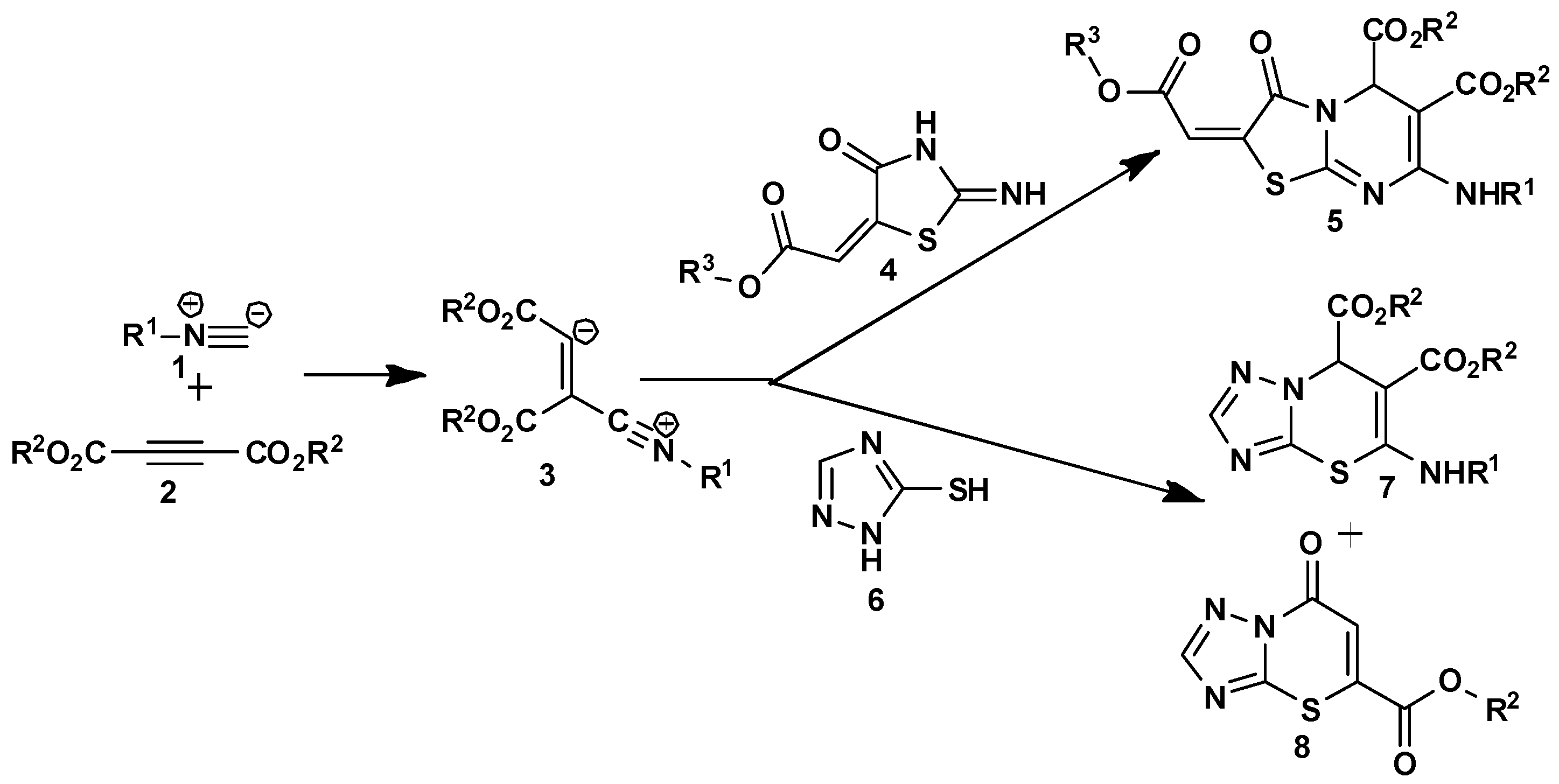
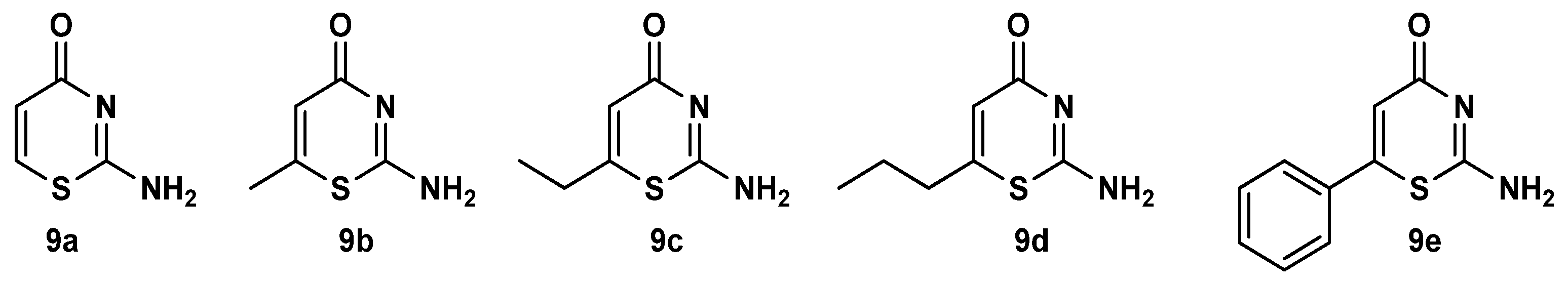
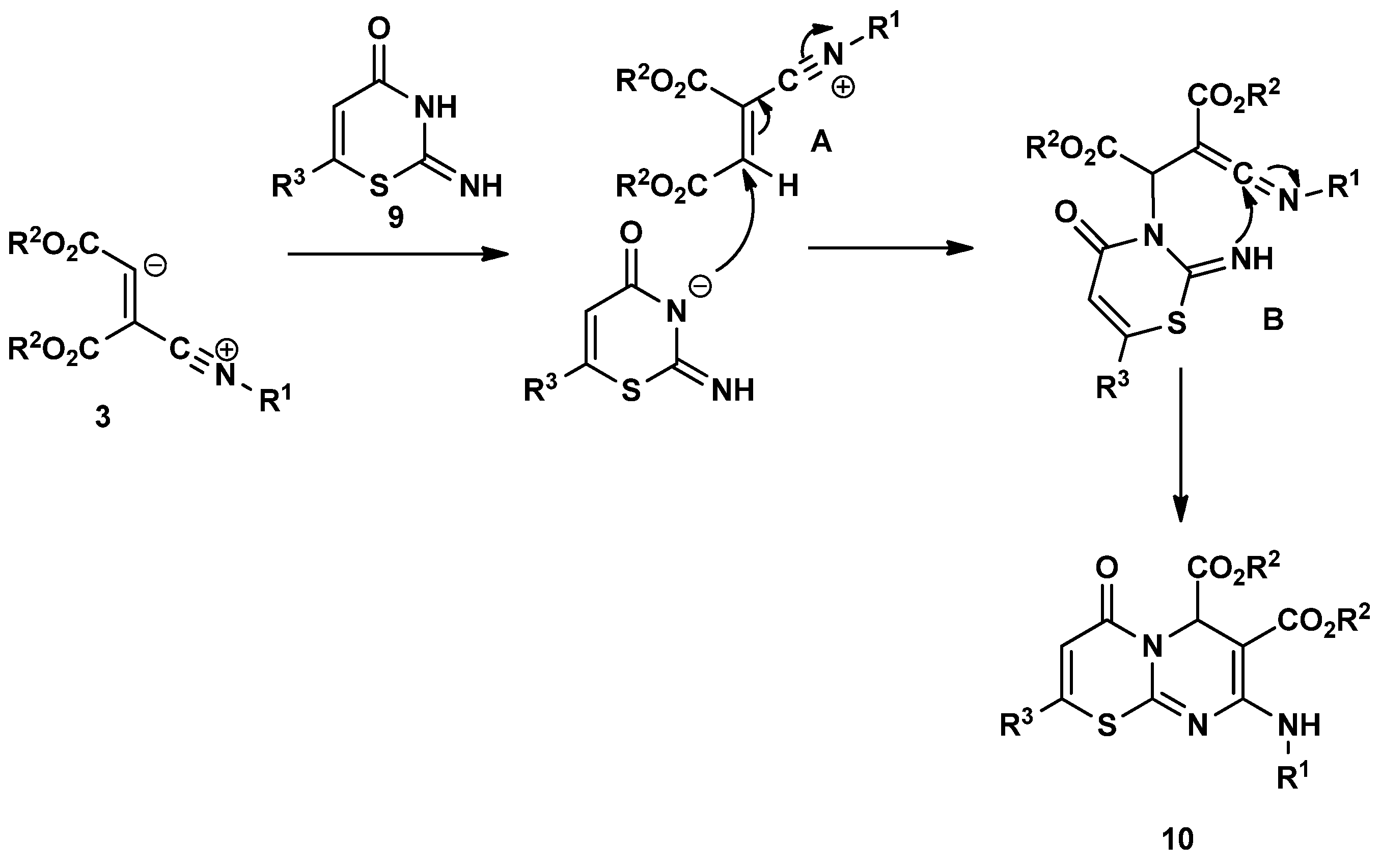
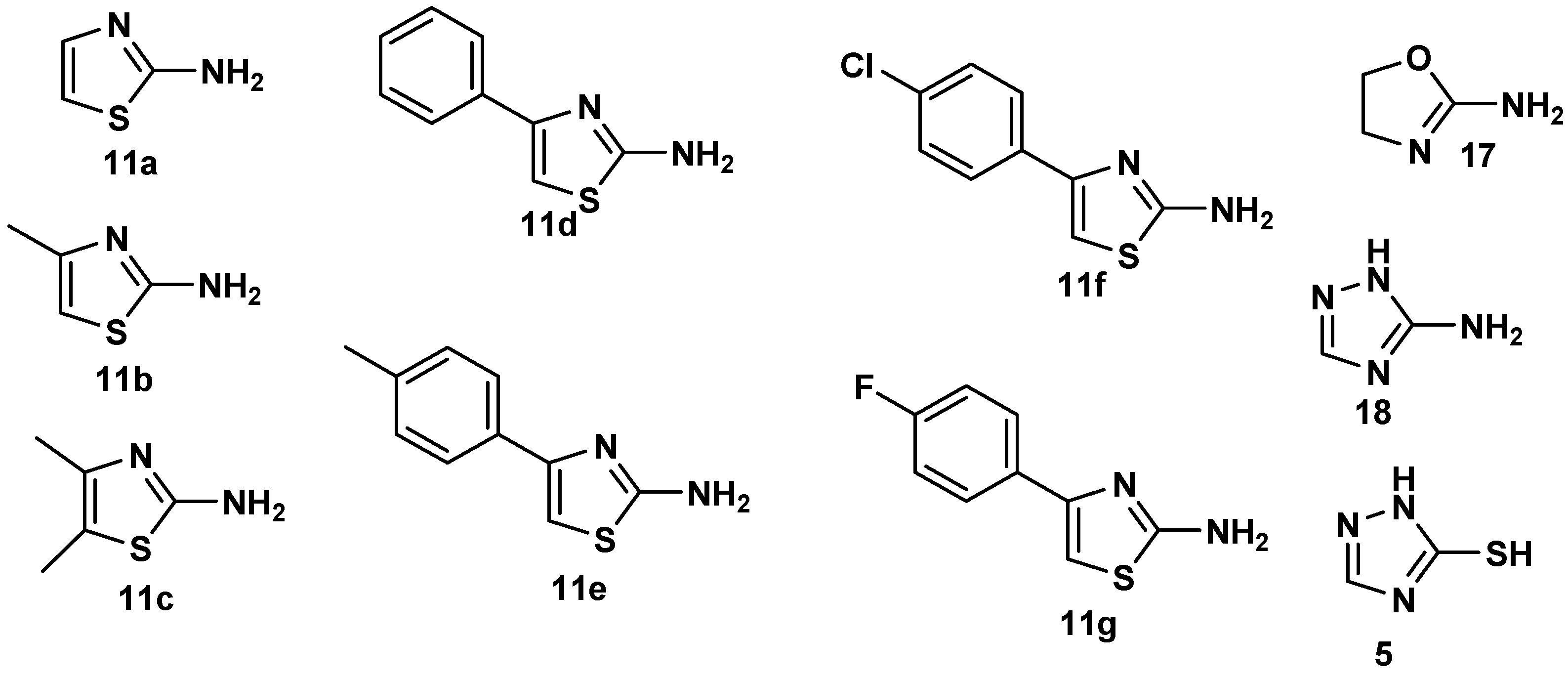
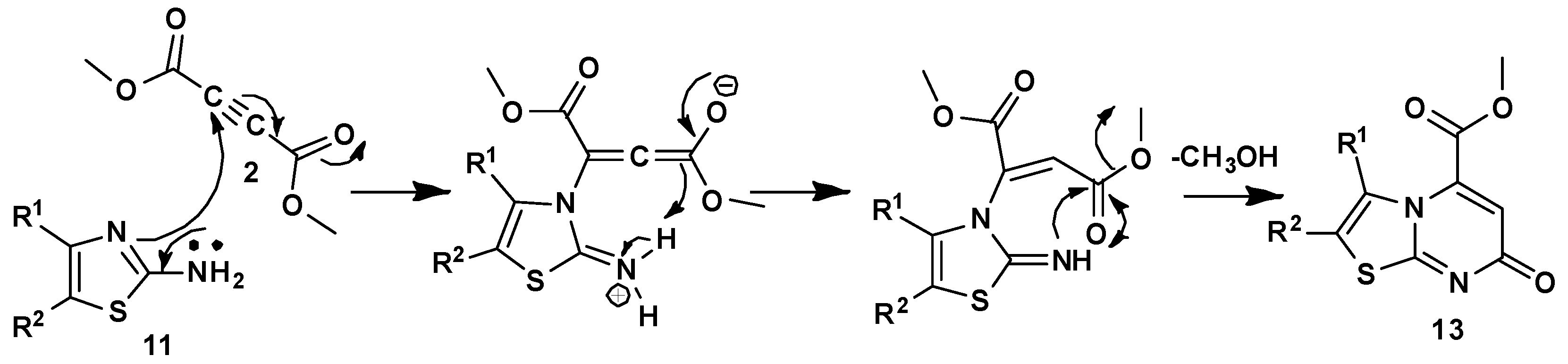
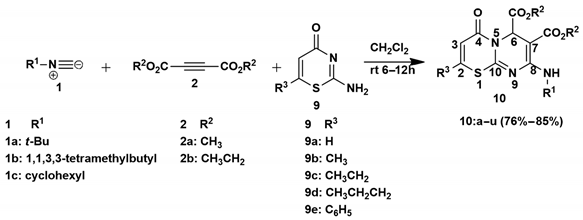
3.1.2. General Synthesis of 4-Oxo-4,6-Dihydropyrimido[2,1-b][1,3]Thiazine-6,7-Dicarboxylate Derivatives (10a–u)
In a round-bottomed flask equipped with a magnetic stirrer, isocyanide derivative (1a–c) in dry CH2Cl2 (2.5 mL) was slowly added to DMAD (2a) or DEtAD (2b) in dry CH2 Cl2 (5 mL) at 0 °C, under inert argon, and the solution was allowed to stir for 0.5 h. Then 2-amino-4H-1,3-thiazin-4-one derivatives 9a–e in dry CH2Cl2 (2.5 mL) were slowly added to the solution and stirred at room temperature under argon for 6–24 h. The solvent was removed under vacuum, and the residue was purified by silica gel column chromatography, using hexane-ethyl acetate as eluent to afford the products.
Dimethyl 8-(tert-butylamino)-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10a); 2-amino-4H-1,3-thiazin-4-one (9a) (0.25 g, 1.95 mmol), tert-butyl isocyanide (1a) (0.16 g, 1.95 mmol) and dimethyl acetylenedicarboxylate (2a) (0.28 g, 1.95 mmol). Physical characteristics: yellow solid; Yield: 0.55 g, 81%; Mp: 145–147 °C, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.73 (1H, br s, NH), 7.36 (1H, d, J = 10.8 Hz, Ar-CH), 6.51 (1H, d, J = 10.4 Hz, Ar-CH), 6.35 (1H, s, CH), 3.75 (3H, s, OCH3), 3.71 (3H, s, OCH3), 1.42 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 170.9 (C=O), 168.4 (C=O), 160.7 (C=O), 157.5 (C-10), 154.9 (C-8), 136.8 (C-3), 118.2 (Ar-CH), 72.3 (C-7), 52.9 (C-6), 52.7 (OCH3), 51.1 (OCH3), 50.8(C(CH3)3), 30.9 (C(CH3)3); FTIR νmax/cm−1: 2982, 2913, 1728, 1690, 1648, 1600, 1533, 1472, 1431; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C15H20N3O5S+ 354.1118 found 354.1113.
Diethyl 8-(tert-butylamino)-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10b); 2-amino-4H-1,3-thiazin-4-one (9a) (0.25 g, 1.95 mmol), tert-butyl isocyanide (1a) (0.16 g, 1.95 mmol) and diethyl acetylenedicarboxylate (2b) (0.33 g, 1.95 mmol). Physical characteristics: yellow solid; Yield: 0.63 g, 85%; Mp: 143–145 °C, Rf: 0.6, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.75 (1H, br s, NH), 7.34 (1H, d, J = 10.8 Hz, Ar-CH), 6.51 (1H, d, J = 10.4 Hz, Ar-CH), 6.34 (1H, s, CH), 4.31–4.11 (4H, m, 2 × CH2), 1.43 (9H, s, C(CH3)3), 1.33 (3H, t, J = 7.2 Hz, CH3), 1.26 (3H, t, J = 7.2 Hz, CH3); 13C NMR (101 MHz, CDCl3) δ 170.6 (C=O), 168.1 (C=O), 160.8 (C=O), 157.3 (C-10), 154.8 (C-8), 136.7 (C-3), 118.2 (Ar-CH), 72.5 (C-7), 61.9 (O-CH2), 59.3 (O-CH2), 52.6 (C-6),) 51.3(C(CH3)3, 30.9 (C(CH3)3, 14.9(CH3), 14.2 (CH3); FTIR νmax/cm−1: 3067, 2983, 2864, 1728, 1690, 1647, 1599, 1534, 1431; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C17H24N3O5S+ 382.1431 found 382.1439.
Dimethyl 8-(tert-butylamino)-2-methyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10c); 2-amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), tert-butyl isocyanide (1a) (0.15 g, 1.76 mmol) and dimethyl acetylenedicarboxylate (2a) (0.25 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.53 g, 82%; Mp: 149–151 °C, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.63 (1H, br s, NH), 6.24 and 6.23 (2H, 2 × s, Ar-CH and H-6), 3.66 (3H, s, O-CH3), 3.61 (3H, s, O-CH3), 2.17 (Ar-CH3), 1.33 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.5 (C=O), 161.2 (C=O), 157.8 (C-10), 155.3 (C-8), 149.7 (C-2), 116.1 (C-3), 72.1 (C-7), 52.9 (C-6), 52.6 (OCH3), 51.0 (OCH3), 50.8 (C(CH3)3), 30.8 (C(CH3)3) 22.1 (Ar-CH3); FTIR νmax/cm−1: 2992, 2963, 2902, 1734, 1648, 1682, 1653, 1602, 1525; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C16H22N3O5S+ 368.1275 found 368.1279.
Diethyl 8-(tert-butylamino)-2-methyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10d); 2-amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), tert-butyl isocyanide (1a) (0.15 g, 1.76 mmol) and diethyl acetylenedicarboxylate (2b) (0.30 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.57 g, 83%; Mp: 135–137 °C, Rf: 0.6, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.72 (1H, br s, NH), 6.28 (2H, 2 × s, Ar-CH and H-6), 4.27–4.06 (4H, m, 2 × CH2), 2.22 (3H, Ar-CH3), 1.39 (9H, s, C(CH3)3), 1.29 (3H, t, J = 7.2 Hz, CH3), 1.22 (3H, t, J = 7.2 Hz, CH3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.4 (C=O), 157.7 (C-10), 155.2 (C-8), 149.7 (C-2), 116.1 (C-3), 72.4 (C-7), 61.8 (O-CH2), 59.2 (O-CH2), 52.6 (C-6), 51.3 (N-C(CH3)3), 30.9 (C(CH3)3, 22.1 (Ar-CH3), 14.9 (CH3), 14.2 (CH3); FTIR νmax/cm−1: 3285, 2975, 2890, 1735, 1690, 1643, 1600, 1535, 14377; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C18H26N3O5S+ 396.1588 found 396.1597.
Dimethyl 2-methyl-4-oxo-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido [2,1-b][1,3]thiazine-6,7-dicarboxylate (10e); 2-Amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.25 g, 1.76 mmol) and dimethyl acetylenedicarboxylate (2a) (0.25 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.56 g, 76%; Mp: 114–116 °C, Rf: 0.6, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.74 (1H, br s, NH), 6.34 and 6.33 (2H, 2 × s, Ar-CH and H-6), 3.76 (3H, s, OCH3), 3.69 (3H, s, OCH3), 2.27 (3H, Ar-CH3), 1.83 (CH2), 1.48 and 1.46 (6H, 2 × s, 2CH3), 0.97 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.5 (C=O), 161.3 (C=O), 157.6 (C-10), 155.4 (C-8), 149.8, (C-2), 116.1 (C-3), 72.0 (C-7), 56.2 (C(CH3)3), 53.3 (CH2), 52.9 (C-6), 51.1 (OCH3), 50.8 (OCH3), 31.7 (C(CH2)2), 31.8 (C(CH3)3), 31.6 (CH3), 31.5 (CH3), 22.1 (Ar-CH3); FTIR νmax/cm−1: 3285, 2951, 2899, 1739, 1687, 1649, 1599, 1535, 1442; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C20H30N3O5S+ 424.1901 found 424.1906.
Diethyl 2-methyl-4-oxo-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10f); 2-amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.25 g, 1.76 mmol) and dimethyl acetylenedicarboxylate (2a) (0.25 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.61 g, 77%; Mp: 112–114 °C, Rf: 0.7, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.73 (1H, br s, NH), 6.29 (2H, 2 × s, Ar-CH, H-6), 4.26–4.01 (4H, m, 2 × CH2O) 2.23 (3H, s, Ar-CH3), 1.80 (2H, q, J = 14.4 Hz, CH2), 1.44 and 1.42 (6H, 2 × s, 2 × CH3), 1.31 (3H, t, J = 6.8 Hz, CH3), 1.21 (3H, t, J = 7.2 Hz, CH3), 0.94 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.4 (C=O), 157.4 (C-10), 155.3 (C-8), 149.6 (C-2), 116.1 (C-3), 72.3 (C-7), 61.8 (O-CH2), 59.1 (O-CH2), 56.2 (C-(CH3)3), 53.1 (CH2), 51.3 (C-6), 31.6 (C-(CH3)3), 22.1 (Ar-CH3), 14.9 (CH3CH2), 14.2 (CH3CH2); FTIR νmax/cm−1: 3285, 2975, 2890, 1735, 1690, 1643, 1600, 1535, 1477; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C22H34N3O5S+ 452.2214 found 452.2223.
Dimethyl 8-(cyclohexylamino)-2-methyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10g); 2-amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), cyclohexyl isocyanide (1c) (0.19 g, 1.76 mmol) and dimethyl acetylenedicarboxylate (2a) (0.25 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.69 g, 84%; Mp: 148–150 °C, Rf: 0.42, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.52 (1H, br s, NH), 6.29 (2H, 2 × s, Ar-CH, H-6), 3.90 (1H, s N-CH), 3.72 (3H, s, OCH3), 3.67 (3H, s, OCH3), 2.23 (CH3, s, Ar-CH3), 1.93–1.70 (5H, m, cyclohexyl) 1.67–1.32 (5H, m, cyclohexyl); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.3 (C=O), 161.2 (C=O), 159.1 (C-10), 154.2 (C-8), 149.6 (C-2), 116.0 (C-3), 71.5 (C-7), 52.9 (O-CH3), 51.2 (C-6), 50.7 (O-CH3), 49.6 (CH-NH), 34.3 (CH2, cyclohexyl), 33.7 (CH2, cyclohexyl), 25.7 (CH2, cyclohexyl), 24.8 (CH2, cyclohexyl), 24.7 (CH2, cyclohexyl), 22.1 (Ar-CH3). FTIR νmax/cm−1: 3285, 2946, 2858, 1762, 1730, 1692, 1653, 1618, 1590, 1525, 1445; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C18H24N3O5S+ 394.1431 found 394.1433.
Diethyl 8-(cyclohexylamino)-2-methyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10h); 2-amino-6-methyl-4H-1,3-thiazin-4-one (9b) (0.25 g, 1.76 mmol), cyclohexyl isocyanide (1c) (0.19 g, 1.76 mmol) and diethyl acetylenedicarboxylate (2b) (0.30 g, 1.76 mmol). Physical characteristics: yellow solid; Yield: 0.63 g, 85%; Mp: 170–172 °C, Rf: 0.50, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.55 (1H, br s, NH), 6.28 (2H, 2 × s, Ar-CH, H-6), 4.27–4.09 (4H, m, 2 × CH2), 3.92 (1H, s, CH), 2.23 (3H, s, Ar-CH3), 1.93–1.69 (4H, m, cyclohexyl), 1.66–1.55 (4H, m, cyclohexyl), 1.41–1.33 (2H, m, cyclohexyl) 1.34 (3H, s, J = 7.2 Hz, CH3), 1.28 (3H, s, J = 6.8 Hz, CH3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.3 (C=O), 158.9 (C-10), 154.1 (C-8), 149.6 (C-2), 115.9 (C-3), 71.8 (C-7), 61.7 (O-CH2), 59.1 (O-CH2), 51.4 (C-6), 49.6 (CH), 34.3(CH2, cyclohexyl), 33.7 (CH2, cyclohexyl), 25.7 (CH2, cyclohexyl), 24.8 (CH2, cyclohexyl), 24.7 (CH2, cyclohexyl), 22.1 (Ar-CH3), 14.9 (CH2CH3), 14.1 (CH2CH3); FTIR νmax/cm−1: 2992, 2945, 2867, 1735, 1689, 1648, 1588, 1540, 1440; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C20H28N3O5S+ 422.1744 found 422.1712.
Dimethyl 8-(tert-butylamino)-2-ethyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10i); 2-amino-6-ethyl-4H-1,3-thiazin-4-one (9c) (0.25 g, 1.60 mmol), tert-butyl isocyanide (1a) (0.13 g, 1.60 mmol) and dimethyl acetylenedicarboxylate (2a) (0.22 g, 1.60 mmol). Physical characteristics: yellow solid; Yield: 0.51 g, 84%; Mp: 135–137 °C, Rf: 0.48, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.70 (1H, br s, NH), 6.31 (2H, 2 × s, Ar-CH, H-6), 3.73 (3H, s, OCH3), 3.68 (3H, s, OCH3), 2.50 (2H, q, J = 7.2 Hz, Ar-CH2), 1.40 (9H, s, C(CH3)3), 1.28 (3H, t, J = 7.6 Hz, CH3); 13C NMR (101 MHz, CDCl3) δ 171.2 (C=O), 168.5 (C=O), 161.5 (C=O), 157.9 (C-10), 155.8 (C-8), 155.3 (C-2), 114.4 (C-3), 72.1 (C-7), 52.9 (OCH3), 52.6 (C-(CH3)3), 51.2 (C-6), 50.8 (OCH3), 30.8 (C(CH3)3), 29.4 (CH2CH3), 12.5 (CH2CH3); FTIR νmax/cm−1: 3273, 2967, 2876, 1739, 1674, 1644, 1616, 1591, 1531, 1438; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C17H24N3O5S+ 382.1430 found 382.1427.
Diethyl 8-(tert-butylamino)-2-ethyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10j); 2-amino-6-ethyl-4H-1,3-thiazin-4-one (9c) (0.25 g, 1.60 mmol), tert-butyl isocyanide (1a) (0.13 g, 1.60 mmol) and diethyl acetylenedicarboxylate (2b) (0.27 g, 1.60 mmol). Physical characteristics: yellow solid; Yield: 0.52 g, 79%; Mp: 94–96 °C, Rf: 0.6, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.72 (1H, br s, NH), 6.30 and 6.28 (2H, 2 × s, Ar-CH, H-6), 4.27–4.09 (4H, m, 2 × CH2), 2.50 (2H, q, J = 7.6 Hz, Ar-CH2), 1.40 (9H, s, C(CH3)3), 1.29–1.26 (6H, m, 2 × CH3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.6 (C=O), 157.8 (C-10), 155.7 (C-8), 155.2 (C-2), 114.4 (C-3), 72.31 (C-7), 61.8 (O-CH2), 59.2 (O-CH2), 52.5 (C(CH3)3), 51.4 (C-6), 30.9 (C(CH3)3, 29.3 (Ar-CH2), 14.9 (CH3), 14.2 (CH3), 12.5 (CH3); FTIR νmax/cm−1: 3285, 2975, 2890, 1735, 1690, 1643, 1600, 1535, 14377; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C19H28N3O5S+ 410.1744 found 410.1749.
Dimethyl 8-((2,4-dimethylpentan-2-yl)amino)-2-ethyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10k); 2-amino-6-ethyl-4H-1,3-thiazin-4-one (9c) (0.25 g, 1.60 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.22 g, 1.76 mmol) and dimethyl acetylenedicarboxylate (2a) (0.23 g, 1.60 mmol). Physical characteristics: yellow paste; Yield: 0.57 g, 81%, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.64 (1H, br s, NH), 6.25 and 6.24 (2H, 2 × s, Ar-CH, H-6), 3.66 (3H, s, OCH3), 3.59 (3H, s, OCH3), 2.44 (2H, q, J = 7.6 Hz, Ar-CH2), 1.73 (CH2), 1.38 and 1.36 (6H, 2 × s, 2 × CH3), 1.21 (3H, t, J = 7.2 Hz, CH3CH2), 0.87 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.4 (C=O), 161.4 (C=O), 157.7 (C-10), 155.8 (C-8), 155.4 (C-2), 114.3 (C-3), 71.9 (C-7), 56.2 (C(CH3)3), 53.3 (CH2), 52.8 (OCH3), 51.1 (C-6), 50.7 (OCH3), 31.8 (C(CH3)3), 31.7, 31.6 (2 × CH3) 29.3 (Ar-CH2), 12.4 (CH3); FTIR νmax/cm−1: 3285, 2950, 2902, 1780, 1738, 1687, 1649, 1598, 1532; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C21H32N3O5S+ 438.2057 found 438.2021.
Diethyl 8-((2,4-dimethylpentan-2-yl)amino)-2-ethyl-4-oxo-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10l); 2-amino-6-ethyl-4H-1,3-thiazin-4-one (9c) (0.25 g, 1.60 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.22 g, 1.60 mmol) and diethyl acetylenedicarboxylate (2b) (0.27 g, 1.60 mmol). Physical characteristics: yellow solid; Yield: 0.61 g, 81%; Mp: 98–100 °C, Rf: 0.6, hexane-ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.73 (1H, br s, NH), 6.30 and 6.29 (2H, 2 × s, Ar-CH, H-6), 4.26–4.08 (4H, m, 2 × CH2O), 2.50 (2H, q, J = 7.2 Hz, Ar-CH2), 1.80 (2H, q, J = 14.8 Hz, CH2), 1.45 and 1.42 (6H, 2 × s, (CH3)2-C), 1.31–1.25 (6H, m, 2 × OCH2CH3), 1.21 (3H, s, J = 7.2 Hz, ArCH2CH3), 0.94 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.6 (C=O), 157.6 (C-10), 155.7 (C-8), 155.3 (C-2), 114.4 (C-3), 72.2 (C-7), 61.8 (O-CH2), 59.1 (O-CH2), 56.1 (C(CH3)3), 53.3 (Ar-CH2), 51.4 (C-6), 31.7 (C(CH3)2), 29.3 (C-(CH3)3), 29.3 (CH2), 14.9 (CH3), 14.2 (CH3), 12.5 (CH3); FTIR νmax/cm−1: 3270, 2973, 2913, 1737, 1688, 1646, 1600, 1535, 1432; HRMS (ESI–TOF) m/z: [M + H]+ Calculated for C23H36N3O5S+ 466.2370 found 466.2333.
Dimethyl 8-(tert-butylamino)-4-oxo-2-propyl-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10m); 2-amino-6-propyl-4H-1,3-thiazin-4-one (9d) (0.25 g, 1.47 mmol), tert-butyl isocyanide (1a) (0.12 g, 1.47 mmol) and dimethyl acetylenedicarboxylate (2a) (0.21 g, 1.47 mmol). Physical characteristics: yellow paste; Yield: 0.48 g, 84%, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.72 (1H, br s, NH), 6.32 (2H, s, Ar-CH, H-6), 3.74 (3H, s, OCH3), 3.69 (3H, s, OCH3), 2.46 (2H, t, J = 7.6 Hz, Ar-CH2), 1.71 (2H, sextet, J = 7.6 Hz, CH2), 1.42 (9H, s, C(CH3)3), 1.03 (3H, t, J = 7.2 Hz, CH3CH2); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.4 (C=O), 161.3 (C=O), 157.9 (C-10), 155.3 (C-8), 154.4 (C-2), 115.1 (C-3), 72.0 (C-7), 52.9 (OCH3), 52.6 (C(CH3)3), 51.1 (C-6), 50.8 (OCH3), 37.9 (C(CH3)3), 30.8 (Ar-CH2), 21.6 (CH3CH2) 13.4 (CH3); FTIR νmax/cm−1: 3282, 2960, 2916, 1739, 1685, 1647, 1597, 1567, 1531, 1441; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C18H26N3O5S+ 396.1588 found 396.1545.
Diethyl 8-(tert-butylamino)-4-oxo-2-propyl-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10n); 2-amino-6-propyl-4H-1,3-thiazin-4-one (9d) (0.25 g, 1.47 mmol), tert-butyl isocyanide (1a) (0.12 g, 1.47 mmol) and diethyl acetylenedicarboxylate (2b) (0.25 g, 1.60 mmol). Physical characteristics: yellow paste; Yield: 0.53 g, 85%, Rf: 0.56, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.74 (1H, br s, NH), 6.30 (2H, s, Ar-CH, H-6), 4.29–4.13 (4H, m, 2 × CH2), 2.46 (2H, t, J = 7.6 Hz, Ar-CH2), 1.71 (2H, sextet, J = 7.2 Hz, CH2), 1.42 (9H, s, C(CH3)3), 1.34–1.32 (6H, m, 2 × CH3), 1.32 (3H, t, J = 7.2 Hz, CH3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.5 (C=O), 157.8 (C-10), 155.2 (C-8), 154.3 (C-2), 115.2 (C-3), 72.3 (C-7), 61.7 (O-CH2), 59.1 (O-CH2), 52.5 (C(CH3)3), 51.3 (C-6), 38.0 (Ar-CH2), 30.8 (C(CH3)3, 21.6 (CH3CH2), 14.9, 14.1 and 13.4 (3 × CH3); FTIR νmax/cm−1: 3285, 2975, 2910, 2870, 1737, 1687, 1646, 1600, 1535, 1432; HRMS (ESI–TOF) m/z: [M+H]+ calculated for C20H30N3O5S+ 424.1901 found 424.1873.
Dimethyl 4-oxo-2-propyl-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido [2,1-b][1,3]thiazine-6,7-dicarboxylate (10o); 2-amino-6-propyl-4H-1,3-thiazin-4-one (9d) (0.25 g, 1.47 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.21 g, 1.47 mmol) and dimethyl acetylenedicarboxylate (2a) (0.21 g, 1.47 mmol). Physical characteristics: yellow paste; Yield: 0.52 g, 79%, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.64 (1H, br s, NH), 6.24 (2H, 2 × s, Ar-CH, H-6), 3.65 (3H, s, OCH3), 3.59 (3H, s, OCH3), 2.38 (2H, t, J = 7.6 Hz, Ar-CH2), 1.73 (2H, s, C-CH2), 1.64 (2H, sextet, J = 7.2 Hz, CH3CH2), 1.38 and 1.36 (6H, 2 × s, 2 × CH3), 0.94 (3H, J = 7.2 Hz, CH3), 0.87 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.4 (C=O), 161.3 (C=O), 157.8 (C-10), 155.4 (C-8), 154.4 (C-2), 115.1 (C-3), 71.9 (C-7), 56.2 (C(CH3)3), 53.3 (Ar-CH2), 52.8 (OCH3), 51.1 (C-6), 50.7 (OCH3), 37.9 (C-CH2), 31.8 (C(CH3)3), 31.7, 31.6 (2 × CH3) 21.6 (CH3CH2), 13.4 (CH2CH3); FTIR νmax/cm−1: 3279, 2951, 2873, 1739, 1686, 1648, 1598, 1598, 1532, 1442; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C22H34N3O5S+ 452.2214 found 452.2190.
Diethyl 4-oxo-2-propyl-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10p); 2-amino-6-propyl-4H-1,3-thiazin-4-one (9d) (0.25 g, 1.47 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.21 g, 1.47 mmol) and diethyl acetylenedicarboxylate (2b) (0.25 g, 1.60 mmol). Physical characteristics: yellow paste; Yield: 0.55 g, 79%, Rf: 0.6, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.75 (1H, br s, NH), 6.30 (2H,2 × s, Ar-CH, H-6), 4.29–4.09 (4H, m, 2 × CH2CH3), 2.46 (2H, t, J = 7.2 Hz, Ar-CH2), 1.85–1.69 (2 × 2H, m, 2 x CH2), 1.46 and 1.44 (6H, 2 × s, (CH3)2-C), 1.33 and 1.21 (6H, 2 × s, 2 × CH3), 1.31 (3H, t, J = 6.8 Hz, CH3), 1.22 (3H, t, J = 7.2 Hz, CH3), 1.03 (3H, t, J = 7.2 Hz, CH3), 0.96 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.1(C=O), 161.5 (C=O), 157.6 (C-10), 155.3 (C-8), 154.3 (C-2), 115.1 (C-3), 72.1 (C-7), 61.7 (O-CH2), 59.1 (O-CH2), 56.1 (C(CH3)3), 53.2 (O-CH2), 51.3 (C-6), 37.9 (Ar-CH2), 31.6 (C(CH3)3), 21.6 (CH3CH2), 14.9 (CH3), 14.1 (CH3), 13.4 (CH3); FTIR νmax/cm−1: 3285, 2957, 2910, 2870, 1737, 1687, 1646, 1600, 1535, 1432; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C24H38N3O5S+ 480.2527 found 480.2526.
Dimethyl 8-(tert-butylamino)-4-oxo-2-phenyl-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10q); 2-amino-6-phenyl-4H-1,3-thiazin-4-one (9e) (0.25 g, 1.22 mmol), tert-butyl isocyanide (1a) (0.10 g, 1.22 mmol) and dimethyl acetylenedicarboxylate (2a) (0.17 g, 1.22 mmol). Physical characteristics: yellow solid; Yield: 0.40 g, 77%; Mp: 99–101 °C, Rf: 0.58, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.74 (1H, br s, NH), 7.59–7.48 (5H, m, Ar-H) 6.69 (1H, s, Ar-CH), 6.36 (1H, s, H-6), 3.75 (3H, s, OCH3), 3.71 (3H, s, OCH3), 1.43 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.5 (C=O), 161.8 (C=O), 157.7 (C-10), 155.1 (C-8), 150.9 (C-2), 133.9 (Ar-C), 132.0 (Ar-C), 129.6 (Ar-CH), 126.6 (Ar-CH), 126.5 (Ar-CH), 113.9 (C-3), 72.1 (C-7), 53.0 (C-6), 52.7 (OCH3), 51.3 (OCH3), 50.9 (C(CH3)3), 30.9 (C(CH3)3); FTIR νmax/cm−1: 2977, 2841, 1735, 1683, 1582, 1448, 1370; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C21H24N3O5S+ 430.1431 found 430.1445.
Diethyl 8-(tert-butylamino)-4-oxo-2-phenyl-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10r); 2-amino-6-phenyl-4H-1,3-thiazin-4-one (9e) (0.25 g, 1.22 mmol), tert-butyl isocyanide (1a) (0.10 g, 1.22 mmol) diethyl acetylenedicarboxylate (2b) (0.21 g, 1.22 mmol). Physical characteristics: yellow solid; Yield: 0.45 g, 80%; Mp: 145–147 °C, Rf: 0.5, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.76 (1H, br s, NH), 7.59-7.47 (5H, m, Ar-H), 6.68 (1H, s, Ar-CH), 6.34 (1H, s, H-6), 4.29–4.10 (4H, m, 2 × CH2), 1.43 (9H, s, C(CH3)3), 1.33–1.22 (6H, m, 2 × CH3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.8 (C=O), 157.5 (C-10), 155.2 (C-8), 150.9 (C-2), 133.9 (Ar-C), 131.9 (Ar-C), 129.6 (Ar-C), 126.4 (ArCH), 113.9 (C-3), 72.4 (C-7), 61.9 (O-CH2), 59.2 (O-CH2), 52.6 (C-6),) 51.5 (C(CH3)3), 30.9 (C(CH3)3), 14.9 (CH3), 14.2 (CH3); FTIR νmax/cm−1: 3279, 2976, 2873, 1734, 1676, 1645, 1606, 1535, 1462; HRMS (ESI–TOF) m/z: [M+H]+ calculated for C23H28N3O5S+ 458.1744 found 458.1744.
Dimethyl 4-oxo-2-phenyl-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10s); 2-amino-6-phenyl-4H-1,3-thiazin-4-one (9e) (0.25 g, 1.22 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.17 g, 1.22 mmol) and dimethyl acetylenedicarboxylate (2b) (3.(0.17 g, 1.22 mmol). Physical characteristics: yellow solid; Yield: 0.45 g, 76%; Mp: 162–164 °C, Rf: 0.4, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.77 (1H, br s, NH), 7.62–7.49 (5H, m, Ar-H), 6.71 (1H, s, Ar-CH), 6.39 (1H, s, H-6), 3.77 (3H, s, OCH3), 3.71 (3H, s, OCH3) 1.85 (CH2), 1.50 and 1.48 (6H, 2 × s, 2 × CH3), 0.98 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 171.0 (C=O), 168.4 (C=O), 161.6 (C=O), 157.4 (C-10), 155.4 (C-8), 150.9 (C-2), 133.8(Ar-C), 131.9 (Ar-CH), 129.5 (Ar-CH), 126.4 (Ar-CH), 113.9 (C-3), 71.2 (C-7), 56.2 (C(CH3)3), 53.3 (CH2), 52.9 (C-6), 51.3 and 50.7 (2 × OCH3), 31.8 (C(CH3)2), 31.7 (C(CH3)3), 31.6 (CH3O), 31.5 (CH3O); FTIR νmax/cm−1: 2953, 2878, 1732, 1681, 1651, 1613, 1542, 1450;–HRMS (ESI–TOF) m/z: [M + H]+ calculated for C25H32N3O5S+ 486.2057 found 486.2044.
Diethyl 4-oxo-2-phenyl-8-((2,4,4-trimethylpentan-2-yl)amino)-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10t); 2-amino-6-phenyl-4H-1,3-thiazin-4-one (9e) (0.25 g, 1.22 mmol), 1,1,3,3-tetramethylbutyl isocyanide (1b) (0.17 g, 1.22 mmol) and dimethyl acetylenedicarboxylate (2b) (0.21 g, 1.22 mmol). Physical characteristics: yellow solid; Yield: 0.52 g, 83%; Mp: 88–90 °C, Rf: 0.5, hexane-ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.00 (1H, br s, NH), 7.63–7.50 (5H, m, Ar-H), 6.72 (1H, s, Ar-CH), 6.37 (1H, s, H-6), 4.34–4.11 (4H, m, 2 × O-CH2), 1.87 (2H, q, J = 14.8 Hz, CH2), 1.50 and 1.48 (6H, 2 × s, 2 × CH3), 1.34 (3H, t, J = 7.2 Hz, CH2CH3), 1.26 (3H, t, J = 6.8 Hz, CH2CH3), 0.98 (9H, s, C(CH3)3); 13C NMR (101 MHz, CDCl3) δ 170.7 (C=O), 168.2 (C=O), 161.8 (C=O), 157.31 (C-10), 155.3 (C-8), 150.9 (C-2), 133.9 (Ar-C), 131.9 (Ar-CH), 129.6 (Ar-CH), 126.4 (Ar-CH), 113.9 (C-3), 72.2 (C-7), 61.8 (O-CH2), 59.2 (O-CH2), 56.2 (C(CH3)3), 53.3 (O-CH2), 51.5 (C-6), 31.8 (C(CH3)3), 31.8 (CH3), 31.7 (CH3), 14.9 (CH3), 14.2 (CH3); FTIR νmax/cm−1: 2963, 2925, 2864, 1735, 1671, 1641, 1611, 1535, 1441; HRMS (ESI–TOF) m/z: [M + H]+ Calculated for C27H36N3O5S+ 514.2370 found 514.2350.
Dimethyl 8-(cyclohexylamino)-4-oxo-2-phenyl-4,6-dihydropyrimido[2,1-b][1,3]thiazine-6,7-dicarboxylate (10u); 2-amino-6-phenyl-4H-1,3-thiazin-4-one (9e) (0.25 g, 1.22 mmol), cyclohexyl isocyanide (1c) (0.13 g, 1.22 mmol) and dimethyl acetylenedicarboxylate (2a) (0.17 g, 1.22 mmol). Physical characteristics: yellow solid; Yield: 0.44 g, 79%; Mp: 168–170 °C, Rf: 0.4, hexane/ethyl acetate (90%:10%); 1H NMR (400 MHz, CDCl3) δ 8.59 (1H, br s, NH), 7.63–7.51 (5H, m, Ar-H), 6.71 (1H, s, Ar-CH), 6.39 (1H, s, CH-6), 3.99 (1H, s, N-CH), 3.78 (3H, s, OCH3), 3.73 (3H, s, OCH3), 1.99–1.59 (6H, m, cyclohexyl), 1.45–1.24 (4H, m, cyclohexyl); 13C NMR (101 MHz, CDCl3) δ 171.1 (C=O), 168.4 (C=O), 161.7 (C=O), 159.0 (C-10), 154.2 (C-8), 150.9 (C-2), 133.9 (Ar-C), 132.0 (Ar-CH), 129.6 (Ar-CH), 126.4 (Ar-CH), 113.8 (C-3), 71.4 (C-7), 52.9 (O-CH3), 51.5 (C-6), 50.8 (O-CH3), 49.7 (CHNH), 34.3 (CH2, cyclohexyl), 33.7 (CH2, cyclohexyl), 25.7 (CH2, cyclohexyl), 24.8 (CH2, cyclohexyl), 24.7 (CH2, cyclohexyl); FTIR νmax/cm−1: 3282, 2918, 2846, 1740, 1677, 1651, 1604, 1533, 1444; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C23H26N3O5S+ 456.1588 found 456.1592.
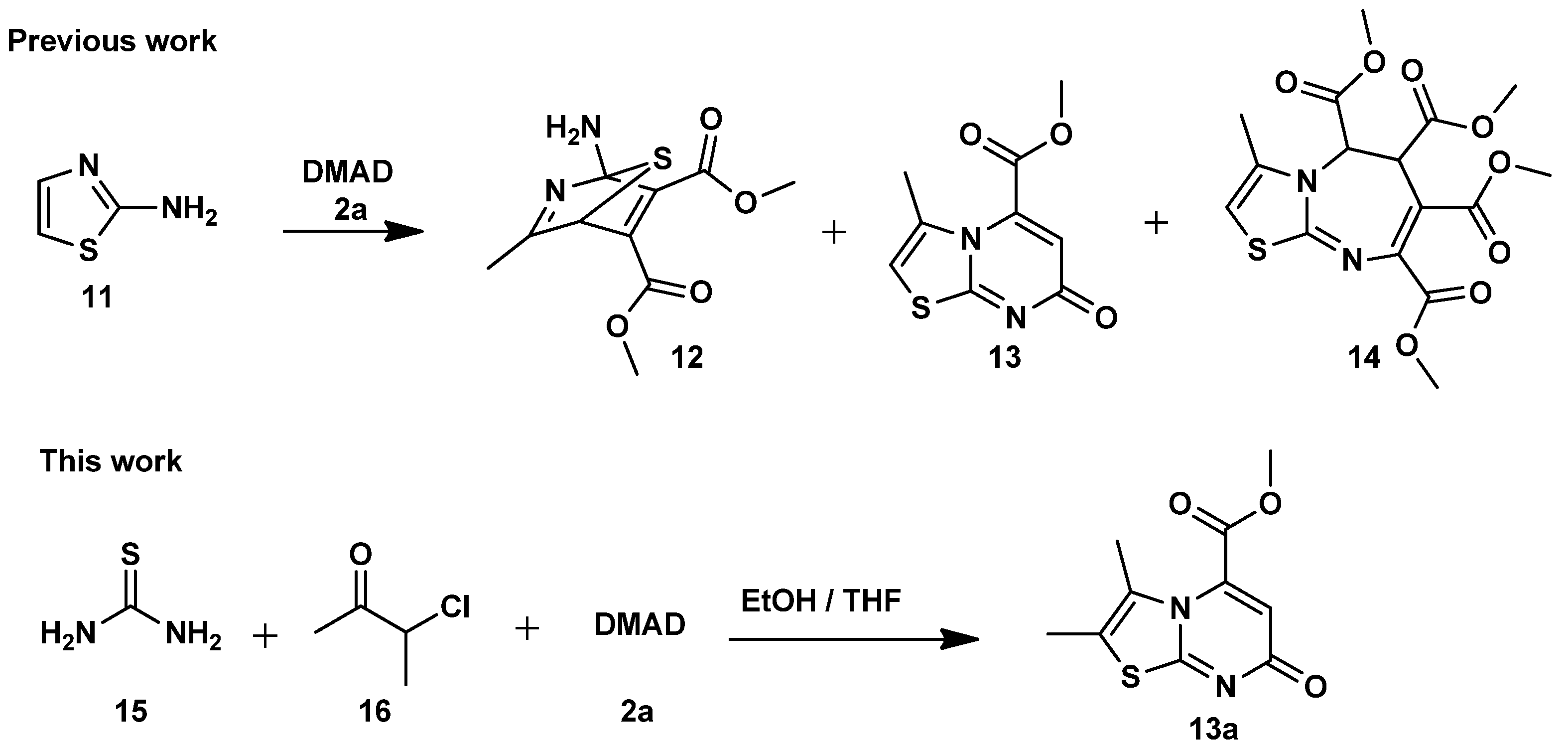
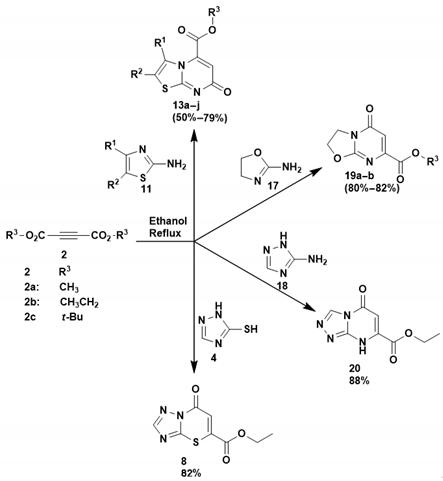
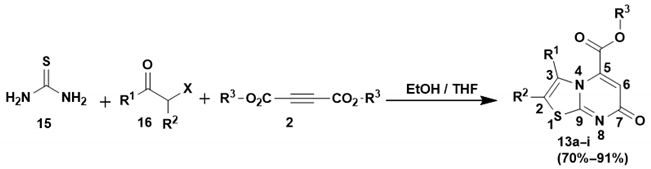
3.1.3. Synthesis of Methyl 7-Oxo-7H-Thiazolo[3,2-a]Pyrimidine-5-Carboxylate Derivatives (13a–j). Method B (3-CR)
In a round-bottomed flask equipped with a magnetic stirrer bar, thiourea (15) was added to a solution of α-haloketone (16) in absolute ethanol (15 mL) and tetrahydrofuran (THF) (15 mL), and the reaction mixture was allowed to stir at room temperature for at least 15 min. After this time, dimethyl acetylenedicarboxylate (DMAD) or diethyl acetylenedicarboxylate (DEtAD) (2) was slowly added over at least 10 min, and the reaction mixture was heated at 80 °C for 6–12 h while being monitored by TLC. After completion, the reaction was cooled, and the solvents were removed under reduced pressure to obtain a residue which was washed with cold ethanol and filtered to give a solid product.
Methyl 2,3-dimethyl-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13a). Thiourea (15) (0.36 g, 4.6 mmol), 3-chloro-2-butanone (16c) (0.50 g, 4.6 mmol) and DMAD (2a) (0.65 g, 4.6 mmol). Physical properties brown solid; Yield: 0.89 g, 82%; Mp: 165–167 °C; Rf 0.5, hexane/ethyl acetate (40%:60 %); 1H NMR (400 MHz, DMSO-d6), 6.50 (1H, s, H-6), 3.95 (3H, s, O-CH3), 2.28 (3H, s, CH3), 2.10 (3H, s, CH3); 13C NMR (101 MHz, DMSO-d6) δ 164.9 (C=O), 164.7 (C=O), 161.5 (C-9), 138.9 (C-5), 127.9 (C-2), 116.7 (C-3), 112.1 (C-6), 54.0 (O-CH3), 12.6 (CH3), 11.8 (CH3); FTIR νmax/cm−1: 2993, 1734, 1638, 1608, 1491, 1413; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C10H11N2O3S+ 239.0485, found 239.0486.
Methyl 7-oxo-3-(p-tolyl)-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13b). Thiourea (15) (0.19 g, 2.4 mmol), 2-bromo-4-methylacetophenone (16d) (0.52 g, 2.4 mmol) and DMAD (2a) (0.34 g, 2.4 mmol). Physical properties: orange solid; Yield: 0.51 g, 71%; Mp: 176–178 °C; Rf 0.4, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.35–7.33 (2H, m, ArH), 7.30–7.27 (3H, m, ArH), 6.47 (1H, s, H-6), 3.15 (3H, s, O-CH3), 2.35 (3H, s, CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 165.3 (C=O), 160.6 (C-9), 139.4 (C-5), 138.9 (Ar-C), 136.6 (Ar-C), 129.4 (Ar-CH), 127.5 (Ar-C), 127.2 (ArCH), 113.01 (C-6), 108.14 (C-2), 52.8 (O-CH3); FTIR νmax/cm−1: 3041, 1742, 1639, 1587, 1509, 1485, 1416; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C15H13N2O3S+ 301.0641, found 301.0648.
Methyl 3-(4-fluorophenyl)-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13c). Thiourea (15) (0.19 g, 2.3 mmol), 2-bromo-4-fluoroacetophenone (16e) (0.51 g, 2.3 mmol) and DMAD (2a) (0.33 g, 2.3 mmol). Physical properties: yellow solid; Yield: 0.49 g, 70%; Mp: 220–222 °C; Rf 0.5, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.56–7.53 (2H, m, ArH), 7.37–7.34 (3H, m, ArH), 6.49 (1H, s, H-6), 3.23 (3H, s, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.7 (C=O), 165.3 (C=O), 162.6 (d, JCF = 246 Hz, Ar-C), 161.3 (ArC), 160.6 (ArC), 138.7 (Ar-C),135.4 (ArC), 129.9 (d, JCF = 8.1 Hz, Ar-CH), 126.9 (d, JCF = 3.0 Hz, Ar-C), 115.9 (d, JCF = 22.1 Hz, Ar-CH), 113.1 (C-6), 109.0 (C-2), 52.9 (O-CH3); FTIR νmax/cm−1: 3058, 1742, 1640, 1581, 1489; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C14H10FN2O3S+ 305.0391, found 305.0391.
Methyl 3-(4-chlorophenyl)-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13d). Thiourea (15) (0.16 g, 2.1 mmol), 2-bromo-4-chloroacetophenone (16f) (0.50 g, 2.1 mmol) and DMAD (2a) (0.30 g, 2.1 mmol). Physical properties: yellow solid; Yield: 0.48 g, 71%; Mp: 200–202 °C; Rf 0.8, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.58 (2H, d, J = 8.4 Hz, ArH), 7.50 (2H, d, J = 8.4 Hz, ArH), 7.39 (1H, s, H-2), 6.50 (1H, s, H-6), 3.23 (3H, s, O-CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 165.3 (C=O), 160.6 (C-9), 138.6 (Ar-C), 135.2 (Ar-C), 134.3 (Ar-C), 129.3 (Ar-C), 129.1 (Ar-CH), 129.0 (Ar-CH), 128.7 (Ar-CH), 127.4 (Ar-CH), 113.2 (C-6), 109.5 (C-2), 52.90 (O-CH3); FTIR νmax/cm−1: 3049, 1734, 1633, 1583, 1494, 1481; HRMS (ESI–TOF) m/z: [M+H]+ calculated for C14H10ClN2O3S+ 321.0095, found 321.0087.
Ethyl 7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13e). Thiourea (15) (0.31 g, 4.1 mmol), chloroacetaldehyde (16a) (0.50 g, 4.1 mmol) and DEtAD (2b) (0.69 g, 4.1 mmol); Physical properties: yellow solid; Yield: 0.84 g, 91%; Mp: 180–182 °C; Rf 0.4, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 8.30 (1H, d, J = 4.8 Hz, H-2), 7.36 (1H, d, J = 4.8 Hz, H-3), 6.76 (1H, s, H-6), 4.38 (2H, q, J = 6.8 Hz, O-CH2), 1.34 (3H, t, J = 7.2 Hz, CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 166.0 (C=O), 160.3 (C-9), 136.6 (C-5), 124.6 (C-2), 114.3 (C-6), 110.0 (C-3), 62.9 (O-CH2), 13.7 (CH3); FTIR νmax/cm−1: 3176, 3077, 2978, 1720, 1621, 1557, 1471; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C9H9N2O3S+ 225.0328, found 225.0339.
Ethyl 3-methyl-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13f). Thiourea (15) (0.41 g, 5.4 mmol), chloroacetone (16b) (0.5 g, 5.4 mmol) and DEtAD (2b) (0.92 g, 5.4 mmol); Physical properties: brown solid; Yield: 1.1 g, 86%; Mp: 150–152 °C; Rf 0.5, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.10 (1H, s, H-2), 6.53 (1H, s, H-6), 4.38 (2H, q, J = 6.8 Hz, O-CH2), 2.23 (3H, s, CH3) 1.34 (3H, t, J = 6.8 Hz, CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 165.0 (C=O), 160.9 (C-9), 139.2 (Ar-C), 132.9 (Ar-C), 111.9 (C-6), 107.1 (C-2), 63.5 (CH2), 15.3 (CH3) 13.6 (CH3); FTIR νmax/cm−1: 2973, 1731, 1634, 1493; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C10H11N2O3S+ 239.0485, found 239.0497.
Ethyl 2,3-dimethyl-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13g). Thiourea (15) (0.36 g, 4.6 mmol), 3-chloro-2-butanone (16c) (0.50 g, 4.6 mmol) and DEtAD (2b) (0.78 g, 4.6 mmol). Physical properties: brown solid; Yield: 0.94 g, 81%; Mp: 153–155 °C; Rf 0.5, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6), 6.5 (1H, s, H-6), 4.40 (2H, q, J = 5.2 Hz, CH2), 2.28 (3H, s, CH3), 2.12 (3H, s, CH3), 1.33 (3H, t, J = 7.6 Hz, CH2CH3); 13C NMR (101 MHz, DMSO-d6) δ 165.0 (C=O), 164.7 (C=O), 161.0 (C-9), 139.2 (Ar-C), 127.9 (Ar-C), 116.7 (Ar-C), 112.1 (C-6), 63.5 (O-CH2), 13.6, 12.7, (2 × CH3), 11.8 (CH2CH3); FTIR νmax/cm−1: 2988, 1732, 1641, 1489, 1452; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C11H13N2O3S+ 253.0641, found 253.0652.
Ethyl 3-(4-fluorophenyl)-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13h). Thiourea (15) (0.19 g, 2.3 mmol), 2-bromo-4-fluoroacetophenone (16e) (0.51 g, 2.3 mmol) and DEtAD (2b) (0.39 g, 2.3 mmol). Physical properties: yellow solid, Yield 0.63 g, 86%; Mp: 180–182 °C; Rf 0.5, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.58–7.52 (2H, m, ArH), 7.37–7.27 (3H, m, ArH), 6.49 (1H, s, H-6), 3.61 (2H, q, J = 7.2 Hz, O-CH2) 1.04 (3H, t, J = 7.2 Hz, CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 165.4 (C=O), 162.6 (d, JCF = 246 Hz, Ar-C), 161.4 (Ar-C), 160.2 (Ar-C), 138.9 (Ar-C), 135.4 (Ar-C), 129.9 (d, JCF = 9.1 Hz, Ar-CH), 128.0 (d, JCF = 8.1 Hz, Ar-CH), 126.9 (d, JCF = 4.0 Hz, Ar-CH), 115.9 (d, JCF = 22.2 Hz, Ar-CH) 113.1 (C-6), 109.1 C-2), 62.7 (CH2-O), 13.3 (O-CH3); FTIR νmax/cm−1: 3061, 1733, 1639, 1489; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C15H12FN2O3S+ 319.0547, found 319.0565.
Ethyl 3-(4-chlorophenyl)-7-oxo-7H-thiazolo[3,2-a]pyrimidine-5-carboxylate (13i). Thiourea (15) (0.16 g, 2.1 mmol), 2-bromo-4-chloroacetophenone (16f) (0.50 g, 2.1 mmol) and DEtAD (2b) (0.36 g, 2.1 mmol). Physical properties: yellow solid, Yield: 0.59 g, 84%; Mp: 198–200 °C; Rf 0.9, hexane/ethyl acetate (40%:60%); 1H NMR (400 MHz, DMSO-d6) 7.58 (2H, d, J = 8.4 Hz, Ar-H), 7.51 (2H, d, J = 8.4 Hz, Ar-H), 7.39 (1H, s, H-2), 6.51 (1H, s, H-6), 3.63 (2H, q, J = 7.2 Hz, O-CH2), 1.03 (3H, t, J = 6.8, Hz, CH3); 13C NMR (101 MHz, DMSO-d6) δ 166.8 (C=O), 165.3 (C=O), 160.1 (C-9), 138.7 (Ar-C), 135.3 (Ar-CH), 134.3 (Ar-C), 129.4 (Ar-C), 129.1 (Ar-CH), 128.9 (Ar-CH), 113.2 (C-6), 109.38 (C-2), 62.7 (CH2-O), 13.2 (CH3); FTIR νmax/cm−1: 3057, 2986, 1733, 1640, 1580, 1501, 1482; HRMS (ESI–TOF) m/z: [M + H]+ calculated for C15H12ClN2O3S+ 335.0252, found 335.0267.