Azocalix[4]arene-Rhodamine Supramolecular Hypoxia-Sensitive Systems: A Search for the Best Calixarene Hosts and Rhodamine Guests
Abstract
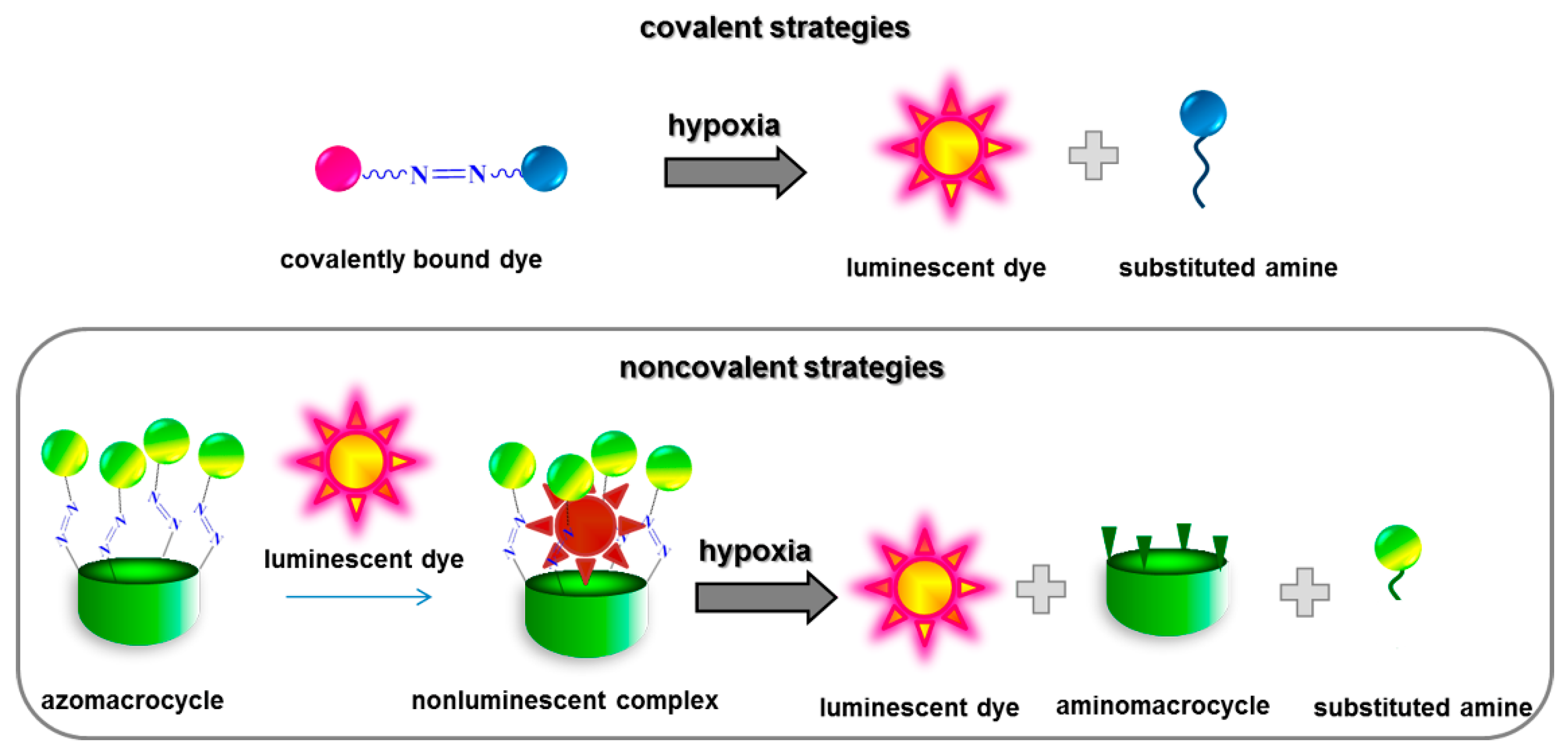
:1. Introduction
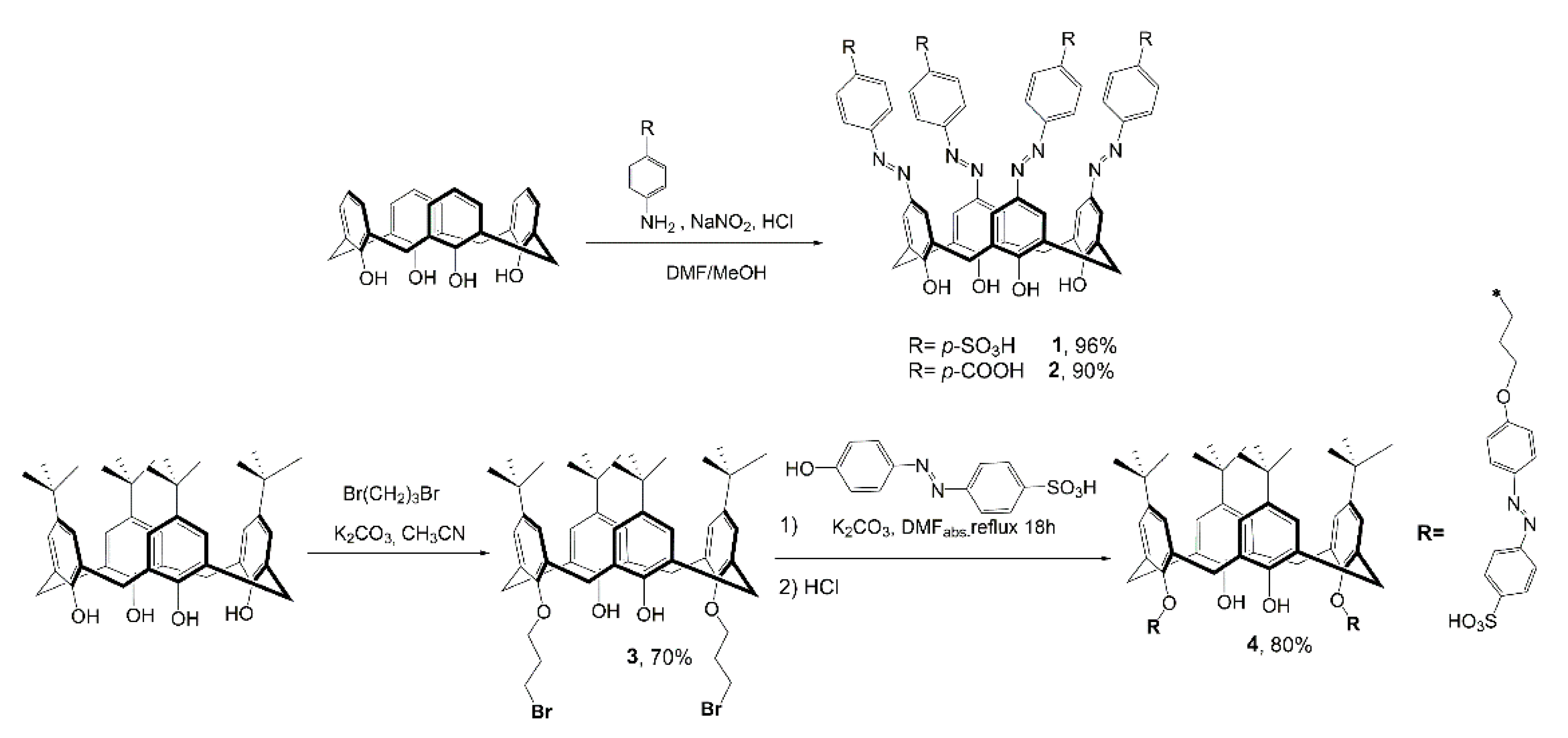
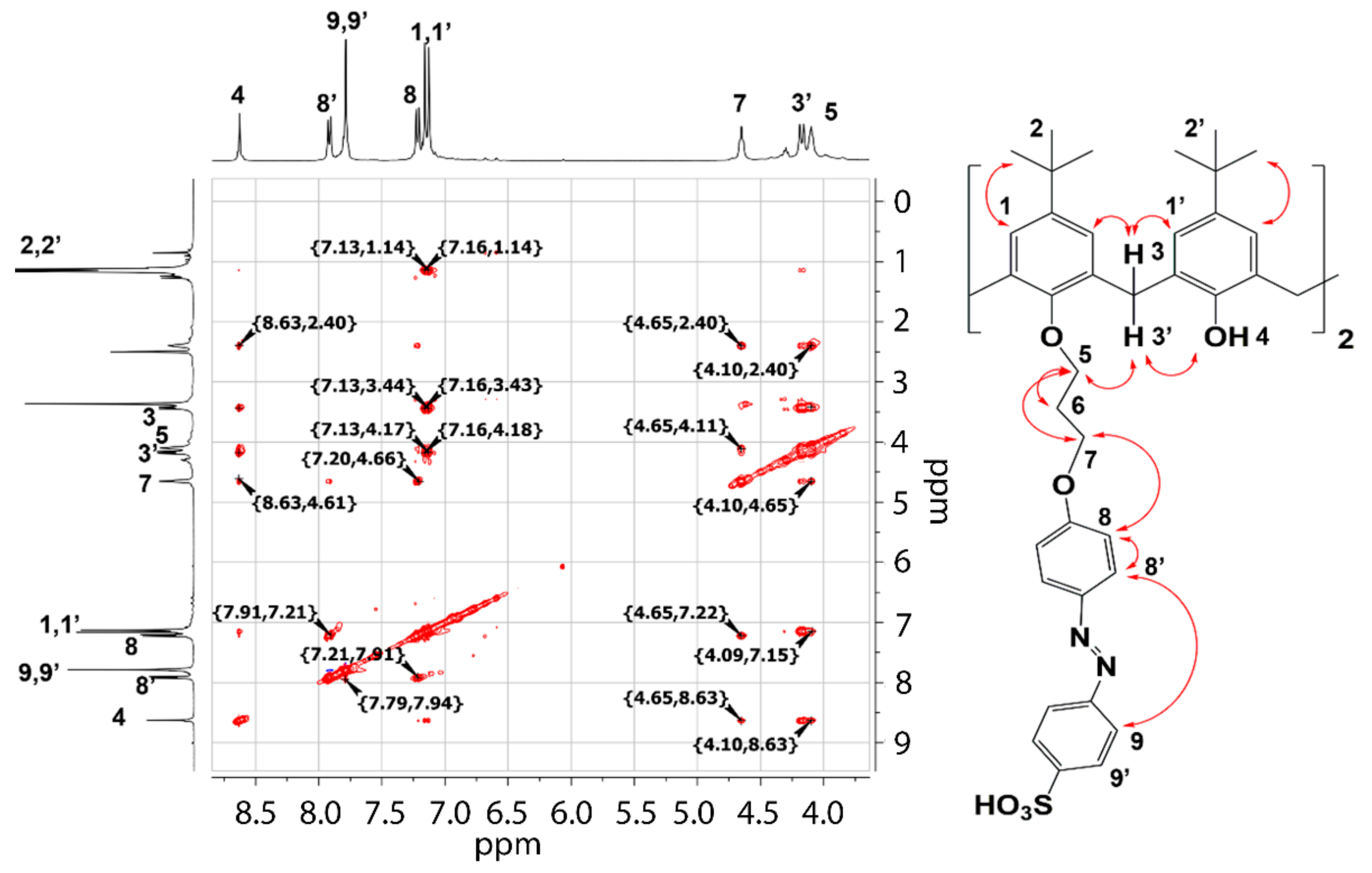
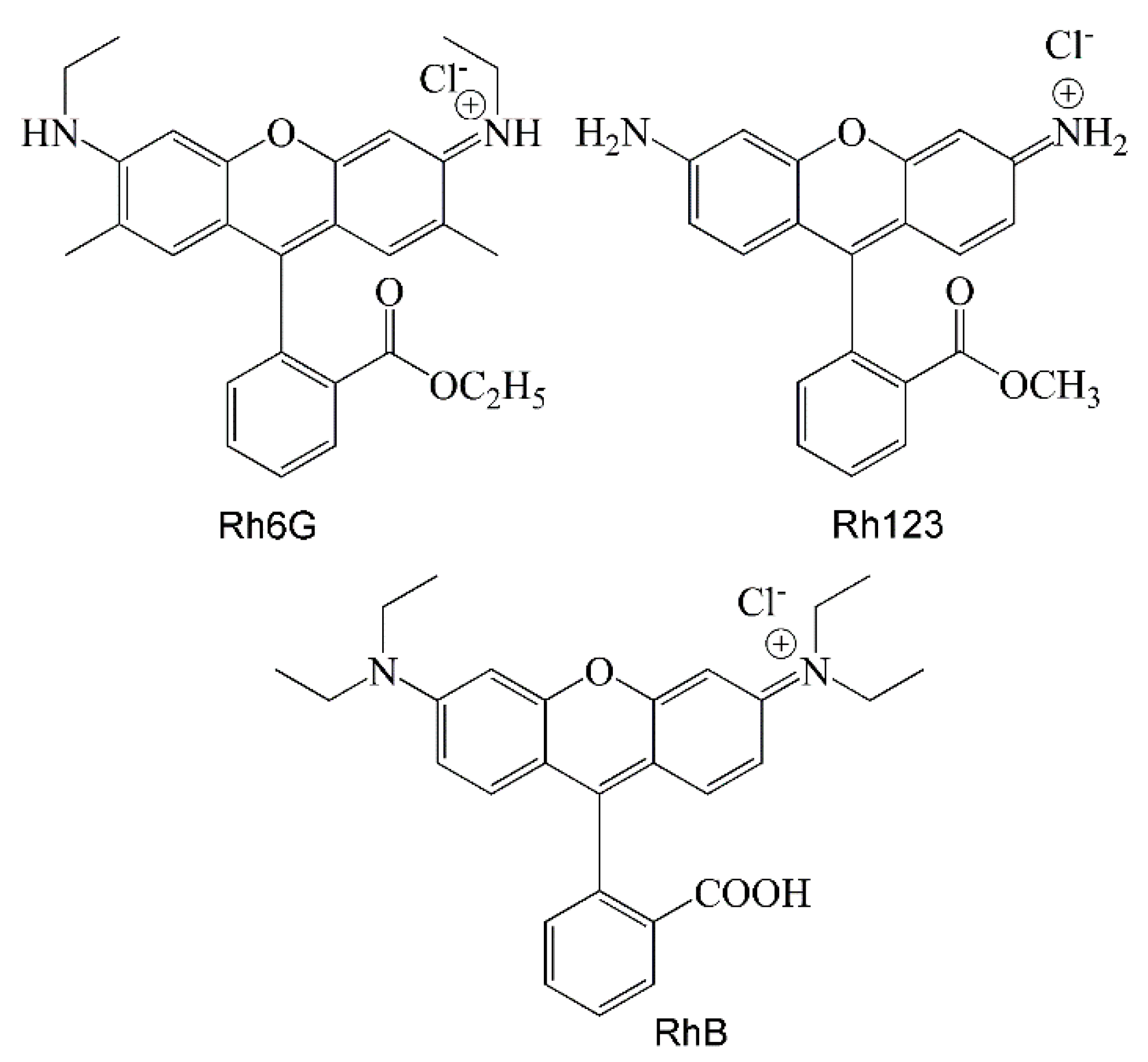
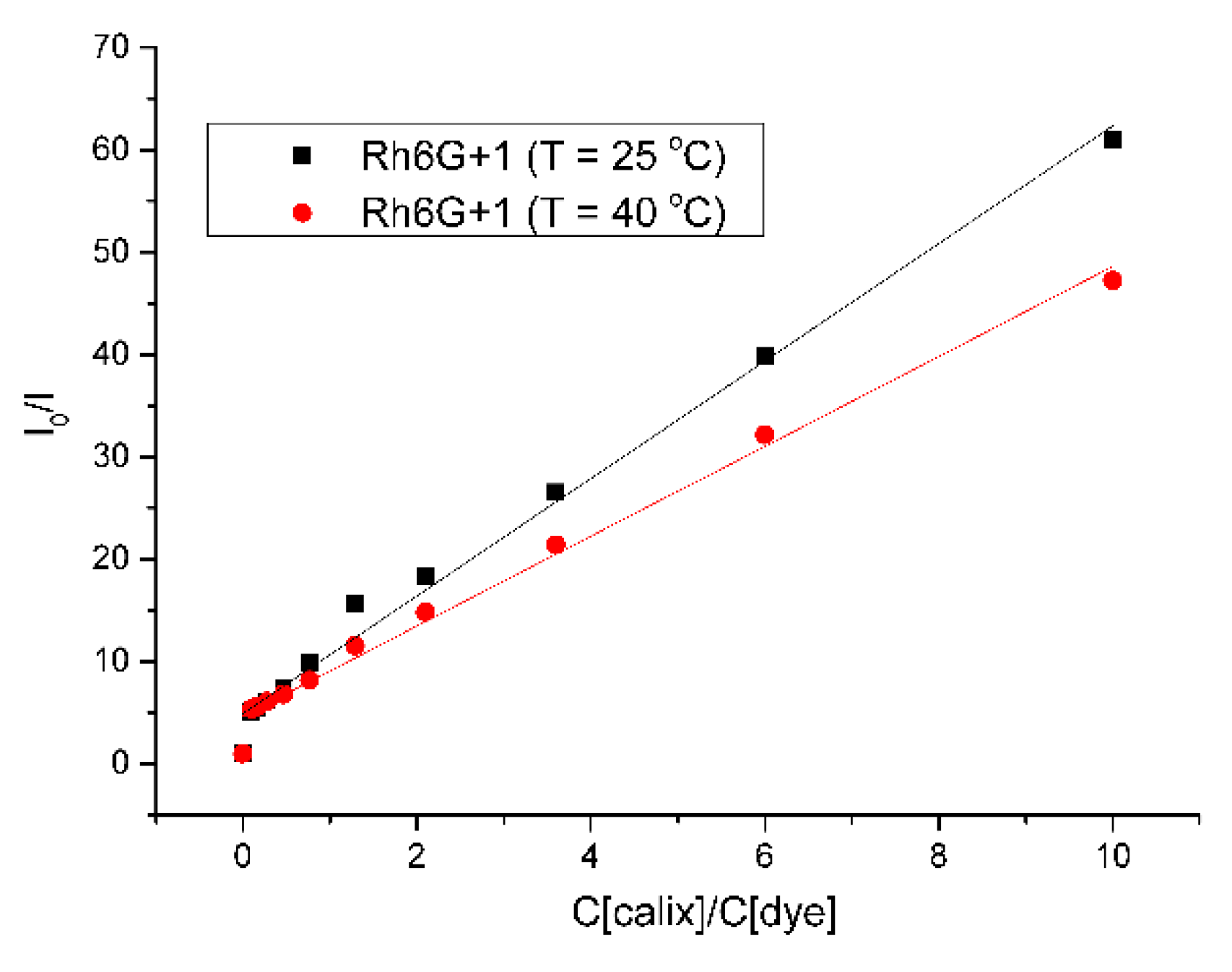
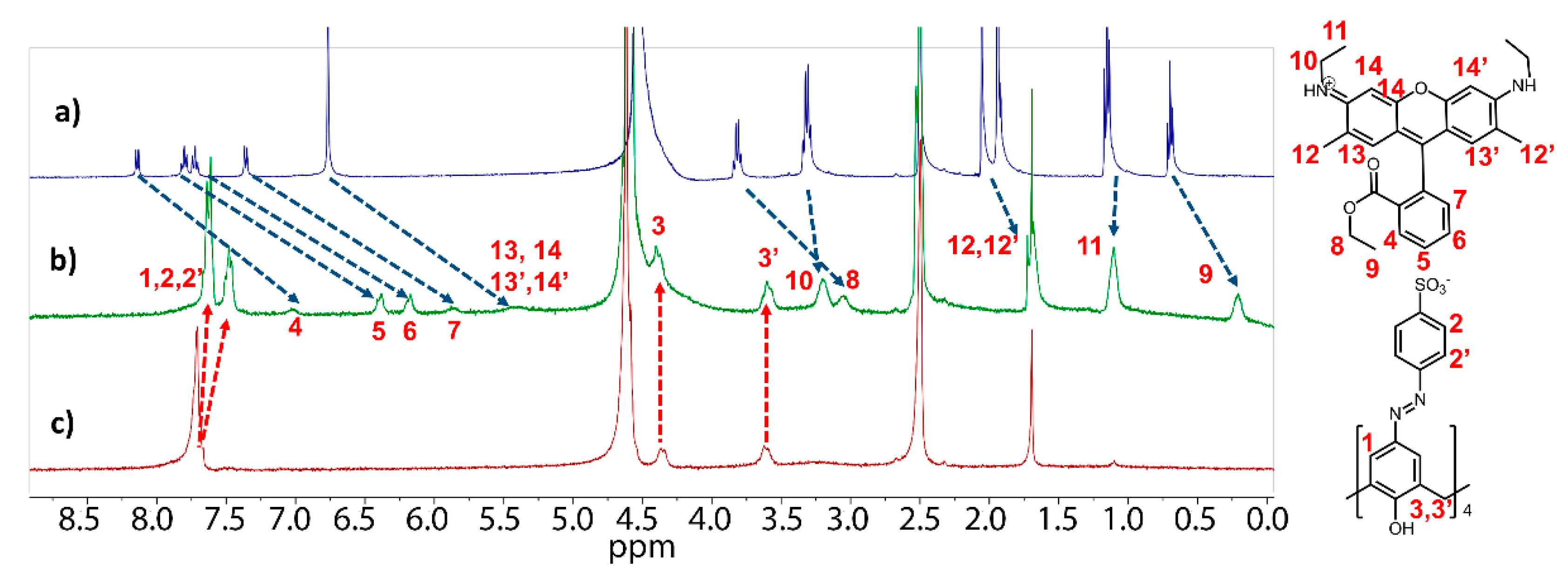
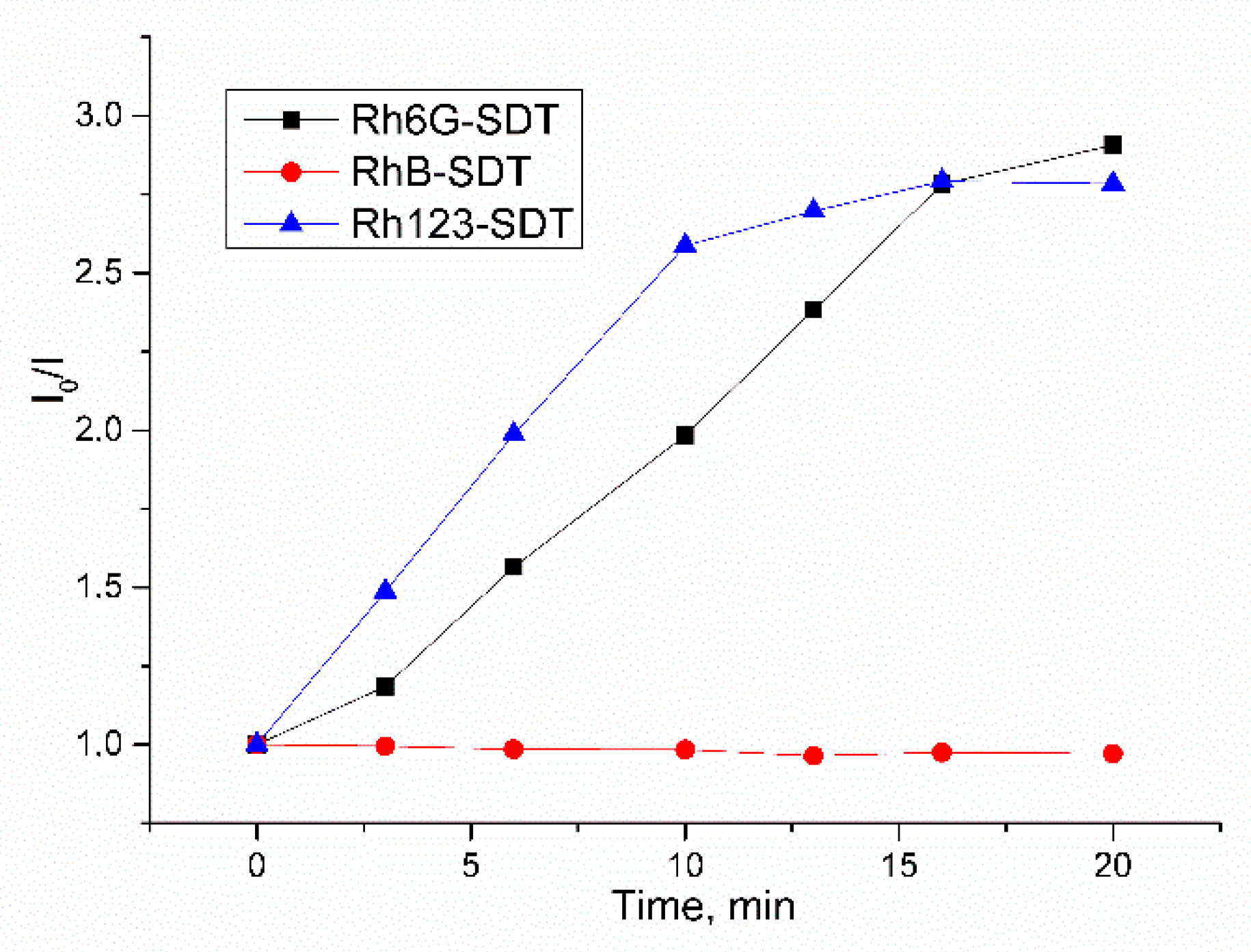
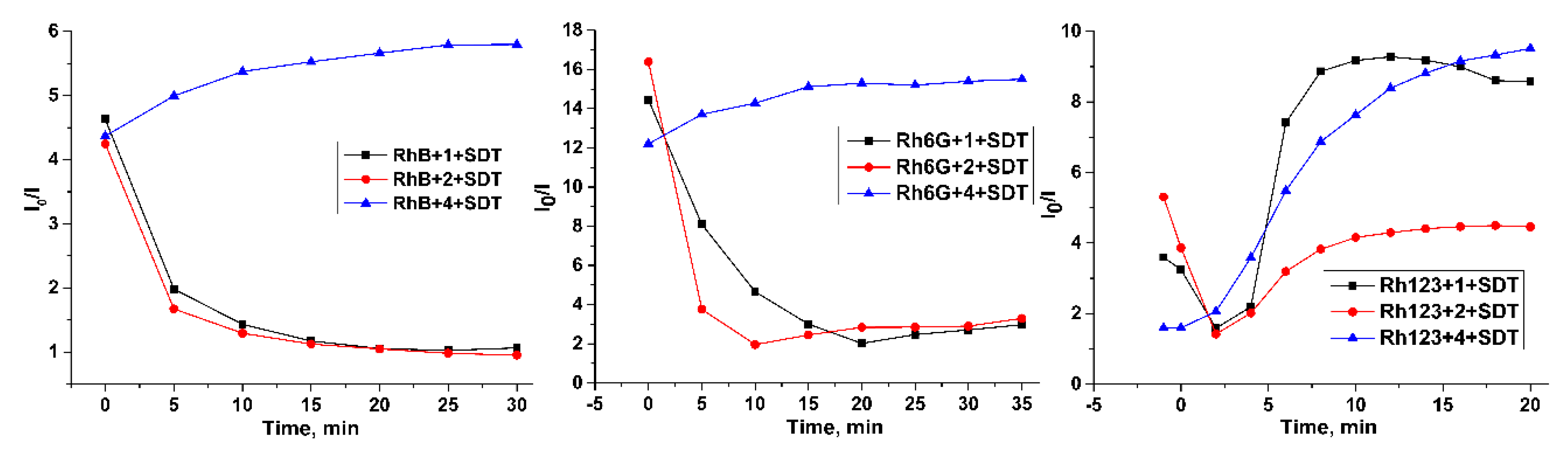
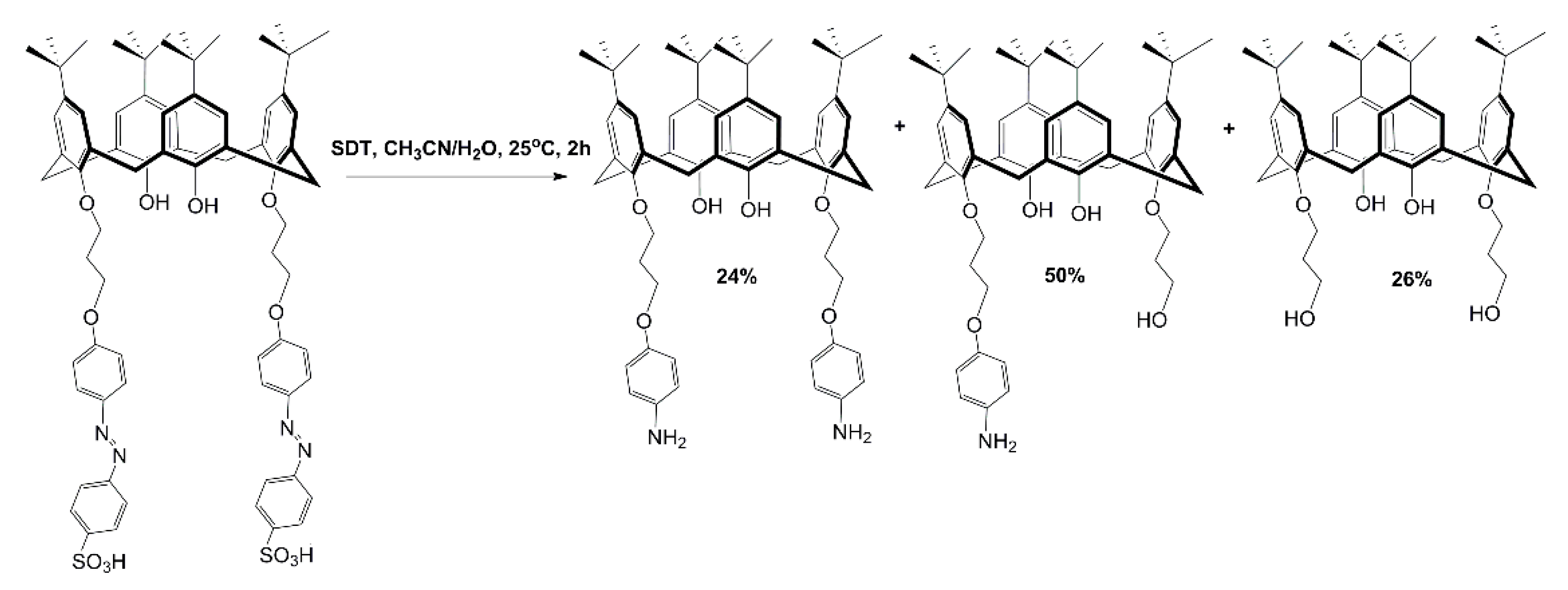
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Apparatus
3.3. Quantum Chemical Calculations
3.4. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. A microenvironmental model of carcinogenesis. Nat. Rev. Cancer 2008, 8, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Busk, M.; Overgaard, J.; Horsman, M.R. Imaging of Tumor Hypoxia for Radiotherapy: Current Status and Future Directions. Semin. Nucl. Med. 2020, 50, 562–583. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia—a key regulatory factor in tumour growth. Nat. Rev. Cancer 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-X.; Zhang, Z.-Z.; Yue, Y.-X.; Hu, X.-Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.-S. A General Hypoxia-Responsive Molecular Container for Tumor-Targeted Therapy. Adv. Mater. 2020, 32, 1908435. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Hu, X.-Y.; Guo, D.-S. Biomedical Applications of Calixarenes: State of the Art and Perspectives. Angew. Chem. Int. Ed. 2020, 60, 2768–2794. [Google Scholar] [CrossRef]
- Chevalier, A.; Piao, W.; Hanaoka, K.; Nagano, T.; Renard, P.-Y.; Romieu, A. Azobenzene-caged sulforhodamine dyes: A novel class of ‘turn-on’ reactive probes for hypoxic tumor cell imaging. Methods Appl. Fluores. 2015, 3, 044004. [Google Scholar] [CrossRef]
- Sun, L.; Li, G.; Chen, X.; Chen, Y.; Jin, C.; Ji, L.; Chao, H. Azo-Based Iridium(III) Complexes as Multicolor Phosphorescent Probes to Detect Hypoxia in 3D Multicellular Tumor Spheroids. Sci. Rep. 2015, 5, 14837. [Google Scholar] [CrossRef] [Green Version]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of Azo-Based Fluorescent Probes to Detect Different Levels of Hypoxia. Angew. Chem. Int. Ed. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Uddin, M.I.; Evans, S.M.; Craft, J.R.; Marnett, L.J.; Uddin, M.J.; Jayagopal, A. Applications of Azo-Based Probes for Imaging Retinal Hypoxia. ACS Med. Chem. Lett. 2015, 6, 445–449. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, J.; Li, Y.; Wu, X.; Yuan, Q.; Yang, R.; Zheng, J. Hypoxia-responsive fluorescent nanoprobe for imaging and cancer therapy. TrAC Trends Anal. Chem. 2020, 131, 116010. [Google Scholar] [CrossRef]
- Kim, H.S.; Sharma, A.; Ren, W.X.; Han, J.; Kim, J.S. COX-2 Inhibition mediated anti-angiogenic activatable prodrug potentiates cancer therapy in preclinical models. Biomaterials 2018, 185, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qi, D.; Li, J. Design, synthesis and applications of responsive macrocycles. Commun. Chem. 2020, 189, 1–14. [Google Scholar]
- Wang, Y.-Y.; Kong, Y.; Zheng, Z.; Geng, W.-C.; Zhao, Z.-Y.; Sun, H.; Guo, D.-S. Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response. Beilstein J. Org. Chem. 2019, 15, 1394–1406. [Google Scholar] [CrossRef]
- Geng, W.-C.; Jia, S.; Zheng, Z.; Li, Z.; Ding, D.; Guo, D.-S. A Noncovalent Fluorescence Turn-on Strategy for Hypoxia Imaging. Angew. Chem. Int. Ed. 2019, 58, 2377–2381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, T.-X.; Zheng, Y.; Wang, C.; Kang, Z.; Zhao, Y.; Chai, J.; Li, H.-B.; Guo, D.-S.; Liu, Y.; et al. Calixarene-Embedded Nanoparticles for Interference-Free Gene–Drug Combination Cancer Therapy. Small 2021, 17, 2006223. [Google Scholar] [CrossRef]
- Morita, Y.; Agawa, T.; Kai, Y.; Kanehisa, N.; Kasai, N.; Nomura, E.; Taniguchi, H. Syntheses and Crystal Structure of Calix[4]quinone. Chem. Lett. 1989, 18, 1349–1352. [Google Scholar] [CrossRef]
- Menon, S.K.; Patel, R.V.; Panchal, J.G. Self-Aggregation and Solubilizing Properties of the Supramolecular System Based on Azobenzenesulfonate Calix[4]arene and CTAB. J. Incl. Phenom. Macrocycl. Chem. 2009, 67, 73–79. [Google Scholar] [CrossRef]
- Li, Z.-T.; Ji, G.-Z.; Zhao, C.-X.; Yuan, S.-D.; Ding, H.; Huang, C.; Du, A.-L.; Wei, M. Self-Assembling Calix[4]arene [2]Catenanes. Preorganization, Conformation, Selectivity, and Efficiency. J. Org. Chem. 1999, 64, 3572–3584. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Bai, S.; Wang, M.; Wang, L. Nanosized Difunctional Photo Responsive Magnetic Imprinting Polymer for Electrochemically Monitored Light-Driven Paracetamol Extraction. ACS Appl. Mater. Interfaces. 2017, 9, 44114–44123. [Google Scholar] [CrossRef]
- Rajasekar, M. Recent Trends in Rhodamine derivatives as fluorescent probes for biomaterial applications. J. Mol. Struct. 2021, 1235, 130232. [Google Scholar] [CrossRef]
- Gill, H.; Gokel, M.R.; McKeever, M.; Negin, S.; Patel, M.B.; Yin, S.; Gokel, G.W. Supramolecular pore formation as an antimicrobial strategy. Coord. Chem. Rev. 2020, 412, 213264. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, US, 2006. [Google Scholar]
- Zana, R.; Schmidt, J.; Talmon, Y. Tetrabutylammonium Alkyl Carboxylate Surfactants in Aqueous Solution: Self-Association Behavior, Solution Nanostructure, and Comparison with Tetrabutylammonium Alkyl Sulfate Surfactants. Langmuir 2005, 21, 11628–11636. [Google Scholar] [CrossRef]
- Reshetnyak, E.A.; Chernysheva, O.S.; Nikitina, N.A.; Loginova, L.P.; Mchedlov-Petrosyan, N.O. Activity coefficients of alkyl sulfate and alkylsulfonate ions in aqueous and water-salt premicellar solutions. Colloid J. 2014, 76, 358–365. [Google Scholar] [CrossRef]
- Burilov, V.A.; Mironova, D.A.; Ibragimova, R.R.; Nugmanov, R.I.; Solovieva, S.E.; Antipin, I.S. Detection of sulfate surface-active substances via fluorescent response using new amphiphilic thiacalix[4]arenes bearing cationic headgroups with Eosin Y dye. Colloids Surf. A Physicochem. Eng. Aspects 2017, 515, 41–49. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, A.; Yang, W.; Araya, T.; Huang, Y.; Zhao, P.; Johnson, D.; Wang, J.; Ren, Z.J. Complex Formation via Hydrogen bonding between Rhodamine B and Montmorillonite in Aqueous Solution. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cheng, M.; Zhang, Z. On different photodecomposition behaviors of rhodamine B on laponite and montmorillonite clay under visible light irradiation. J. Saudi Chem. Soc. 2014, 18, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Harbron, R.L.; Weaver, J.V.M.; Binks, B.P.; Mann, S. Electrostatically gated membrane permeability in inorganic protocells. Nature Chem. 2013, 5, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, A.R.; Conway, L.K.; Kirkhouse, J.B.A.; McCarney, K.M.; Puissegur, O.; Staunton, E.; Teat, S.J.; Warren, J.E. Monosulfonated Azo Dyes: A Crystallographic Study of the Molecular Structures of the Free Acid, Anionic and Dianionic Forms. Crystals 2020, 10, 662. [Google Scholar] [CrossRef]
- Jaffe, C.L.; Lis, H.; Sharon, N. New cleavable photoreactive heterobifunctional cross linking reagents for studying membrane organization. Biochemistry 1980, 19, 4423–4429. [Google Scholar] [CrossRef]
- Leriche, G.; Budin, G.; Brino, L.; Wagner, A. Optimization of the Azobenzene Scaffold for Reductive Cleavage by Dithionite; Development of an Azobenzene Cleavable Linker for Proteomic Applications. Eur. J. Org. Chem. 2010, 2010, 4360–4364. [Google Scholar] [CrossRef]
- Brienza, F.; Van Aelst, K.; Thielemans, K.; Sels, B.F.; Debecker, D.P.; Cybulska, I. Enhancing lignin depolymerization via a dithionite-assisted organosolv fractionation of birch sawdust. Green Chem. 2021, 23, 3268–3276. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013; Available online: https://gaussian.com (accessed on 20 May 2020).
- Rdkit. Available online: https://www.rdkit.org/ (accessed on 20 May 2020).



| System | Ka, M−1 |
|---|---|
| 1-Rh123 | 5.0 ± 0.3 × 105 |
| 2-Rh123 | 2.3 ± 0.6 × 104 |
| 4-Rh123 | 2.4 ± 0.5 × 105 |
| 1-Rh6G | 1.5 ± 0.1 × 107 |
| 2-Rh6G | 1.0 ± 0.4 × 105 |
| 4-Rh6G | 1.0 ± 0.1 × 106 |
| 1-RhB | 7.0 ± 0.1 × 105 |
| 2-RhB | 2.6 ± 0.1 × 104 |
| 4-RhB | 3.6 ± 0.4 × 105 |
| System | Ka, M−1 |
|---|---|
| 1-Rh123 | 1.1 ± 0.1 × 106 |
| 1-Rh6G | 6.8 ± 0.7 × 106 |
| 1-RhB | 1.5 ± 0.1 × 106 |
| Macrocycle | k, min−1 |
|---|---|
| 1 | 0.350 (Adj. R2 > 0.991) |
| 2 | 0.403 (Adj. R2 > 0.987) |
| 4 | 0.614 (Adj. R2 > 0.982) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironova, D.; Burilov, V.; Galieva, F.; Khalifa, M.A.M.; Kleshnina, S.; Gazalieva, A.; Nugmanov, R.; Solovieva, S.; Antipin, I. Azocalix[4]arene-Rhodamine Supramolecular Hypoxia-Sensitive Systems: A Search for the Best Calixarene Hosts and Rhodamine Guests. Molecules 2021, 26, 5451. https://doi.org/10.3390/molecules26185451
Mironova D, Burilov V, Galieva F, Khalifa MAM, Kleshnina S, Gazalieva A, Nugmanov R, Solovieva S, Antipin I. Azocalix[4]arene-Rhodamine Supramolecular Hypoxia-Sensitive Systems: A Search for the Best Calixarene Hosts and Rhodamine Guests. Molecules. 2021; 26(18):5451. https://doi.org/10.3390/molecules26185451
Chicago/Turabian StyleMironova, Diana, Vladimir Burilov, Farida Galieva, Mohamed Ali Mohamed Khalifa, Sofia Kleshnina, Alsu Gazalieva, Ramil Nugmanov, Svetlana Solovieva, and Igor Antipin. 2021. "Azocalix[4]arene-Rhodamine Supramolecular Hypoxia-Sensitive Systems: A Search for the Best Calixarene Hosts and Rhodamine Guests" Molecules 26, no. 18: 5451. https://doi.org/10.3390/molecules26185451
APA StyleMironova, D., Burilov, V., Galieva, F., Khalifa, M. A. M., Kleshnina, S., Gazalieva, A., Nugmanov, R., Solovieva, S., & Antipin, I. (2021). Azocalix[4]arene-Rhodamine Supramolecular Hypoxia-Sensitive Systems: A Search for the Best Calixarene Hosts and Rhodamine Guests. Molecules, 26(18), 5451. https://doi.org/10.3390/molecules26185451







