Miniaturized Salinity Gradient Energy Harvesting Devices
Abstract
:1. Introduction
1.1. The Blue Energy: Salinity Gradient Energy
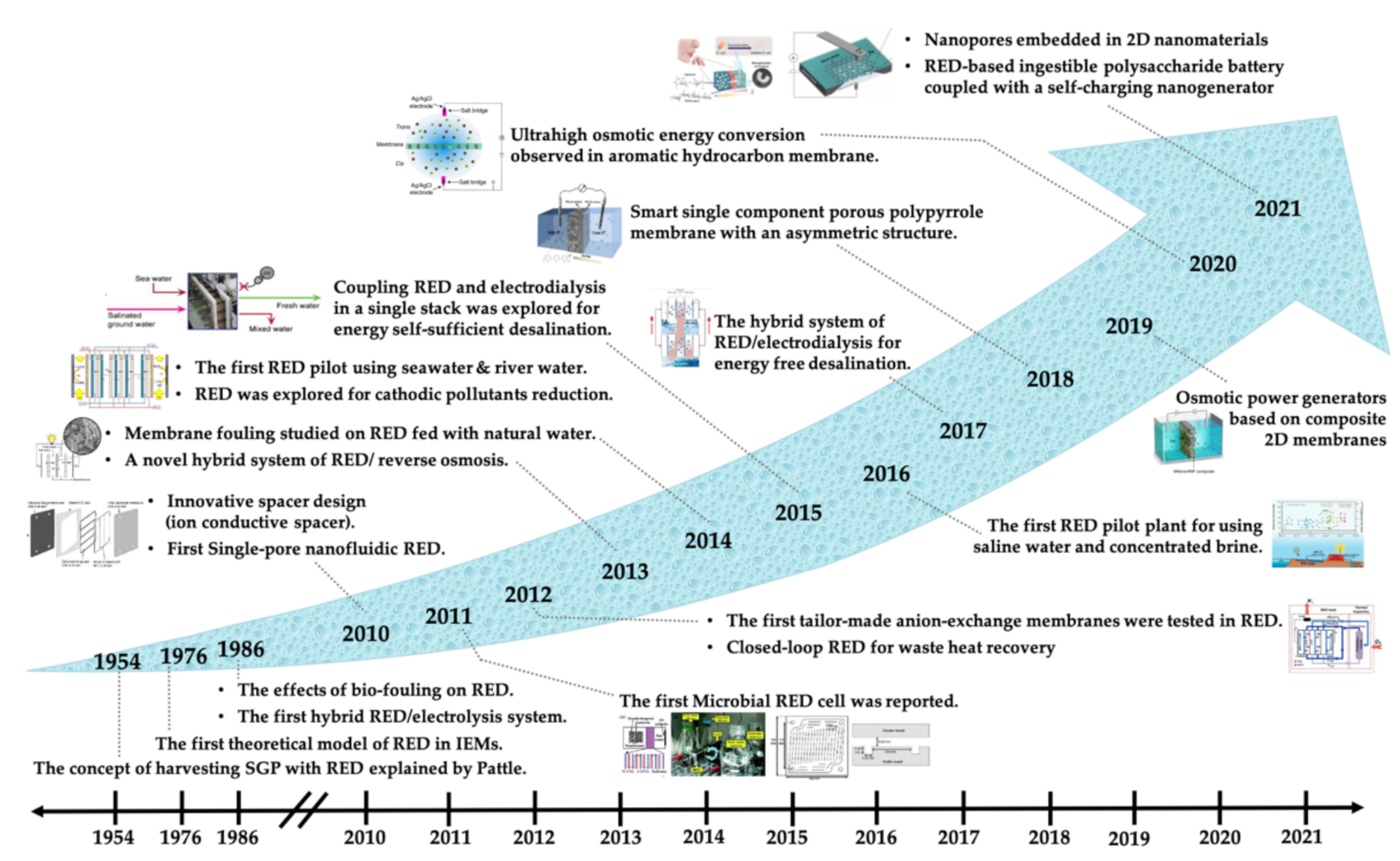
1.2. The History of the Development of Salinity Gradient Energy Devices
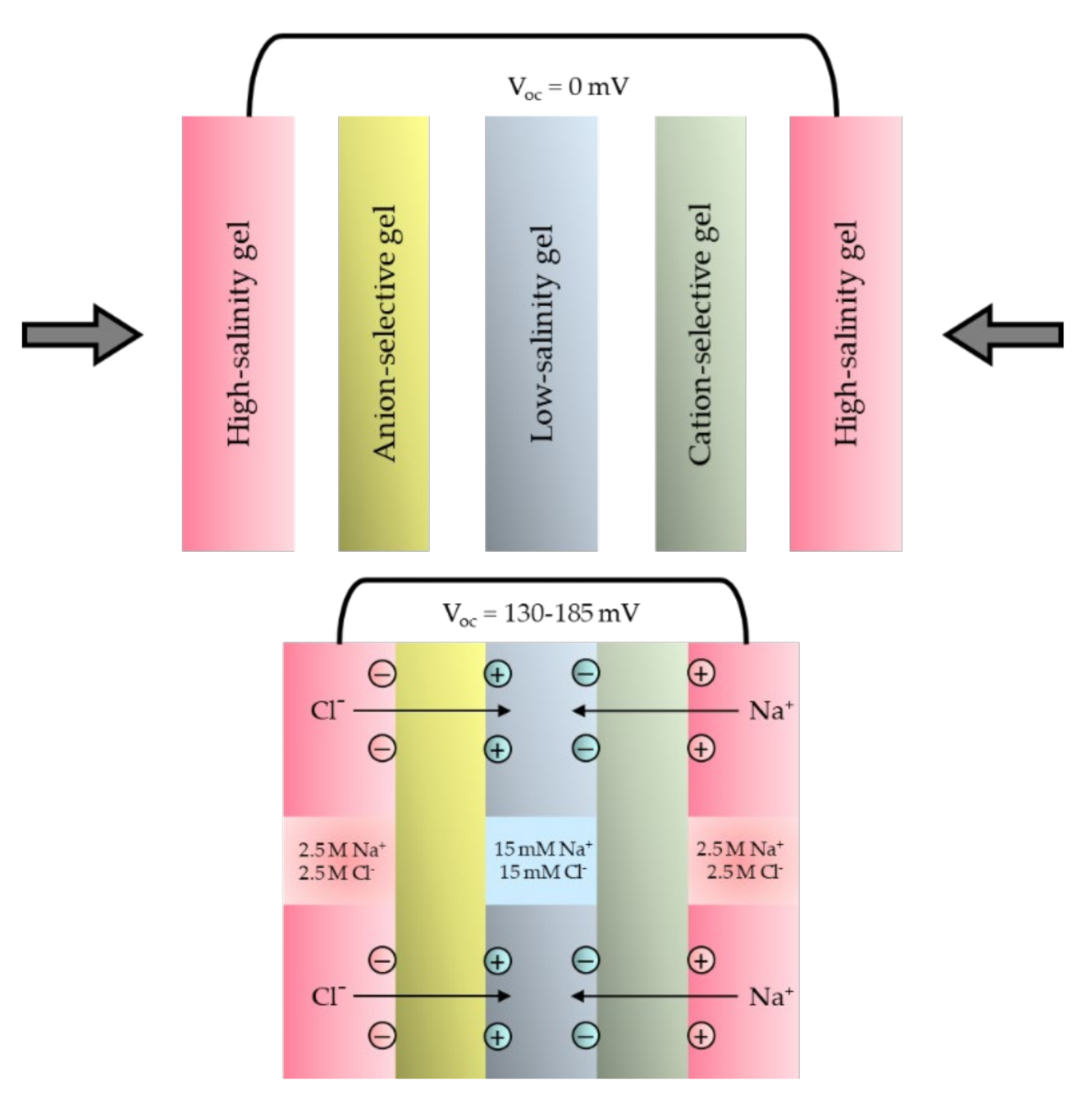
2. Miniaturized SGE Devices
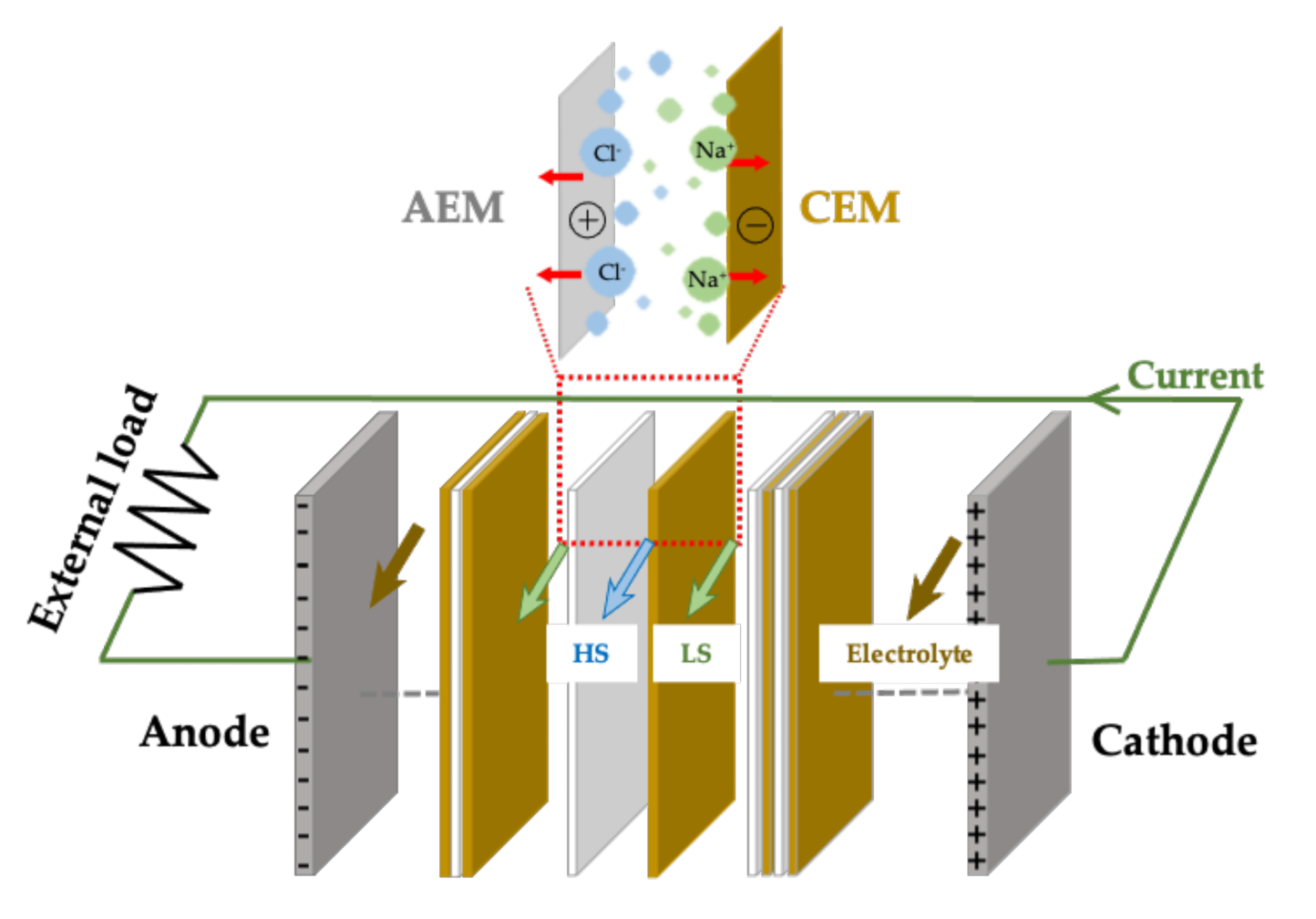
2.1. Membrane-Based Salinity Gradient Energy Devices
2.1.1. Salinity Power Generation Using Biocompatible Asymmetric Polypyrrole Membrane
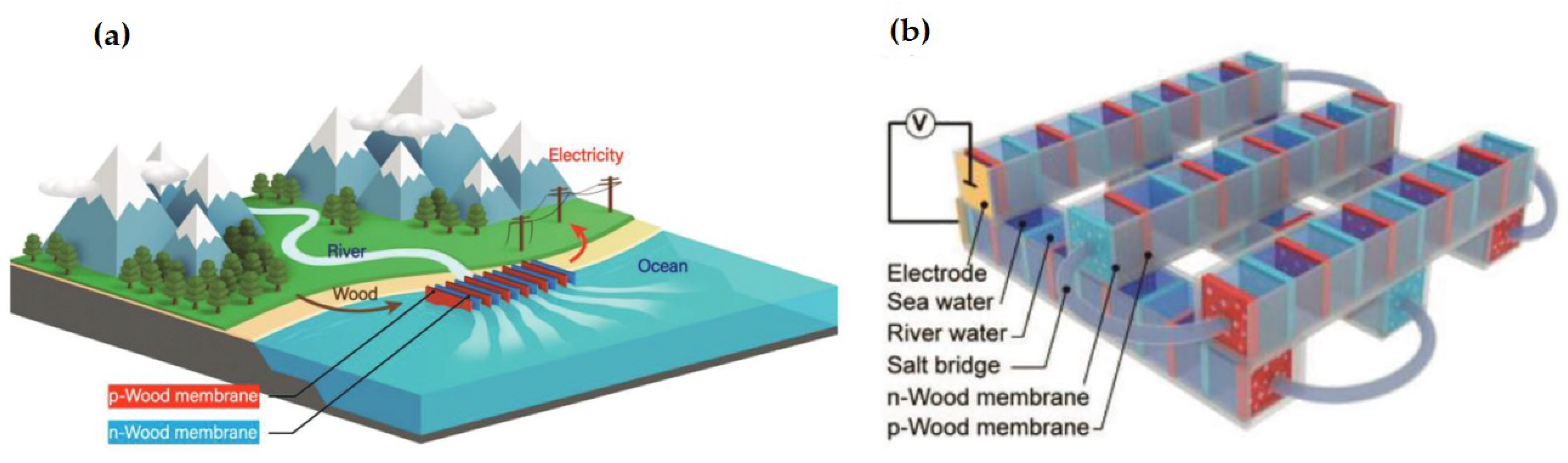
2.1.2. Osmotic Power Generation with Ionized Wood Membranes as Micro- or Nanofluidic Membranes
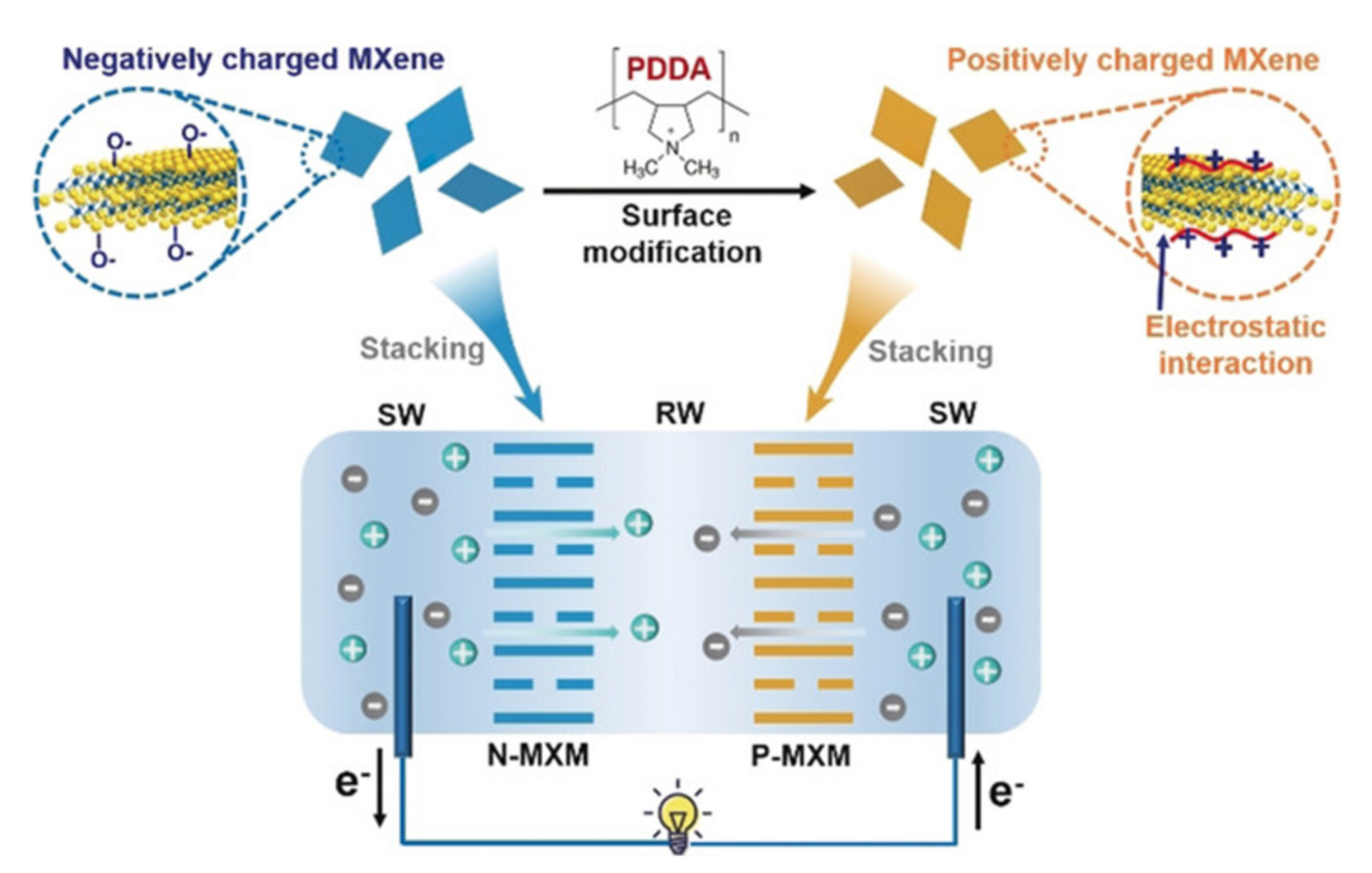
2.1.3. Enhanced Osmotic Energy Harvesting Using 2D-Composites as Nanofluidic Channels
2.2. Colloid-Based Salinity Gradient Energy Devices
2.3. Other Salinity Gradient Energy Devices
3. Discussion and Outlook
4. Conclusions
5. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Tong, X.; Liu, S.; Crittenden, J.; Chen, Y. Nanofluidic Membranes to Address the Challenges of Salinity Gradient Power Harvesting. ACS Nano 2021, 15, 5838–5860. [Google Scholar] [CrossRef]
- Ramon, G.Z.; Feinberg, B.J.; Hoek, E.M.V.V. Membrane-based production of salinity-gradient power. Energy Environ. Sci. 2011, 4, 4423. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-based processes for sustainable power generation using water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Schaetzle, O.; Buisman, C.J.N. Salinity Gradient Energy: Current State and New Trends. Engineering 2015, 1, 164–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Savenije, H.H.G. Thermodynamics of saline and fresh water mixing in estuaries. Earth Syst. Dyn. 2018, 9, 241–247. [Google Scholar] [CrossRef]
- Mei, Y.; Tang, C.Y. Recent developments and future perspectives of reverse electrodialysis technology: A review. Desalination 2018, 425, 156–174. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wang, C.; Wang, R.; Chen, C.; Gao, J.; Dai, J.; Liu, D.; Lin, Z.; Hu, L. Salinity-Gradient Power Generation with Ionized Wood Membranes. Adv. Energy Mater. 2020, 10, 1902590. [Google Scholar] [CrossRef]
- Budi, S.H.; Susanto, H.; Hermawan. The potential recovery energy of SWD (sea water desalination) by SGP (salinity gradient power). J. Phys. Conf. Ser. 2021, 1858, 012078. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, L.; Jiang, L. Nanofluidics for osmotic energy conversion. Nat. Rev. Mater. 2021, 6, 622–639. [Google Scholar] [CrossRef]
- Zhang, Z.; He, L.; Zhu, C.; Qian, Y.; Wen, L.; Jiang, L. Improved osmotic energy conversion in heterogeneous membrane boosted by three-dimensional hydrogel interface. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, Q.; Qian, Y.; Xin, W.; Hao, D.; Zhao, X.; Zhu, C.; Kong, X.Y.; Lu, B.; Jiang, L.; et al. Improved Ion Transport in Hydrogel-Based Nanofluidics for Osmotic Energy Conversion. ACS Cent. Sci. 2020, 6, 2097–2104. [Google Scholar] [CrossRef]
- Yip, N.Y.; Brogioli, D.; Hamelers, H.V.M.; Nijmeijer, K. Salinity gradients for sustainable energy: Primer, progress, and prospects. Environ. Sci. Technol. 2016, 50, 12072–12094. [Google Scholar] [CrossRef]
- Han, G.; Liu, J.T.; Lu, K.J.; Chung, T.S. Advanced Anti-Fouling Membranes for Osmotic Power Generation from Wastewater via Pressure Retarded Osmosis (PRO). Environ. Sci. Technol. 2018, 52, 6686–6694. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Zhang, S.; Li, X.; Chung, T.S. Progress in pressure retarded osmosis (PRO) membranes for osmotic power generation. Prog. Polym. Sci. 2014, 51, 1–27. [Google Scholar] [CrossRef]
- Alsawaftah, N.; Abuwatfa, W.; Darwish, N.; Husseini, G. A comprehensive review on membrane fouling: Mathematical modelling, prediction, diagnosis, and mitigation. Water 2021, 13, 1327. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Bajracharya, S.; Sales, B.B.; Saakes, M.; Hamelers, B.; Nijmeijer, K. Clean energy generation using capacitive electrodes in reverse electrodialysis. Energy Environ. Sci. 2013, 6, 643–651. [Google Scholar] [CrossRef]
- Veerman, J.; Saakes, M.; Metz, S.J.; Harmsen, G.J. Reverse electrodialysis: Evaluation of suitable electrode systems. J. Appl. Electrochem. 2010, 40, 1461–1474. [Google Scholar] [CrossRef] [Green Version]
- Arribas, P.; Khayet, M.; García-Payo, M.C.; Gil, L. Novel and emerging membranes for water treatment by electric potential and concentration gradient membrane processes. In Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications; Wiley-VCH Verlag: Weinheim, Germany, 2015; Volume 10, pp. 287–325. ISBN 9781782421269. [Google Scholar]
- Curto, D.; Franzitta, V.; Guercio, A. A review of the water desalination technologies. Appl. Sci. 2021, 11, 670. [Google Scholar] [CrossRef]
- Su, Y.S.; Hsu, S.C.; Peng, P.H.; Yang, J.Y.; Gao, M.; Yeh, L.H. Unraveling the anomalous channel-length-dependent blue energy conversion using engineered alumina nanochannels. Nano Energy 2021, 84, 105930. [Google Scholar] [CrossRef]
- Zhang, Z.; Sui, X.; Li, P.; Xie, G.; Kong, X.Y.; Xiao, K.; Gao, L.; Wen, L.; Jiang, L. Ultrathin and Ion-Selective Janus Membranes for High-Performance Osmotic Energy Conversion. J. Am. Chem. Soc. 2017, 139, 8905–8914. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Xiao, H.; Kong, X.Y.; Chen, J.; Yang, L.; Niu, B.; Qian, Y.; Teng, Y.; Jiang, L.; Wen, L. Biomimetic Nacre-Like Silk-Crosslinked Membranes for Osmotic Energy Harvesting. ACS Nano 2020, 14, 9701–9710. [Google Scholar] [CrossRef]
- Chen, C.; Liu, D.; Qing, X.; Yang, G.; Wang, X.; Lei, W. Robust Membrane for Osmotic Energy Harvesting from Organic Solutions. ACS Appl. Mater. Interfaces 2020, 12, 52771–52778. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, B.; Lim, G.; Han, J. A multiscale-pore ion exchange membrane for better energy efficiency. J. Mater. Chem. A 2018, 6, 7714–7723. [Google Scholar] [CrossRef] [Green Version]
- Macha, M.; Marion, S.; Nandigana, V.V.R.; Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 2019, 4, 588–605. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Z.; Li, C.; Gao, L.; Zhao, Y.; Yang, L.; Wen, L.; Jiang, L. Engineered Nanochannel Membranes with Diode-like Behavior for Energy Conversion over a Wide pH Range. ACS Appl. Mater. Interfaces 2019, 11, 23815–23821. [Google Scholar] [CrossRef]
- Pattle, R.E. Production of electric power by mixing fresh and salt water in the hydroelectric pile. Nature 1954, 174, 660. [Google Scholar] [CrossRef]
- Yang, H.-C.C.; Xie, Y.; Hou, J.; Cheetham, A.K.; Chen, V.; Darling, S.B. Janus Membranes: Creating Asymmetry for Energy Efficiency. Adv. Mater. 2018, 30, 1801495. [Google Scholar] [CrossRef]
- Brauns, E. Salinity gradient power by reverse electrodialysis: Effect of model parameters on electrical power output. Desalination 2009, 237, 378–391. [Google Scholar] [CrossRef]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Korayem, A.H.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folaranmi, G.; Bechelany, M.; Sistat, P.; Cretin, M.; Zaviska, F. Towards Electrochemical Water Desalination Techniques: A Review on Capacitive Deionization, Membrane Capacitive Deionization and Flow Capacitive Deionization. Membranes 2020, 10, 96. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Y.; Hu, Y.; Zhu, C.; Guo, H.; Kong, X.-Y.; Luo, E.; Jiang, L.; Wen, L. Thermoenhanced osmotic power generator via lithium bromide and asymmetric sulfonated poly(ether ether ketone)/poly(ether sulfone) nanofluidic membrane. NPG Asia Mater. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Wen, L.; Sui, X.; Cheng, Y.; Gao, L.; Jiang, L. Large-scale, robust mushroom-shaped nanochannel array membrane for ultrahigh osmotic energy conversion. Sci. Adv. 2021, 7, eabg2183. [Google Scholar] [CrossRef]
- Yazda, K.; Bleau, K.; Zhang, Y.; Capaldi, X.; St-Denis, T.; Grutter, P.; Reisner, W.W. High Osmotic Power Generation via Nanopore Arrays in Hybrid Hexagonal Boron Nitride/Silicon Nitride Membranes. Nano Lett. 2021, 21, 4152–4159. [Google Scholar] [CrossRef]
- Lin, Z.H.; Hsu, W.S.; Preet, A.; Yeh, L.H.; Chen, Y.H.; Pao, Y.P.; Lin, S.F.; Lee, S.; Fan, J.C.; Wang, L.; et al. Ingestible polysaccharide battery coupled with a self-charging nanogenerator for controllable disinfection system. Nano Energy 2021, 79, 105440. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J. General Principles of Cell Communication. In Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002; 1464p. [Google Scholar]
- Xiao, K.; Jiang, L.; Antonietti, M. Ion Transport in Nanofluidic Devices for Energy Harvesting. Joule 2019, 3, 2364–2380. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, H.; Khan, M.A.; Zou, W.; Xu, J.; Zhang, L.; Zhang, J. Recent progress in advanced electrode materials, separators and electrolytes for lithium batteries. J. Mater. Chem. A 2018, 6, 20564–20620. [Google Scholar] [CrossRef]
- Laucirica, G.; Albesa, A.G.; Toimil-Molares, M.E.; Trautmann, C.; Marmisollé, W.A.; Azzaroni, O. Shape matters: Enhanced osmotic energy harvesting in bullet-shaped nanochannels. Nano Energy 2020, 71, 104612. [Google Scholar] [CrossRef]
- Guo, W.; Cao, L.; Xia, J.; Nie, F.Q.; Wen, M.; Xue, J.; Song, Y.; Zhu, D.; Wang, Y.; Jiang, L. Energy harvesting with single-ion-selective nanopores: A concentration-gradient-driven nanofluidic power source. Adv. Funct. Mater. 2010, 20, 1339–1344. [Google Scholar] [CrossRef]
- He, Y.; Huang, Z.; Chen, B.; Tsutsui, M.; Shui Miao, X.; Taniguchi, M. Electrokinetic Analysis of Energy Harvest from Natural Salt Gradients in Nanochannels. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Yeon, S.Y.; Yun, J.; Yoon, S.H.; Lee, D.; Jang, W.; Han, S.H.; Kang, C.M.; Chung, T.D. A miniaturized solid salt reverse electrodialysis battery: A durable and fully ionic power source. Chem. Sci. 2018, 9, 8071–8076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siria, A.; Poncharal, P.; Biance, A.L.; Fulcrand, R.; Blase, X.; Purcell, S.T.; Bocquet, L. Giant osmotic energy conversion measured in a single transmembrane boron nitride nanotube. Nature 2013, 494, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Ajdari, A.; Bocquet, L. Giant amplification of interfacially driven transport by hydrodynamic slip: Diffusio-osmosis and beyond. Phys. Rev. Lett. 2006, 96, 186102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ault, J.T.; Shin, S.; Stone, H.A. Characterization of surface-solute interactions by diffusioosmosis. Soft Matter 2019, 15, 1582–1596. [Google Scholar] [CrossRef]
- Keh, H.J. Diffusiophoresis of charged particles and diffusioosmosis of electrolyte solutions. Curr. Opin. Colloid Interface Sci. 2016, 24, 13–22. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, T.B.H.; Guha, A.; Lamoureux, A.; Vanrenterghem, G.; Sept, D.; Shtein, M.; Yang, J.; Mayer, M. An electric-eel-inspired soft power source from stacked hydrogels. Nature 2017, 552, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tang, K.; Liu, H.; Van derBruggen, B.; Sotto Díaz, A.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Membr. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Ding, L.; Xiao, D.; Lu, Z.; Deng, J.; Wei, Y.; Caro, J.; Wang, H. Oppositely Charged Ti 3 C 2 T x MXene Membranes with 2D Nanofluidic Channels for Osmotic Energy Harvesting. Angew. Chem. 2020, 132, 8798–8804. [Google Scholar] [CrossRef]
- Ji, J.; Kang, Q.; Zhou, Y.; Feng, Y.; Chen, X.; Yuan, J.; Guo, W.; Wei, Y.; Jiang, L. Osmotic Power Generation with Positively and Negatively Charged 2D Nanofluidic Membrane Pairs. Adv. Funct. Mater. 2017, 27, 1603623. [Google Scholar] [CrossRef]
- Yang, G.; Liu, D.; Chen, C.; Qian, Y.; Su, Y.; Qin, S.; Zhang, L.; Wang, X.; Sun, L.; Lei, W. Stable Ti3C2TxMXene-Boron Nitride Membranes with Low Internal Resistance for Enhanced Salinity Gradient Energy Harvesting. ACS Nano 2021, 15, 6594–6603. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Q.; Chen, J.; Zhang, Q.; Zhao, X.; Qian, Y.; Zhu, C.; Yang, L.; Zhao, Y.; Kong, X.Y.; et al. Improved Ion Transport and High Energy Conversion through Hydrogel Membrane with 3D Interconnected Nanopores. Nano Lett. 2020, 20, 5705–5713. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-C.; Yeh, L.-H. Improved Rectification and Osmotic Power in Polyelectrolyte-Filled Mesopores. Micromachines 2020, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Zhang, Z.; Huang, X.; Hu, Y.; Zhou, T.; Zhu, C.; Kong, X.-Y.; Jiang, L.; Wen, L. High-performance silk-based hybrid membranes employed for osmotic energy conversion. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, S.; Zhang, P.; Zhang, J.; Chen, G.; Feng, X. Mechanically strong MXene/Kevlar nanofiber composite membranes as high-performance nanofluidic osmotic power generators. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Cheng, H.; Zhou, Y.; Feng, Y.; Geng, W.; Liu, Q.; Guo, W.; Jiang, L. Electrokinetic Energy Conversion in Self-Assembled 2D Nanofluidic Channels with Janus Nanobuilding Blocks. Adv. Mater. 2017, 29, 1700177. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jiang, J.; Liu, Q.; Xie, Z.; Zhai, J. Hybrid nanochannel membrane based on polymer/MOF for high-performance salinity gradient power generation. Nano Energy 2018, 53, 643–649. [Google Scholar] [CrossRef]
- Kim, D.K.; Duan, C.; Chen, Y.F.; Majumdar, A. Power generation from concentration gradient by reverse electrodialysis in ion-selective nanochannels. Microfluid. Nanofluidics 2010, 9, 1215–1224. [Google Scholar] [CrossRef]
- Hwang, J.; Sekimoto, T.; Hsu, W.L.; Kataoka, S.; Endo, A.; Daiguji, H. Thermal dependence of nanofluidic energy conversion by reverse electrodialysis. Nanoscale 2017, 9, 12068–12076. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.-C.; Liu, C.-W.; Yang, R.-J. Power Generation by Reverse Electrodialysis in a Microfluidic Device with a Nafion Ion-Selective Membrane. Micromachines 2016, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.G.; Chen, Y. Nanocomposite reverse electrodialysis (RED) ion-exchange membranes for salinity gradient power generation. J. Membr. Sci. 2014, 460, 139–147. [Google Scholar] [CrossRef]
- Tufa, R.A.; Piallat, T.; Hnát, J.; Fontananova, E.; Paidar, M.; Chanda, D.; Curcio, E.; di Profio, G.; Bouzek, K. Salinity gradient power reverse electrodialysis: Cation exchange membrane design based on polypyrrole-chitosan composites for enhanced monovalent selectivity. Chem. Eng. J. 2020, 380, 122461. [Google Scholar] [CrossRef]
- Sun, J.; Wang, G.; Zhang, H.; Zhang, B.; Hu, C. Facile fabrication of a conductive polypyrrole membrane for anti-fouling enhancement by electrical repulsion and in situ oxidation. Chemosphere 2021, 270, 129416. [Google Scholar] [CrossRef]
- Filippov, A.N.; Starov, V.M.; Kononenko, N.A.; Berezina, N.P. Asymmetry of diffusion permeability of bi-layer membranes. Adv. Colloid Interface Sci. 2008, 139, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.E.; Pantano, S. Salt induced asymmetry in membrane simulations by partial restriction of ionic motion. J. Chem. Phys. 2009, 130, 195105. [Google Scholar] [CrossRef]
- Zhang, Z.; Kong, X.-Y.Y.; Xiao, K.; Liu, Q.; Xie, G.; Li, P.; Ma, J.; Tian, Y.; Wen, L.; Jiang, L. Engineered Asymmetric Heterogeneous Membrane: A Concentration-Gradient-Driven Energy Harvesting Device. J. Am. Chem. Soc. 2015, 137, 14765–14772. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Y.; Zhang, Z.; Wang, P.; Wang, X. Biomimetic multifunctional nanochannels based on the asymmetric wettability of heterogeneous nanowire membranes. Adv. Mater. 2014, 26, 1071–1075. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wen, L.; Jiang, L. Bioinspired smart asymmetric nanochannel membranes. Chem. Soc. Rev. 2018, 47, 322–356. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wen, L.; Jiang, L. Towards Practical Osmotic Energy Capture by a Layer-by-Layer Membrane. Trends Chem. 2020, 2, 180–182. [Google Scholar] [CrossRef] [Green Version]
- Shaulsky, E.; Karanikola, V.; Straub, A.P.; Deshmukh, A.; Zucker, I.; Elimelech, M. Asymmetric membranes for membrane distillation and thermo-osmotic energy conversion. Desalination 2019, 452, 141–148. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, X.; Wang, C.; Zhou, Y.; Jia, X.; Jiang, L.; Liu, X.; Wallace, G.G. A smart cyto-compatible asymmetric polypyrrole membrane for salinity power generation. Nano Energy 2018, 53, 475–482. [Google Scholar] [CrossRef]
- Chen, K.; Chen, Y.; Deng, Q.; Jeong, S.H.; Jang, T.S.; Du, S.; Kim, H.E.; Huang, Q.; Han, C.M. Strong and biocompatible poly(lactic acid) membrane enhanced by Ti3C2Tz (MXene) nanosheets for Guided bone regeneration. Mater. Lett. 2018, 229, 114–117. [Google Scholar] [CrossRef]
- Huang, H.; Dong, D.; Li, W.; Zhang, X.; Zhang, L.; Chen, Y.; Sheng, X.; Lu, X. Synergistic effect of MXene on the flame retardancy and thermal degradation of intumescent flame retardant biodegradable poly (lactic acid) composites. Chin. J. Chem. Eng. 2020, 28, 1981–1993. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-based wearable biosensor system for high-performance in vitro perspiration analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, K.; Alhabeb, M.; Wang, Z.; Shalaev, V.M.; Gogotsi, Y.; Boltasseva, A. Highly Broadband Absorber Using Plasmonic Titanium Carbide (MXene). ACS Photonics 2018, 5, 1115–1122. [Google Scholar] [CrossRef]
- An, H.; Habib, T.; Shah, S.; Gao, H.; Radovic, M.; Green, M.J.; Lutkenhaus, J.L. Surface-agnostic highly stretchable and bendable conductive MXene multilayers. Sci. Adv. 2018, 4, eaaq0118. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Wang, X.; Yu, L.; Chen, Y.; Shi, J. Two-Dimensional Ultrathin MXene Ceramic Nanosheets for Photothermal Conversion. Nano Lett. 2017, 17, 384–391. [Google Scholar] [CrossRef]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving Through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Hong, S.; Ming, F.; Shi, Y.; Li, R.; Kim, I.S.; Tang, C.Y.; Alshareef, H.N.; Wang, P. Two-Dimensional Ti3C2Tx MXene Membranes as Nanofluidic Osmotic Power Generators. ACS Nano 2019, 13, 8917–8925. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, P.; Yang, S.; Zhang, T.; Löffler, M.; Shi, H.; Lohe, M.R.; Feng, X. Oxidation promoted osmotic energy conversion in black phosphorus membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 13959–13966. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491. [Google Scholar] [CrossRef]
- Zheng, Z.; Grünker, R.; Feng, X. Synthetic Two-Dimensional Materials: A New Paradigm of Membranes for Ultimate Separation. Adv. Mater. 2016, 28, 6529–6545. [Google Scholar] [CrossRef] [Green Version]
- Aliprandi, A.; Pakulski, D.; Ciesielski, A.; Samorì, P. Punctured Two-Dimensional Sheets for Harvesting Blue Energy. ACS Nano 2017, 11, 10654–10658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Graf, M.; Liu, K.; Ovchinnikov, D.; Dumcenco, D.; Heiranian, M.; Nandigana, V.; Aluru, N.R.; Kis, A.; Radenovic, A. Single-layer MoS2 nanopores as nanopower generators. Nature 2016, 536, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Ji, D.; Li, H.; Tang, J.; Feng, Y.; Ding, L.; Cao, L.; Li, N.; Jiang, L.; Guo, W. A general strategy to simulate osmotic energy conversion in multi-pore nanofluidic systems. Mater. Chem. Front. 2018, 2, 935–941. [Google Scholar] [CrossRef]
- De Santana, C.D.; Crampton, W.G.R.; Dillman, C.B.; Frederico, R.G.; Sabaj, M.H.; Covain, R.; Ready, J.; Zuanon, J.; de Oliveira, R.R.; Mendes-Júnior, R.N.; et al. Unexpected species diversity in electric eels with a description of the strongest living bioelectricity generator. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jiang, L. Bioinspired Nanoporous Membrane for Salinity Gradient Energy Harvesting. Joule 2020, 4, 2244–2248. [Google Scholar] [CrossRef]
- Wang, C.; Choi, E.; Park, J. High-voltage nanofluidic energy generator based on ion-concentration-gradients mimicking electric eels. Nano Energy 2018, 43, 291–299. [Google Scholar] [CrossRef]
- Hasson, D.; Beck, A.; Fingerman, F.; Tachman, C.; Shemer, H.; Semiat, R. Simple model for characterizing a Donnan dialysis process. Ind. Eng. Chem. Res. 2014, 53, 6094–6102. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, J.; Yan, C.; Liao, T.; Bell, J.; Sun, Z. Bioinspired 2D Nanomaterials for Sustainable Applications. Adv. Mater. 2020, 32, 1902806. [Google Scholar] [CrossRef] [PubMed]



| Membrane Type | Main Materials | Functional Groups/ Surfaces | Salts | Ref. |
|---|---|---|---|---|
| Anion selective | Ionized Wood Membrane (Positively charged aligned cellulose nanofibers) | Quaternary ammonium groups | NaCl | [8] |
| Anion selective | Hybrid Janus nanochannel membrane composed of two block copolymers (Poly(ethylene oxide)-block-poly(methacrylate) and polystyrene-block-poly(4-vinylpyridine)) | Hybrid Janus nanochannel membrane | NaCl; KCl | [22] |
| Anion selective | 3-acrylamidopropyl) trimethylammonium chloride | -NH2 groups | NaCl | [49] |
| Anion selective | Poly (sodium 4-styrene sulfonate) (PSS), hydroxypropyltrimethyl ammonium chloride chitosan (HACC) | -NH2 groups | Na2SO4 | [50] |
| Anion selective | Ti3C2Tx MXene membrane modified with polydiallyl dimethyl ammonium (PDDA) (positively charged) | Positively charged MXene surfaces (P-MXene) | NaCl | [51] |
| Anion selective | Graphene oxide membrane (GOM) (Positively charged 1-aminopropyl-3-methylimidazolium bromide (APMIB) conjugated onto GO) | Positively charged GO surfaces | NaCl | [52] |
| Cation selective | Ionized Wood Membrane (Negatively charged aligned cellulose nanofibers) | Carboxyl groups | NaCl | [8] |
| Cation selective | Ti3C2Tx MXene−Boron Nitride | -OH groups, –F groups | NaCl | [53] |
| Cation selective | 2-acrylamido-2-methylpropane sulfonic acid | -OH groups | NaCl | [49] |
| Cation selective | 2-hydroxyethyl methacrylate phosphate (HEMAP) hydrogel membrane | -OH groups | KCl | [54] |
| Cation Selective | Poly(styrenesulfonate) (PSS), anodic alumina oxide (AAO) | -OH groups, -COOH groups | KCl | [27] |
| Cation selective | Poly(ethylene terephthalate) (PET) | -COOH groups | KCl | [55] |
| Cation selective | Pristine Ti3C2Tx MXene nanosheets (negatively charged) | Negatively charged MXene surfaces (p-MXene) | NaCl | [51] |
| Cation selective | Silk-based hybrid Membranes (composed of a silk nanofibril membrane and an anodic aluminum oxide membrane) | Negatively charged surface/ -COOH | NaCl; KCl | [56] |
| Cation selective | MXene/Kevlar nanofiber Composite (Ti3C2Tx (MXene) and charged aramid nanofiber (ANF)) | (–O–) and (–OH) groups in MXene; C−N, C=O, and –COOH groups on ANF | NaCl; KCl | [57] |
| Cation selective | Pristine graphene oxide (GO) sheets (Negatively charged) | -COOH groups | NaCl | [52] |
| Cation Selective | 2D kaolinite | Al-OH groups | KCl | [58] |
| Cation selective | Polymer/MOF hybrid membrane composed of polystyrene sulfonate (PSS)/Metal-organic frameworks (MOF) composites and anodic aluminum oxide (AAO) | Positively charged hybrid membrane surfaces | KCl | [59] |
| Cation selective | Ionic diode membrane | Nanochannel surfaces | KCl | [60] |
| Cation selective | Mesoporous Silica Thin film | Negatively charged silica surfaces | KCl | [61] |
| Cation selective | Nafion-filled polydimethylsiloxane (PDMS) Microchannels | Nafion | KCl | [62] |
| Cation selective | Nanocomposite membrane containing iron (III) oxide and sulfonated poly (2,6-dimethyl-1,4-phenylene oxide) (sPPO) polymer | Sulfonate groups (–SO3-) | NaCl | [63] |
| Cation selective | Polypyrrole (PPy)/chitosan (CS) composite | Amino (–NH2) groups | NaCl | [64] |
| Cation selective | Gellan gum (GG) membrane | ‒COOH groups | KCl | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, W.-S.; Preet, A.; Lin, T.-Y.; Lin, T.-E. Miniaturized Salinity Gradient Energy Harvesting Devices. Molecules 2021, 26, 5469. https://doi.org/10.3390/molecules26185469
Hsu W-S, Preet A, Lin T-Y, Lin T-E. Miniaturized Salinity Gradient Energy Harvesting Devices. Molecules. 2021; 26(18):5469. https://doi.org/10.3390/molecules26185469
Chicago/Turabian StyleHsu, Wei-Shan, Anant Preet, Tung-Yi Lin, and Tzu-En Lin. 2021. "Miniaturized Salinity Gradient Energy Harvesting Devices" Molecules 26, no. 18: 5469. https://doi.org/10.3390/molecules26185469
APA StyleHsu, W.-S., Preet, A., Lin, T.-Y., & Lin, T.-E. (2021). Miniaturized Salinity Gradient Energy Harvesting Devices. Molecules, 26(18), 5469. https://doi.org/10.3390/molecules26185469







