A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species
Abstract
:1. Introduction
2. Results
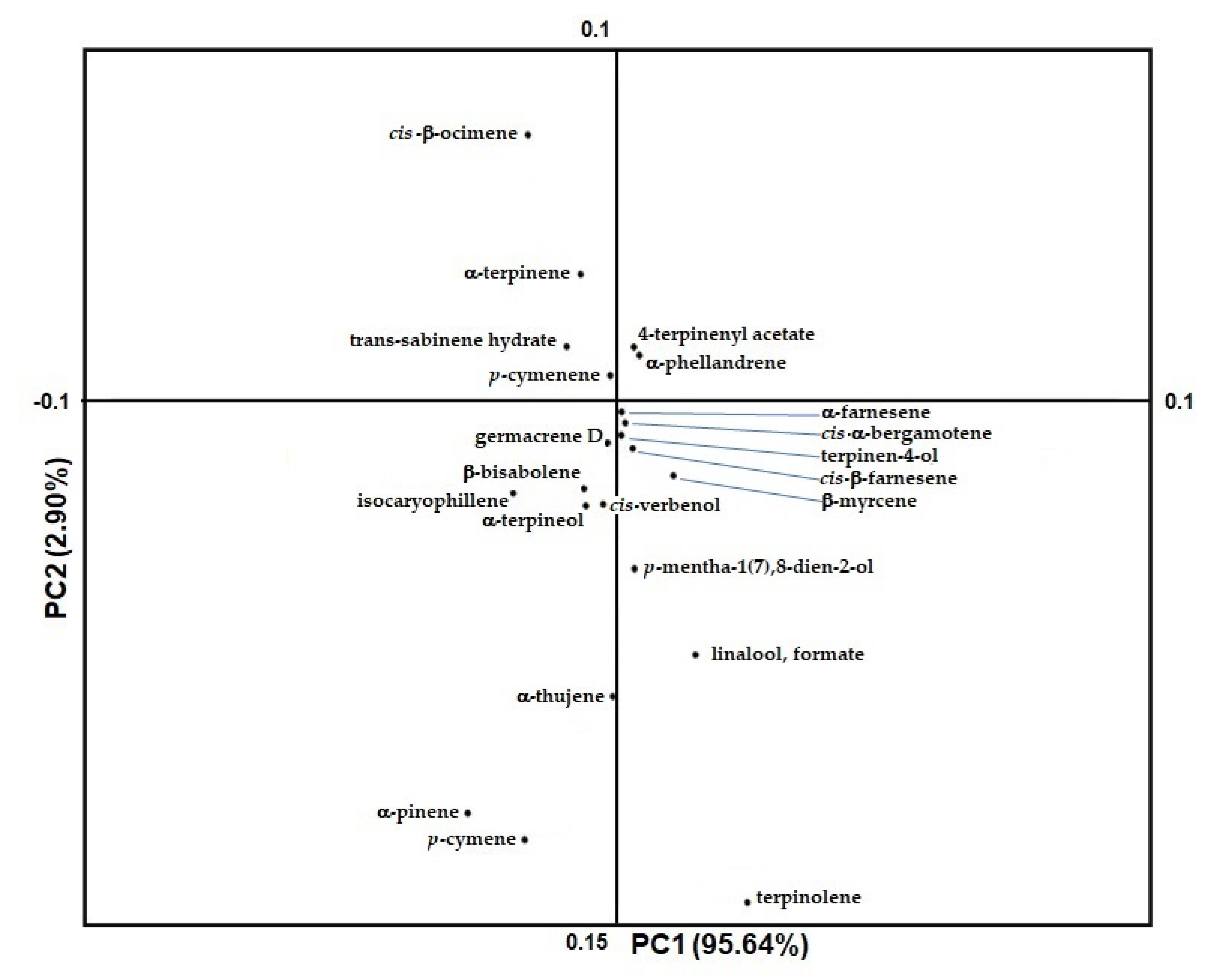
2.1. Juice and Peel Vapor Phase Chemical Volatile Composition
2.2. Total Polyphenols and Flavonoids Content
2.3. Antiradical Activity
3. Discussion
4. Materials and Methods
4.1. General
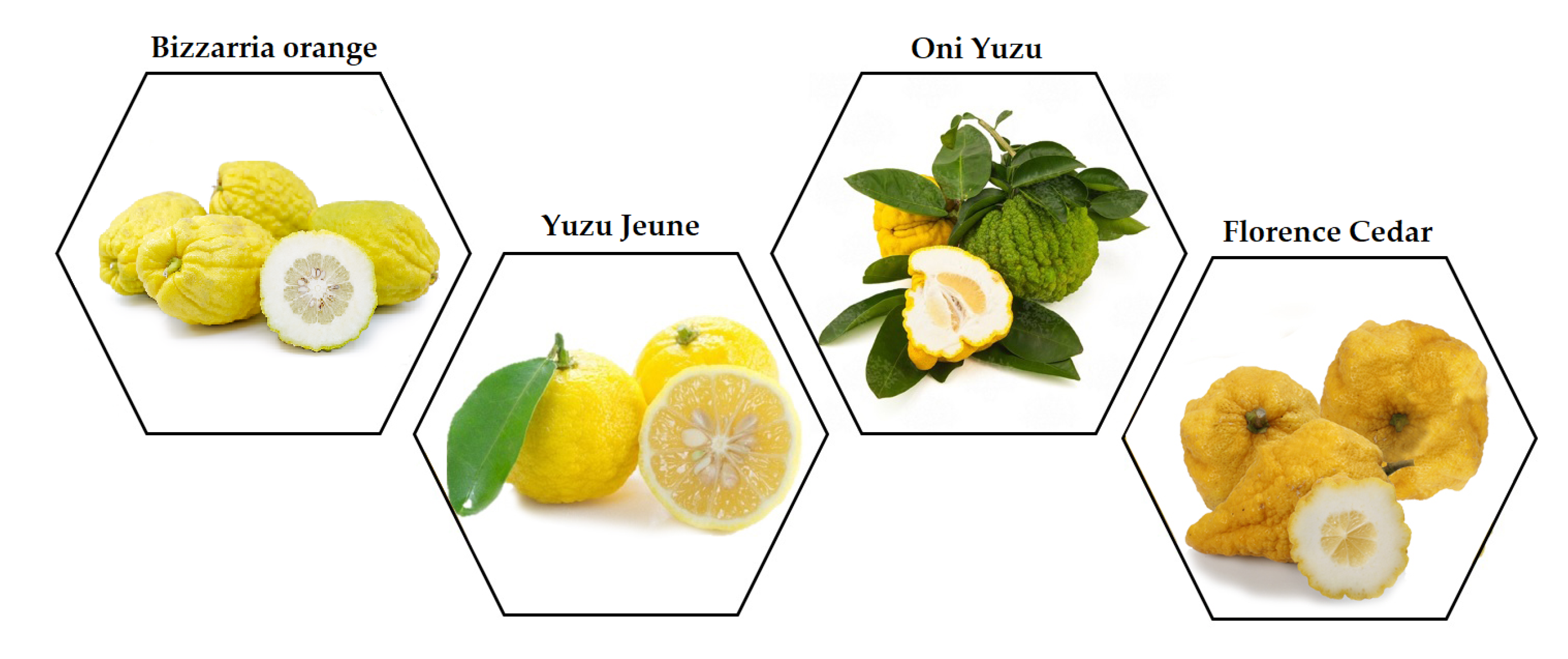
4.2. Citrus Materials (Peels and Juices)
4.3. SPME of Peels and Juices
4.4. GC-MS and GC-FID Chemical Analysis
4.5. Determination of Total Polyphenols
4.6. Determination of Total Flavonoids
4.7. Determination of Antiradical Activity
4.7.1. DPPH• Radical-Scavenging Assay
4.7.2. ABTS•+ Radical-Scavenging Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Faostat. Available online: http://www.fao.org/faostat/en/ (accessed on 16 June 2021).
- Mabberley, D.J. Citrus (Rutaceae): A review of recent advances in etymology, systematics and medical applications. Blumea-Biodivers. Evol. Biogeogr. Plants 2004, 49, 481–498. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hernandez Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, M.F.; Weaver, T.R. Citrus rootstock scion effects and reactions. Punjab Fruit J. 2003, 26–27, 239–246. [Google Scholar]
- Flora of China. Available online: http://www.efloras.org/browse.aspx?flora_id=2&start_taxon_id=107164 (accessed on 8 July 2021).
- Tanaka, T. Bizzarria—A Clear Case of Periclinal Chimera. J. Genet. 1927, 18, 77–85. [Google Scholar] [CrossRef]
- Cameron, J.W.; Frost, H.B. Genetics, Breeding, and Nuclear Embryony. In Citrus Industry. Anatomy, Physiology, Genetics, and Reproduction; UC Regents: Oakland, CA, USA, 1968; Volume 2. [Google Scholar]
- Le Gallerie degli Uffizi. Available online: https://www.uffizi.it/opere/Citrus-medica-florentina#text (accessed on 8 July 2021).
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Nayik, G.A. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Heath, H.B. Flavor Chemistry and Technology; Macmillan Publishers: London, UK, 1986; Volume 4. [Google Scholar]
- Wieczorek, M.N.; Majcher, M.; Jeleń, H. Comparison of Three Extraction Techniques for the Determination of Volatile Flavor Components in Broccoli. Foods 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerry, J.P.; Kerry, J.F.; Ledward, D. Meat Processing, Improvent Quality, 1st ed.; Woodhead Publishing: Cambridge, UK, 2002; p. 480. [Google Scholar]
- Delort, E.; Jaquier, A. Novel terpenyl esters from Australian finger lime (Citrus australasica) peel extract. Flavour Fragr. J. 2009, 24, 123–132. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three Australian finger lime (Citrus australasica) cultivars: Identification of unique Citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef]
- Tomiyama, K.; Aoki, H.; Oikawa, T.; Sakurai, K.; Kasahara, Y.; Kawakami, Y. Characteristic volatile components of Japanese sour Citrus fruits: Yuzu, Sudachi and Kabosu. Flavour Fragr. J. 2012, 27, 341–355. [Google Scholar] [CrossRef]
- Cheong, M.W.; Loke, X.Q.; Liu, S.Q.; Pramudya, K.; Curran, P.; Yu, B. Characterization of Volatile Compounds and Aroma Profiles of Malaysian Pomelo (Citrus grandis (L.) Osbeck) Blossom and Peel. J. Essent. Oil Res. 2011, 23, 34–44. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative Analysis of the Volatile Fraction of Fruit Juice from Different Citrus Species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barboni, T.; Luro, F.; Chiaramonti, N.; Desjobert, J.-M.; Muselli, A.; Costa, J. Volatile composition of hybrids Citrus juices by headspace solid-phase micro extraction/gas chromatography/mass spectrometry. Food Chem. 2009, 116, 382–390. [Google Scholar] [CrossRef]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. Tangerines cultivated on Madeira Island—A high throughput natural source of bioactive compounds. Food Chem. 2020, 9, 1470. [Google Scholar] [CrossRef]
- Hou, J.; Lian, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME-GC-MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, Y.; Huang, L.; Wu, H.; Sun, R.; Li, G. Effect of Peeling Method on the Volatile Flavor of Citrus Fruit. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Changchun, China, 21–23 August 2020; Volume 474, p. 032042. [Google Scholar]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, anti-inflammatory and anti-angiogenic properties of Citrus lumia juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Crowell, P.L.; Elson, C.E.; Bailey, H.H.B.; Elegbede, A.; Haag, J.D. Human metabolism of the experimental cancer therapeutic agent d-limonene. Cancer Chemother. Pharmacol. 1994, 35, 31–37. [Google Scholar] [CrossRef]
- Vigushin, D.M.; Poon, G.K.; Boddy, A.; English, J.; Halbert, G.W.; Pagonis, C.; Jarman, M.; Coombes, R.C. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother. Pharmacol. 1998, 42, 111–117. [Google Scholar] [CrossRef]
- De Oliveira Ramalho, T.R.; de Oliveira, M.T.P.; de Araujo Lima, A.L.; Bezerra-Santos, C.R.; Piuvezam, M.R. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar]
- Wojtunik-Kulesza, K.A.; Targowska-Duda, K.; Klimek, K.; Ginalska, G.; Jóźwiak, K.; Waksmundzka-Hajnos, M.; Cieśla, L. Volatile terpenoids as potential drug leads in Alzheimer’s disease. Open Chem. 2017, 15, 332–343. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, S.; Sawasaki, E.; Okuyama, S.; Miyake, Y.; Yokogoshi, H. Flavor components of monoterpenes in Citrus essential oils enhance the release of monoamines from rat brain slices. Nutr. Neurosci. 2006, 9, 73–80. [Google Scholar] [CrossRef]
- De Brito Passos, F.F.; Lopes, E.M.; De Araújo, J.M.; De Sousa, D.P.; Veras, L.M.C.; Leite, R.S.A.; De Castro Almeida, F.R. Involvement of Cholinergic and Opioid System in γ-Terpinene-Mediated Antinociception. Evid. Based Complement. Altern. Med. 2015, 2015, 829414. [Google Scholar] [CrossRef] [Green Version]
- Do Amaral, J.F.; Silva, M.I.G.; de Aquino Neto, M.R.A.; Neto, P.F.T.; Moura, B.A.; de Melo, C.T.V.; de Araujo, F.L.O.; de Sousa, D.P.; de Vasconcelos, P.F.; de Vasconcelos, S.M.M.; et al. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007, 30, 1217–1220. [Google Scholar] [CrossRef] [Green Version]
- Diep, C.S.; Baranowski, J.; Baranowski, T. The Impact of Fruit and Vegetable Intake on Weight Management. In Managing and Preventing Obesity. Behavioural Factors and Dietary Interventions; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2015; Chapter 4; pp. 59–78. [Google Scholar]
- Grassmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar]
- de Moraes Barros, H.; Pinto de Castro Ferreira, T.A.; Genovese, M.I. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of Citrus from Brazil. Food Chem. 2012, 134, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of flavonoid and antioxidant activity of fruit tissues from 27 Chinese local Citrus cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, V.; Singh Chahal, V.; Kaur Grewal, S.; Singh Gill, P. Effect of fruit development stages on antioxidant properties and bioactive compounds in peel, pulp and juice of grapefruit varieties. J. Food Meas. Charact. 2021, 15, 2531–2539. [Google Scholar] [CrossRef]
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Influence of extraction techniques on antioxidant properties and bioactive compounds of loquat fruit (Eriobotrya japonica Lindl.) skin and pulp extracts. Food Sci. Nutr. 2015, 3, 179–187. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Shah, B.B.; Mehta, A.A. In vitro evaluation of antioxidant activity of d–limonene. Asian J. Pharm. Pharmacol. 2018, 4, 883–887. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and antibacterial activity of seven predominant terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Piccialli, I.; Tedeschi, V.; Caputo, L.; Amato, G.; De Martino, L.; De Feo, V.; Secondo, A.; Pannaccione, A. The antioxidant activity of limonene counteracts neurotoxicity triggered by Aβ1-42 oligomers in primary cortical neurons. Antioxidants 2021, 10, 937. [Google Scholar] [CrossRef] [PubMed]
- Himed, L.; Merniz, S.; Monteagudo-Olivan, R.; Barkat, M.; Coronas, J. Antioxidant activity of the essential oil of Citrus limon before and after its encapsulation in amorphous SiO2. Sci. Afr. 2019, 6, e00181. [Google Scholar] [CrossRef]
- Kirkan, B.; Sarikurkcu, C.; Amarowicz, R. Composition, and antioxidant and enzyme-inhibition activities, of essential oils from Satureja thymbra and Thymbra spicata var. spicata. Flavour Fragr. J. 2019, 34, 436–442. [Google Scholar] [CrossRef]
- Li, G.-X.; Liu, Z.-Q. Unusual Antioxidant Behavior of r- and γ-Terpinene in Protecting Methyl Linoleate, DNA, and Erythrocyte. J. Agric. Food Chem. 2009, 57, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C.; Ingold, K.U. Mechanism of inhibition of lipid peroxidation by γ-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J. Agric. Food Chem. 2003, 51, 2758–2765. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elwahab, S.M.; El-Tanbouly, N.D.; Moussa, M.Y.; Abdel-Monem, A.R.; Fayek, N.M. Antimicrobial and antiradical potential of four agro-waste Citrus peels cultivars. J. Essent. Oil-Bear. Plants 2016, 19, 1932–1942. [Google Scholar] [CrossRef]
- Zardi-Bergaoui, A.; Jelassi, A.; Daami-Remadi, M.; HarzallahSkhiri, F.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Chemical composition and bioactivities of essential oils from Pulicaria vulgaris subsp. dentata (Sm.) Batt. growing in Tunisia. J. Essent. Oil Res. 2020, 32, 111–120. [Google Scholar] [CrossRef]
- Vitalini, S.; Grande, S.; Visioli, F.; Agradi, E.; Fico, G.; Tomè, F. Antioxidant activity of wild plants collected in Valsesia, an alpine region of Northern Italy. Phytother. Res. 2006, 20, 576–580. [Google Scholar] [CrossRef]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. 3 Biotech 2016, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Fracassetti, D.; Gabrielli, M.; Costa, C.; Tomás-Barberán, F.A.; Tirelli, A. Characterization and suitability of polyphenols-based formulas to replace sulfur dioxide for storage of sparkling white wine. Food Control 2016, 60, 606–614. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wine. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Casgrain, P.; Legendre, P. The R Package for Multivariate Analysis, Version 4.0. Available online: http://www.bio.umontreal.ca/casgrain/en/labo/R/v4/index.html (accessed on 3 July 2021).

| N° | Component 1 | LRI 2 | LRI 3 | Yuzu Jeune Juice (%) | Yuzu Jeune Peel (%) | Oni Yuzu Juice (%) | Oni Yuzu Peel (%) | Bizzarria Orange Juice (%) | Bizzarria Orange Peel (%) | Florence Cedar Juice (%) | Florence Cedar Peel (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 918 | 923 | 0.69 ± 0.02a | 0.68 ± 0.03A | 0.20 ± 0.01b | - | 0.09 ± 0.01c | 0.35 ± 0.01B | - | 0.80 ± 0.01C |
| 2 | α-pinene | 940 | 943 | - | - | - | 0.80 ± 0.01A | 0.75 ± 0.01a | 1.00 ± 0.01B | 0.88 ± 0.01b | 1.81 ± 0.01C |
| 3 | camphene | 942 | 946 | - | - | - | - | - | tr | - | - |
| 4 | β-myrcene | 978 | 983 | 0.78 ± 0.01 | 1.40 ± 0.02 | - | - | - | - | - | - |
| 3 | β-pinene | 982 | 986 | 1.13 ± 0.02dc | 1.48 ± 0.02D | 1.17 ± 0.01c | 2.80 ± 0.01B | 1.55 ± 0.02a | 2.30 ± 0.02C | 1.25 ± 0.02b | 3.15 ± 0.01A |
| 5 | α-phellandrene | 1000 | § | 0.96 ± 0.01a | 0.44 ± 0.01A | 0.19 ± 0.01b | tr | 0.13 ± 0.01cd | 0.10 ± 0.01CB | 0.13 ± 0.01d | 0.13 ± 0.01B |
| 6 | α-terpinene | 1002 | 1008 | - | - | - | 0.15 ± 0.01A | - | 0.14 ± 0.01A | 0.54 ± 0.01 | - |
| 7 | p-cymene | 1020 | 1016 | 2.38 ± 0.01a | 2.33 ± 0.01BC | 0.59 ± 0.02ca | 0.38 ± 0.01D | 1.21 ± 0.01b | 1.14 ± 0.01C | 1.23 ± 0.01db | 2.34 ± 0.01A |
| 8 | limonene | 1025 | 1023 | 78.25 ± 0.02b | 78.40 ± 0.02B | 85.74 ± 0.02a | 84.15 ± 0.02A | 70.85 ± 0.01c | 75.40 ± 0.02C | 55.16 ± 0.02d | 54.80 ± 0.02D |
| 9 | cis-β-ocimene | 1029 | 1032 | - | 0.42 ± 0.01A | 0.58 ± 0.02b | - | 0.53 ± 0.01c | - | 1.17 ± 0.02a | 0.46 ± 0.02A |
| 10 | β-terpinene | 1032 | 1036 | - | - | - | - | 0.11 ± 0.01 | - | - | - |
| 11 | trans-sabinene hydrate | 1048 | 1053 | - | - | - | tr | 0.29 ± 0.01a | - | 0.34 ± 0.01b | 0.28 ± 0.01 |
| 12 | γ-terpinene | 1051 | 1054 | 14.43 ± 0.01c | 11.43 ± 0.01C | 9.46 ± 0.01d | 9.18 ± 0.01D | 21.51 ± 0.02b | 13.36 ± 0.02B | 35.21 ± 0.01a | 26.89 ± 0.02A |
| 13 | terpinolene | 1067 | 1078 | 0.46 ± 0.02c | - | 1.91 ± 0.01a | 2.22 ± 0.02A | 1.65 ± 0.01b | 1.33 ± 0.02C | - | 1.52 ± 0.01B |
| 14 | p-cymenene | 1087 | 1083.4 | - | - | - | - | - | - | 0.11 ± 0.02 | - |
| 15 | cis-verbenol | 1125 | 1130 | - | - | - | - | - | 0.15 ± 0.01B | - | 0.26 ± 0.02A |
| 16 | terpinen-4-ol | 1158 | 1160 | - | - | - | - | - | 0.23 ± 0.01 | - | |
| 17 | trans-3-caren-2-ol | 1165 | 1160 | - | - | - | - | - | - | 0.16 ± 0.01 | - |
| 18 | α-terpineol | 1181 | 1183 | - | - | - | - | 0.79 ± 0.01a | 0.51 ± 0.02A | 0.92 ± 0.01b | 0.46 ± 0.02B |
| 19 | p-mentha-1(7),8-dien-2-ol | 1189 | 1186 | - | - | - | - | - | 1.09 ± 0.02 | - | tr |
| 20 | carveol | 1198 | 1201 | - | - | - | - | - | - | 0.11 ± 0.01 | 1.98 ± 0.01 |
| 21 | linalool, formate | 1202 | 1206 | - | 2.81 ± 0.02 | - | - | - | - | - | - |
| 22 | β-citronellol | 1214 | 1212 | - | - | - | - | - | tr | - | tr |
| 23 | perillal | 1275 | 1277 | - | - | - | - | - | tr | - | - |
| 24 | α-citral | 1291 | 1287 | - | - | - | - | - | 1.66 ± 0.01B | 0.21 ± 0.01 | 2.91 ± 0.02A |
| 25 | 4-terpinenyl acetate | 1300 | 1304 | 0.76 ± 0.00 | - | - | - | - | - | - | - |
| 26 | δ-elemene | 1340 | 1347 | - | - | - | tr | - | - | - | - |
| 27 | isocaryophyllene | 1375 | 1379 | - | - | - | - | - | - | 0.64 ± 0.01 | 0.78 ± 0.01 |
| 28 | α-copaene | 1381 | 1379 | - | - | - | - | - | tr | - | - |
| 29 | cis-α-bergamotene | 1402 | 1407 | 0.16 ± 0.01b | - | 0.23 ± 0.01a | 0.46 ± 0.02 | - | - | ||
| 30 | cis-muurola-3,5-diene | 1445 | § | - | - | - | tr | - | - | tr | tr |
| 31 | cis-β-farnesene | 1449 | 1451 | - | 0.53 ± 0.01 | - | - | - | - | - | - |
| 32 | cis-muurola-4(14),5-diene | 1460 | § | - | - | - | - | - | - | tr | tr |
| 33 | germacrene D | 1504 | 1500 | - | - | - | - | - | tr | - | 0.13 ± 0.01 |
| 34 | β-bisabolene | 1507 | 1501 | - | - | - | - | 0.15 ± 0.00a | 0.22 ± 0.01B | 0.13 ± 0.00b | 0.37 ± 0.01A |
| 35 | α-farnesene | 1509 | 1506 | - | - | - | 0.10 ± 0.03 | - | - | - | tr |
| 36 | valencene | 1512 | 1515 | - | - | - | tr | - | - | - | - |
| 37 | δ-cadinene | 1525 | 1530 | - | - | - | tr | - | tr | - | - |
| 38 | SUM | 100.0 | 99.92 | 99.84 | 99.78 | 99.84 | 99.44 | 98.19 | 99.07 | ||
| Monoterpene hydrocarbons | 99.08 | 96.58 | 99.84 | 99.68 | 98.67 | 95.12 | 96.02 | 92.18 | |||
| Oxygenated monoterpenes | 0.76 | 2.81 | - | - | 0.79 | 3.64 | 1.4 | 5.61 | |||
| Sesquiterpene hydrocarbons | 0.16 | 0.53 | - | 0.10 | 0.38 | 0.68 | 0.77 | 1.28 | |||
| Others | - | - | - | - | - | - | - | - |
| Citrus Species | Total Polyphenols (mg GAE/100 mL Juice) | Total Flavonoids (mg QE/100 mL Juice) |
|---|---|---|
| Yuzu Jeune | 146.0 ± 2.12 | 3.3 ± 0.06 |
| Oni Yuzu | 219.5 ± 0.71 | 5.1 ± 0.10 |
| Bizzarria orange | 165.0 ± 4.09 | 1.9 ± 0.10 |
| Florence cedar | 125.2 ± 3.07 | 2.2 ± 0.10 |
| Citrus Species | ABTS (µM Trolox eq./100 mL Juice) | DPPH (µM Trolox eq./100 mL Juice) |
|---|---|---|
| Yuzu Jeune | 242.0 ± 4.00 | 30.0 ± 0.00 |
| Oni Yuzu | 220.0 ± 2.02 | 29.0 ± 0.00 |
| Bizzarria orange | 213.0 ± 5.51 | 25.0 ± 0.00 |
| Florence cedar | 102.0 ± 110 | 10.0 ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitalini, S.; Iriti, M.; Vinciguerra, V.; Garzoli, S. A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species. Molecules 2021, 26, 5378. https://doi.org/10.3390/molecules26175378
Vitalini S, Iriti M, Vinciguerra V, Garzoli S. A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species. Molecules. 2021; 26(17):5378. https://doi.org/10.3390/molecules26175378
Chicago/Turabian StyleVitalini, Sara, Marcello Iriti, Vittorio Vinciguerra, and Stefania Garzoli. 2021. "A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species" Molecules 26, no. 17: 5378. https://doi.org/10.3390/molecules26175378
APA StyleVitalini, S., Iriti, M., Vinciguerra, V., & Garzoli, S. (2021). A Comparative Study of the Chemical Composition by SPME-GC/MS and Antiradical Activity of Less Common Citrus Species. Molecules, 26(17), 5378. https://doi.org/10.3390/molecules26175378









