a Novel Intrinsic Ovarian Regulatory System. Microsc. Res. Tech. 2002, 59, 503–508. [Google Scholar] [CrossRef]
- Krsmanovic, L.Z.; Mores, N.; Navarro, C.E.; Saeed, S.A.; Arora, K.K.; Catt, K.J. Muscarinic Regulation of Intracellular Signaling and Neurosecretion in Gonadotropin-Releasing Hormone Neurons. Endocrinology 1998, 139, 4037–4043. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Riboni, L.; Zipitria, D.; Revilla, R. Is There a Cholinergic Circadian Rhythm throughout the Oestrous Cycle Related to Ovulation in the Rat? J. Endocrinol. 1982, 95, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ledesma, L.; Linares, R.; Rosas, G.; Morán, C.; Chavira, R.; Cárdenas, M.; Domínguez, R. Unilateral Sectioning of the Superior Ovarian Nerve of Rats with Polycystic Ovarian Syndrome Restores Ovulation in the Innervated Ovary. Reprod. Biol. Endocrinol. 2010, 8, 99. [Google Scholar] [CrossRef]
- Venegas-Meneses, B.; Padilla, J.F.; Juárez, C.E.; Morán, J.L.; Morán, C.; Rosas-Murrieta, N.H.; Handal, A.; Domínguez, R. Effects of Ovarian Dopaminergic Receptors on Ovulation. Endocrine 2015, 50, 783–796. [Google Scholar] [CrossRef]
- Morales, L.; Chavez, R.; Ayala, M.; Dominguez, R. Effects of Unilateral or Bilateral Superior Ovarian Nerve Section in Prepubertal Rats on the Ovulatory Response to Gonadotrophin Administration. J. Endocrinol. 1998, 158, 213–219. [Google Scholar] [CrossRef][Green Version]
- Greenwald, G.G. Follicular development and its control. In The Physiology of Reproduction; Raven Press: New York, NY, USA, 1994; Volume 2, pp. 629–724. [Google Scholar]
- Lara, H.E.; Dissen, G.A.; Leyton, V.; Paredes, A.; Fuenzalida, H.; Fiedler, J.L.; Ojeda, S.R. An Increased Intraovarian Synthesis of Nerve Growth Factor and Its Low Affinity Receptor Is a Principal Component of Steroid-Induced Polycystic Ovary in the Rat. Endocrinology 2000, 141, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Morales-Ledesma, L.; Cruz, M.E. Ovarian Asymmetry. Annu. Rev. Biomed. Sci. 2003, 5, 95–104. [Google Scholar]
- Venegas, B.; De León Gordillo, L.Y.; Rosas, G.; Espinoza, J.A.; Morán, C.; Domínguez, R.; Morales-Ledesma, L. In Rats with Estradiol Valerate-Induced Polycystic Ovary Syndrome, the Acute Blockade of Ovarian β-Adrenoreceptors Improve Ovulation. Reprod. Biol. Endocrinol. RBE 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Brawer, J.R.; Munoz, M.; Farookhi, R. Development of the Polycystic Ovarian Condition (PCO) in the Estradiol Valerate-Treated Rat. Biol. Reprod. 1986, 35, 647–655. [Google Scholar] [CrossRef]
- Burden, H.W.; Leonard, M.; Smith, C.P.; Lawrence, I.E. The Sensory Innervation of the Ovary: A Horseradish Peroxidase Study in the Rat. Anat. Rec. 1983, 207, 623–627. [Google Scholar] [CrossRef]
- Trkulja, V.; Lackovic, Z. Vagal Influence on Compensatory Ovarian Growth Is Important Only Briefly after Hemicastration. Exp. Biol. Med. 2001, 226, 776–781. [Google Scholar] [CrossRef]
- Stillman, M.J.; Shukitt-Hale, B.; Kong, R.M.; Levy, A.; Lieberman, H.R. Elevation of Hippocampal Extracellular Acetylcholine Levels by Methoctramine. Brain Res. Bull. 1993, 32, 385–389. [Google Scholar] [CrossRef]
- Toide, K.; Arima, T. Effects of Cholinergic Drugs on Extracellular Levels of Acetylcholine and Choline in Rat Cortex, Hippocampus and Striatum Studied by Brain Dialysis. Eur. J. Pharmacol. 1989, 173, 133–141. [Google Scholar] [CrossRef]
- Damsma, G.; Westerink, B.H.C.; Vries, J.B.; Berg, C.J.; Horn, A.S. Measurement of Acetylcholine Release in Freely Moving Rats by Means of Automated Intracerebral Dialysis. J. Neurochem. 1987, 48, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Barco, A.I.; Flores, A.; Chavira, R.; Damián-Matsumura, P.; Domínguez, R.; Cruz, M.E. Asymmetric Effects of Acute Hemiovariectomy on Steroid Hormone Secretion by the In Situ Ovary. Endocrine 2003, 21, 209–216. [Google Scholar] [CrossRef]
- Burden, H.W.; Lawrence, I.E., Jr. The Effect of Denervation on Compensatory Ovarian Hypertrophy. Neuroendocrinology 1977, 23, 368–378. [Google Scholar] [CrossRef]
- Klein, C.M.; Burden, H.W. Anatomical Localization of Afferent and Postganglionic Sympathetic Neurons Innervating the Rat Ovary. Neurosci. Lett. 1988, 85, 217–222. [Google Scholar] [CrossRef]
- Fritz, S.; Grünert, R.; Stocco, D.M.; Hales, D.B.; Mayerhofer, A. StAR Protein Is Increased by Muscarinic Receptor Activation in Human Luteinized Granulosa Cells. Mol. Cell. Endocrinol. 2001, 171, 49–51. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Dimitrijevic, N.; Kunz, L. The Expression and Biological Role of the Non-Neuronal Cholinergic System in the Ovary. Life Sci. 2003, 72, 2039–2045. [Google Scholar] [CrossRef]
- Fritz, S.; Föhr, K.J.; Boddien, S.; Berg, U.; Brucker, C.; Mayerhofer, A. Functional and Molecular Characterization of a Muscarinic Receptor Type and Evidence for Expression of Choline-Acetyltransferase and Vesicular Acetylcholine Transporter in Human Granulosa-Luteal Cells1. J. Clin. Endocrinol. Metab. 1999, 84, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Kunz, L.; Thalhammer, A.; Berg, F.D.; Berg, U.; Duffy, D.M.; Stouffer, R.L.; Dissen, G.A.; Ojeda, S.R.; Mayerhofer, A. Ca2+-Activated, Large Conductance K+ Channel in the Ovary: Identification, Characterization, and Functional Involvement in Steroidogenesis. J. Clin. Endocrinol. Metab. 2002, 87, 5566–5574. [Google Scholar] [CrossRef]
- Mayerhofer, A.; Kunz, L. A Non-Neuronal Cholinergic System of the Ovarian Follicle. Ann. Anat. Anat. Anz. 2005, 187, 521–528. [Google Scholar] [CrossRef]
- Flores, A.; Meléndez, G.; Palafox, M.T.; Rodríguez, J.O.; Barco, A.I.; Chavira, R.; Domínguez, R.; Cruz, M.E. The Participation of the Cholinergic System in Regulating Progesterone Secretion Through the Ovarian–Adrenal Crosstalk Varies Along the Estrous Cycle. Endocrine 2005, 28, 145–152. [Google Scholar] [CrossRef]
- Flores, A.; Rodríguez, J.O.; Palafox, M.T.; Meléndez, G.; Barco, A.I.; Chavira, R.; Esther Cruz, M.; Domínguez, R. The Acute Asymmetric Effects of Hemiovariectomy on Testosterone Secretion Vary along the Estrous Cycle. The Participation of the Cholinergic System. Reprod. Biol. Endocrinol. 2006, 4, 11. [Google Scholar] [CrossRef][Green Version]
- Dominguez, R.; Cruz-Morales, S.-E. The Ovarian Innervation Participates in the Regulation of Ovarian Functions. Endocrinol. Metab. Syndr. 2011, S4, 1–10. [Google Scholar] [CrossRef]
- Michal, P.; Lysíková, M.; Tuček, S. Dual Effects of Muscarinic M2 Acetylcholine Receptors on the Synthesis of Cyclic AMP in CHO Cells: Dependence on Time, Receptor Density and Receptor Agonists: Muscarinic M2 Receptors on Cyclic AMP Synthesis. Br. J. Pharmacol. 2001, 132, 1217–1228. [Google Scholar] [CrossRef]
- Bódis, J.; Tinneberg, H.R.; Papenfuß, F.; Török, A.; Cledon, P.; Hanf, V.; Schwarz, H. Cholinergic Stimulation of Progesterone and Estradiol Secretion by Human Granulosa Cells Cultured in Serum-Free Medium. Gynecol. Endocrinol. 1993, 7, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kornya, L.; Bódis, J.; Koppán, M.; Tinneberg, H.R.; Török, A. Modulatory Effect of Acetylcholine on Gonadotropin-Stimulated Human Granulosa Cell Steroid Secretion. Gynecol. Obstet. Investig. 2001, 52, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Luck, M.R. Cholinergic Stimulation, through Muscarinic Receptors, of Oxytocin and Progesterone Secretion from Bovine Granulosa Cells Undergoing Spontaneous Luteinization in Serum-Free Culture. Endocrinology 1990, 126, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.E.; Flores, A.; Alvarado, B.E.; Hernández, C.G.; Zárate, A.; Chavira, R.; Cárdenas, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Ovulation Requires the Activation on Proestrus of M1 Muscarinic Receptors in the Left Ovary. Endocrine 2015, 49, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.; Chávez, R.; Domínguez, R. Participation of the Superior Ovarian Nerve in the Regulation of Ovulation in the Prepubertal Rat: Differential Effects of Uni-Lateral and Bilateral Section of the Nerve. Med. Sci. Res. 1993, 21, 15–17. [Google Scholar]
- Riquelme, R.; Ruz, F.; Mayerhofer, A.; Lara, H.E. Huperzine-A Administration Recovers Rat Ovary Function after Sympathetic Stress. J. Neuroendocrinol. 2020, 33, e12914. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares, R.; Acuña, X.N.; Rosas, G.; Vieyra, E.; Ramírez, D.A.; Chaparro, A.; Espinoza, J.A.; Domínguez, R.; Morales-Ledesma, L. Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome. Molecules 2021, 26, 5506. https://doi.org/10.3390/molecules26185506
Linares R, Acuña XN, Rosas G, Vieyra E, Ramírez DA, Chaparro A, Espinoza JA, Domínguez R, Morales-Ledesma L. Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome. Molecules. 2021; 26(18):5506. https://doi.org/10.3390/molecules26185506
Chicago/Turabian StyleLinares, Rosa, Xóchitl N. Acuña, Gabriela Rosas, Elizabeth Vieyra, Deyra A. Ramírez, Andrea Chaparro, Julieta A. Espinoza, Roberto Domínguez, and Leticia Morales-Ledesma. 2021. "Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome" Molecules 26, no. 18: 5506. https://doi.org/10.3390/molecules26185506
APA StyleLinares, R., Acuña, X. N., Rosas, G., Vieyra, E., Ramírez, D. A., Chaparro, A., Espinoza, J. A., Domínguez, R., & Morales-Ledesma, L. (2021). Participation of the Cholinergic System in the Development of Polycystic Ovary Syndrome. Molecules, 26(18), 5506. https://doi.org/10.3390/molecules26185506





