A Novel Synthetic Precursor of Styryl Sulfone Neuroprotective Agents Inhibits Neuroinflammatory Responses and Oxidative Stress Damage through the P38 Signaling Pathway in the Cell and Animal Model of Parkinson’s Disease
Abstract
:1. Introduction
2. Results
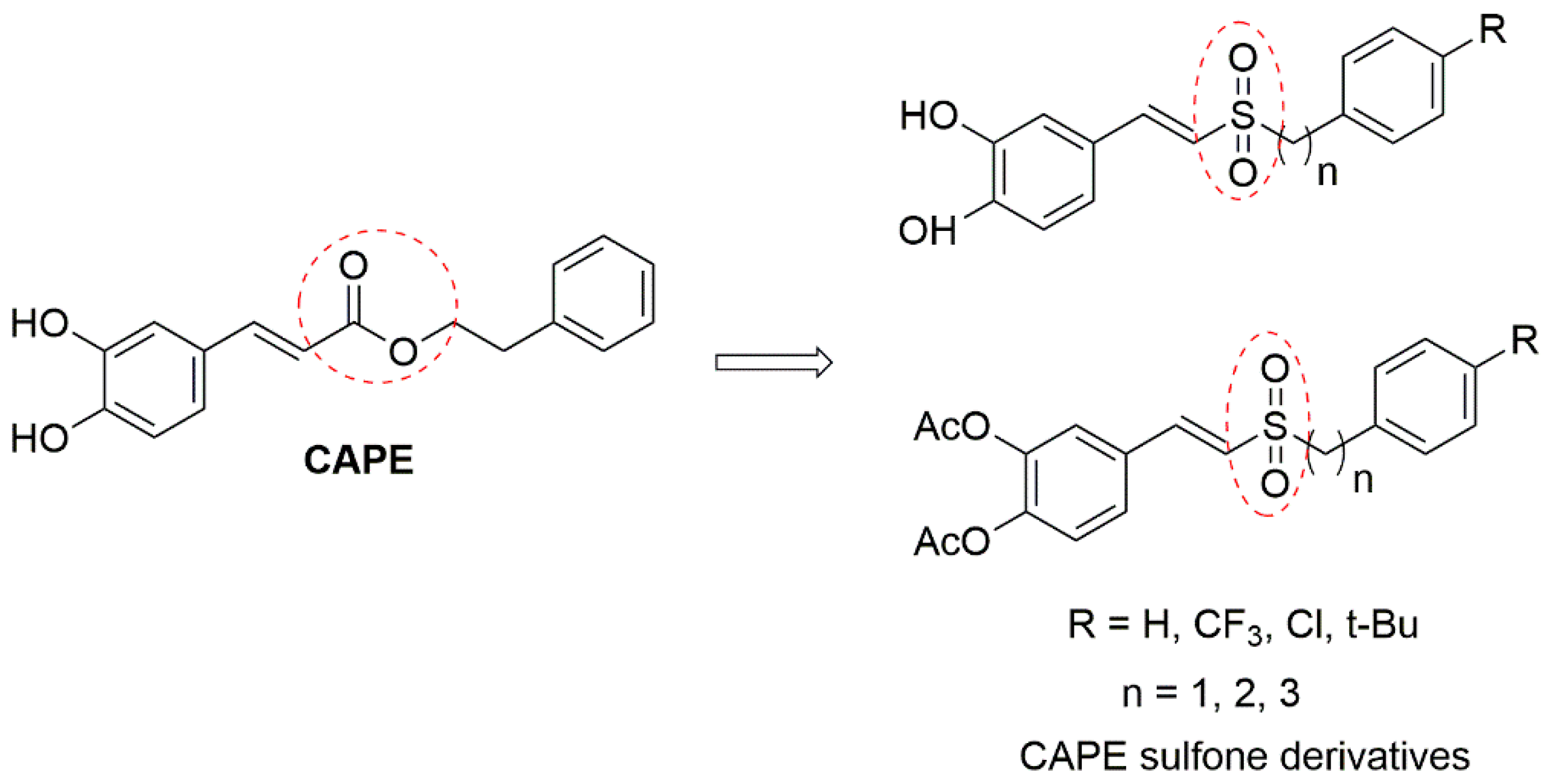
2.1. In Vitro Screening against PD Activity for Styryl Sulfone Compounds
2.1.1. Evaluation of the Styryl Sulfone Compounds for Neurons’ Protection
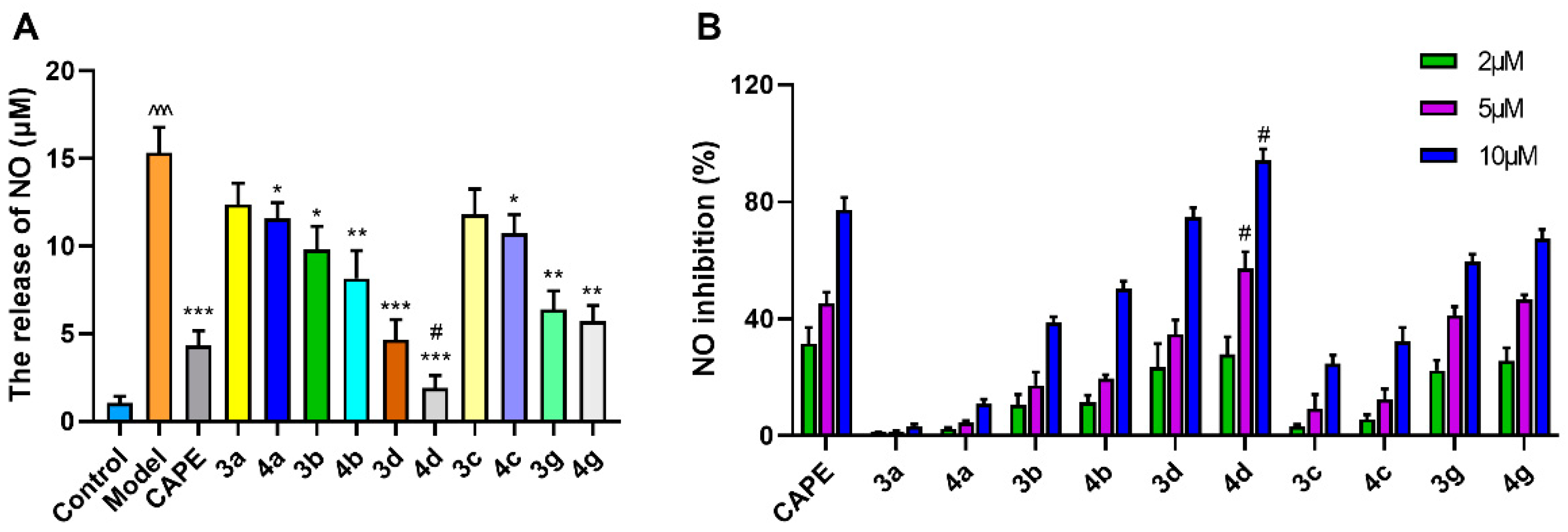
2.1.2. Evaluation of the Styryl Sulfone Compounds for NO Inhibition
2.2. Validation of Anti-PD Biological Activity of 4d in Animal Experiments
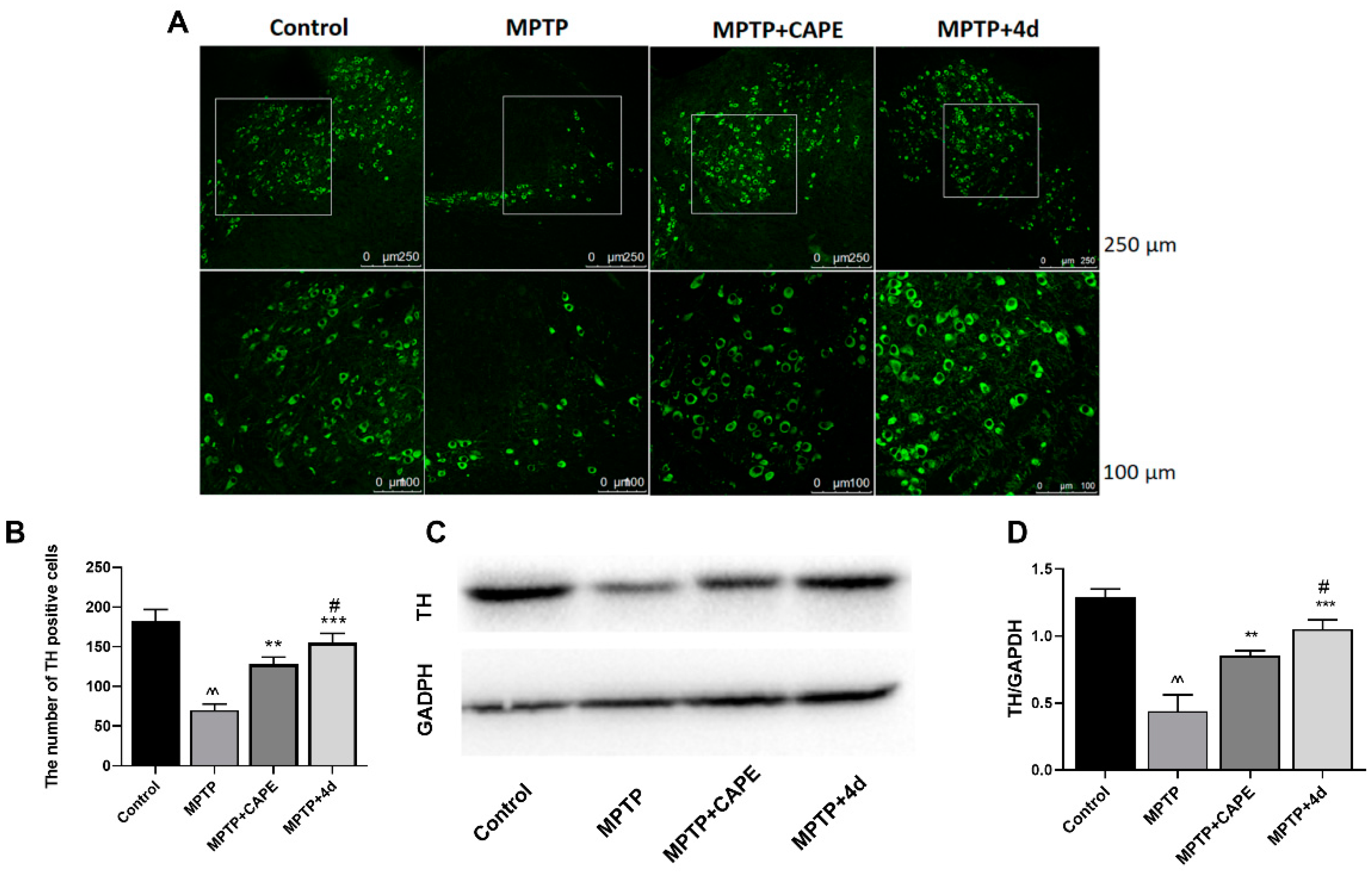
2.2.1. 4d Blocks MPTP-Induced Loss of Dopamine Neurons in Substantia Nigra of Mice
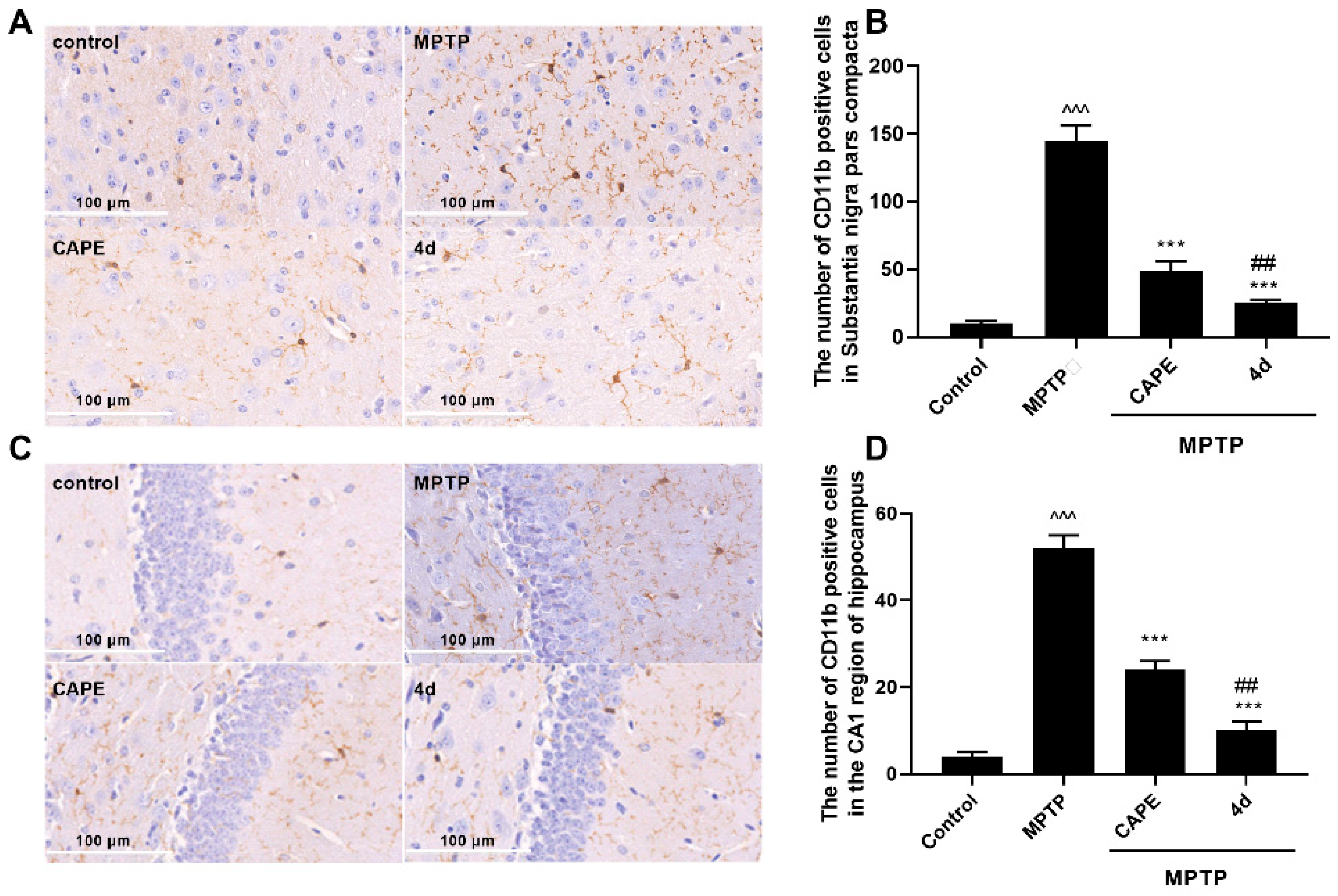
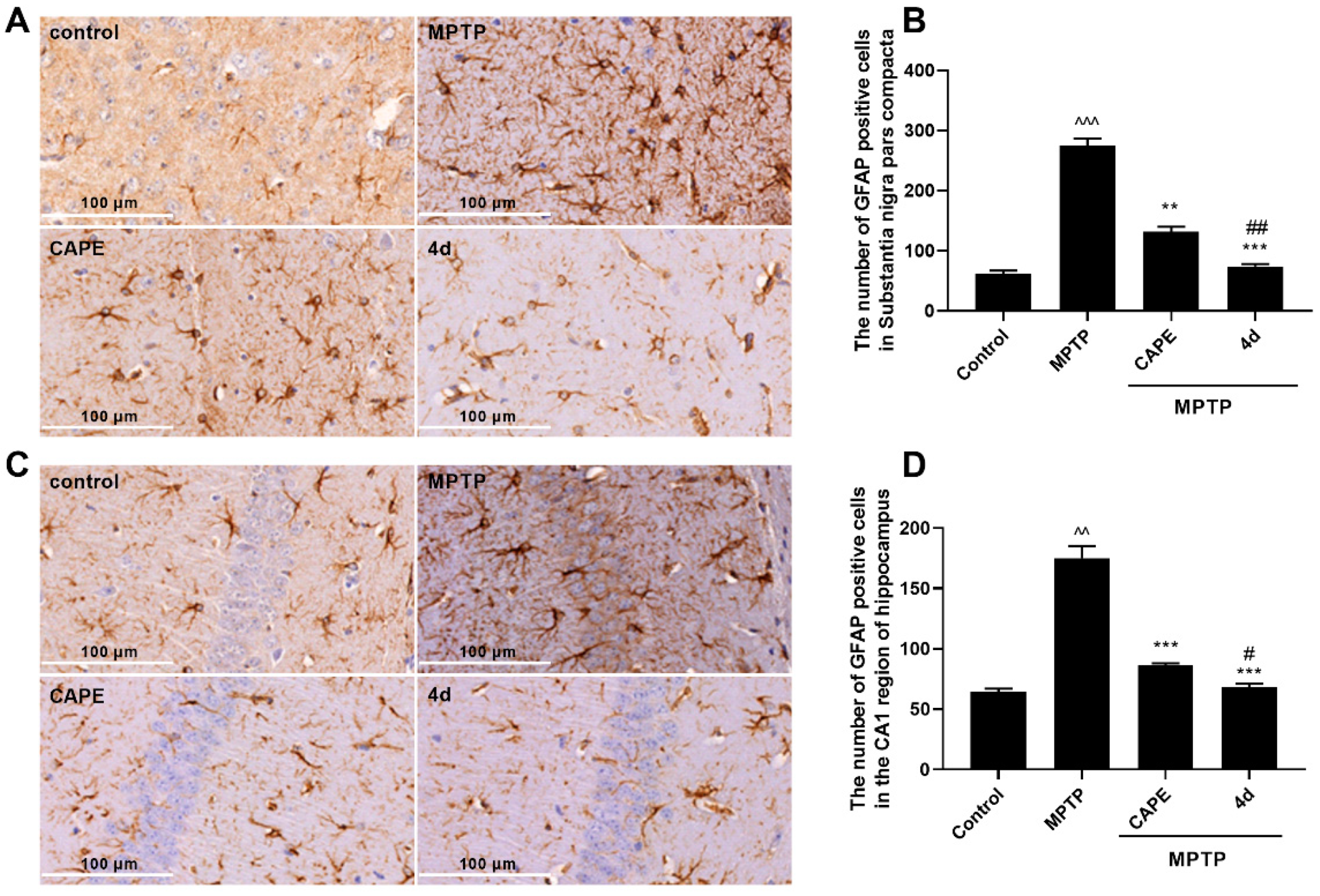
2.2.2. 4d Inhibits MPTP-Induced Activation of Microglia and Astrocytes in Substantia Nigra and Hippocampus of Mice
2.3. 4d Suppresses MPP+-Induced Inflammation by Mechanisms Associated with the p38 Signaling Pathway and Against Oxidative Stress by the Nrf2 Signaling Pathway
2.3.1. 4d Suppresses MPP+-Induced Activation of p38 MAPK and Downstream NF-κB Pathways in Primary Microglia
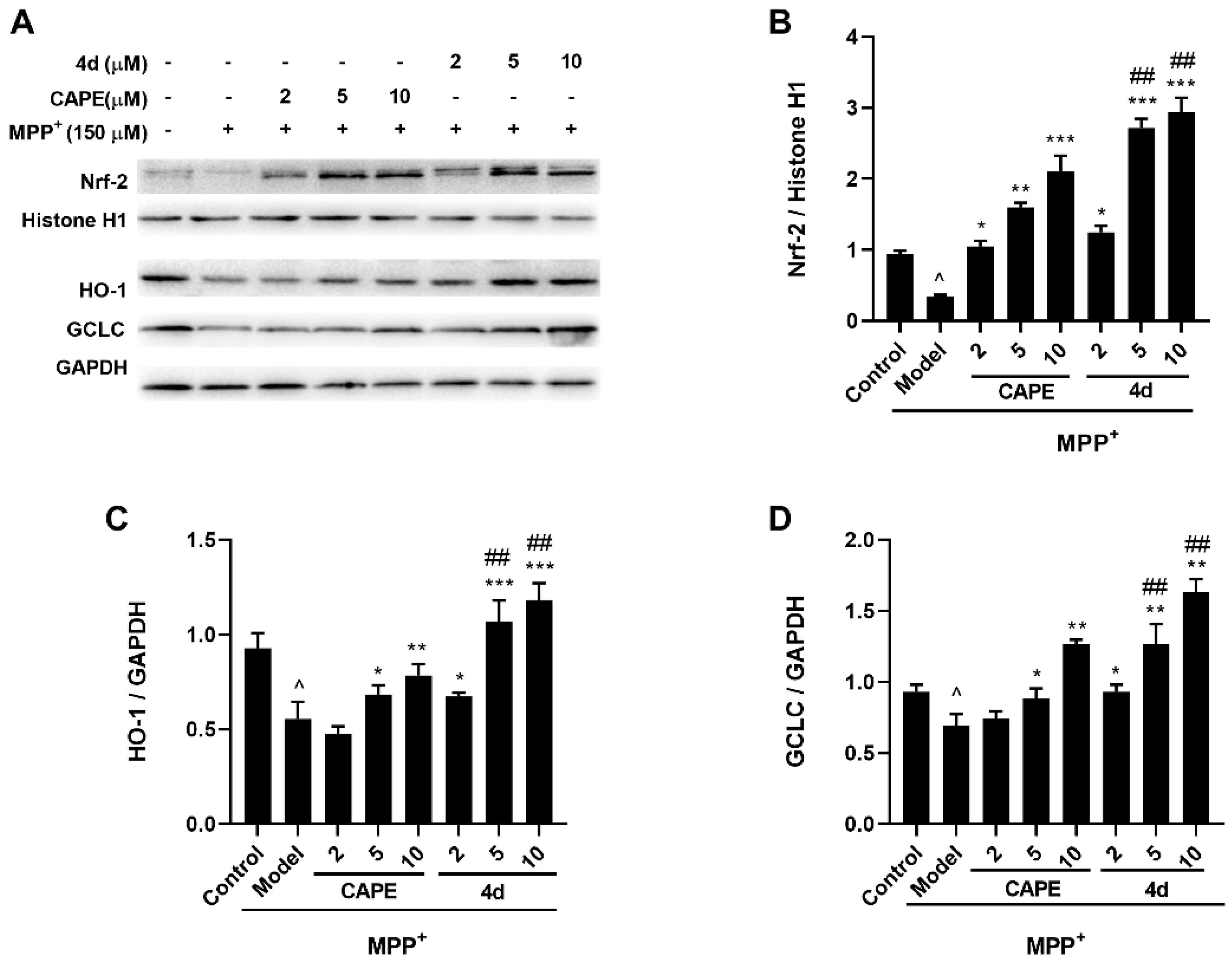
2.3.2. 4d Activates Nrf2 and Upregulates HO-1 and GCLC Protein Expression in MPP+-Induced Microglia
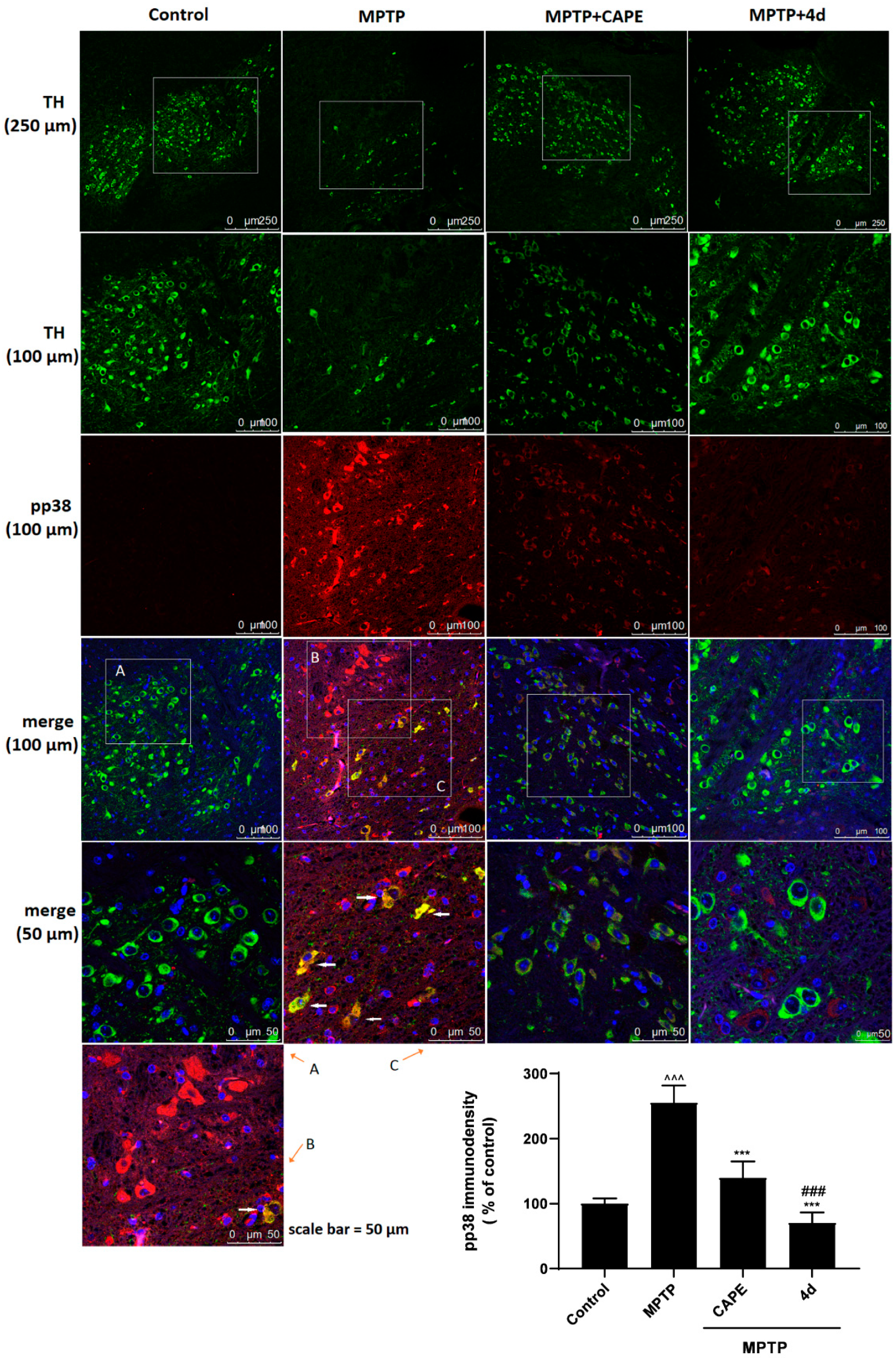
2.3.3. 4d Blocks Phosphorylation of p38 MAPK in an Animal Model of PD
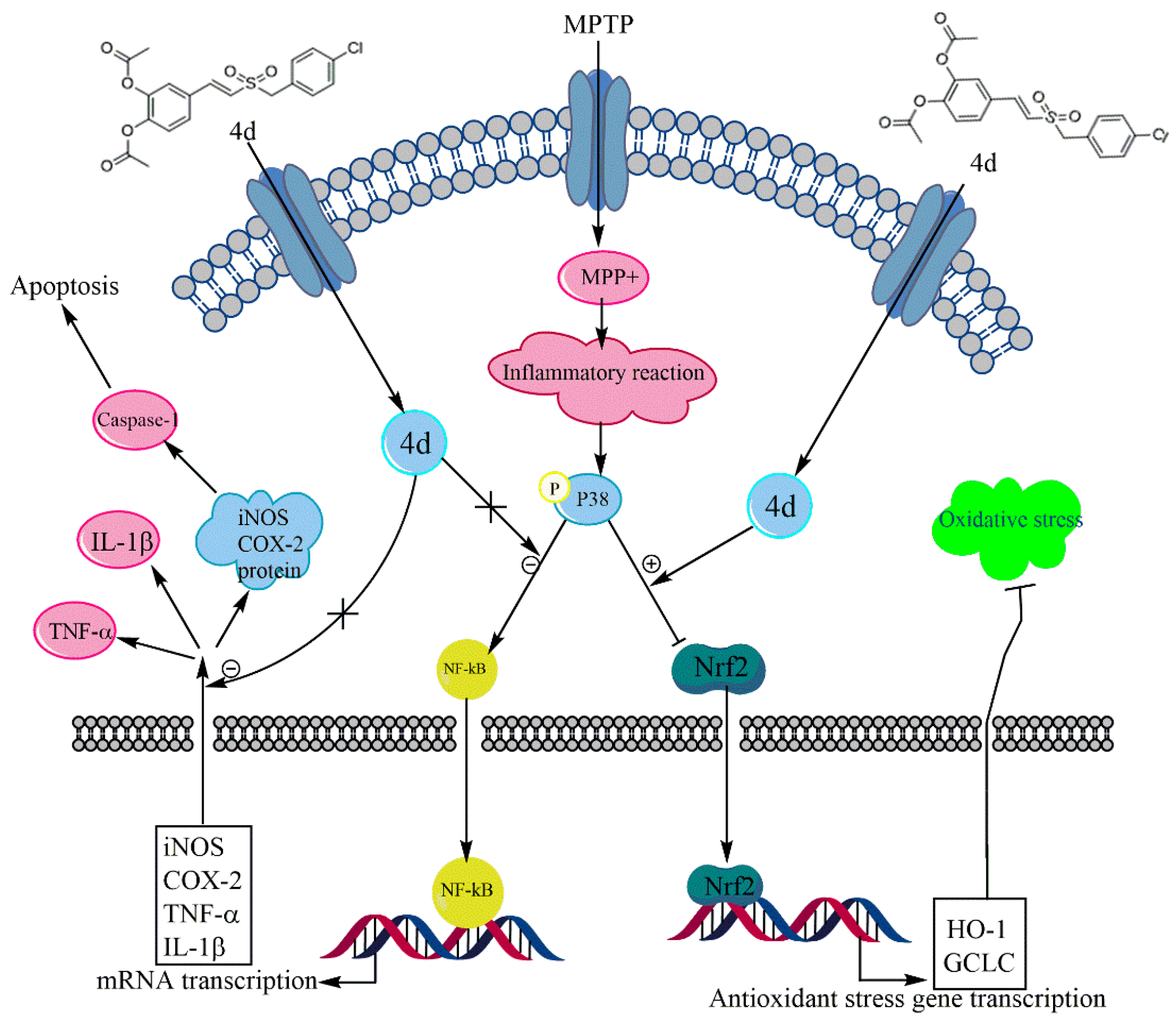
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Neuronal Cell Cultures and Assessment of Cell Viability
4.3. Primary Microglia Cultures and MPP+ Stimulation
4.4. Determination of NO Inhibition
4.5. Animals and Treatment
4.6. Immunohistochemistry
4.7. Immunofluorescence Assay
4.8. Immuno-Positive Cell Counting
4.9. Protein Extraction and Western Blot Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rocha, E.M.; De-Miranda, B.; Sanders, L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Picconi, B.; Hernandez, L.F.; Obeso, J.A.; Calabresi, P. Motor complications in Parkinson’s disease: Striatal molecular and electrophysiological mechanisms of dyskinesias. Mov. Disord. 2018, 33, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Warren-Olanow, C.; Torti, M.; Kieburtz, K.; Leinonen, M.; Vacca, L.; Grassini, P.; Heller, A.; Heller, E.; Stocchi, F. Continuous versus intermittent oral administration of levodopa in Parkinson’s disease patients with motor fluctuations: A pharmacokinetics, safety, and efficacy study. Mov. Disord. 2019, 34, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, K.; Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell Physiol. 2019, 234, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhao, X.; Li, Y.; Li, G.; Liu, X. Damage to dopaminergic neurons by oxidative stress in Parkinson’s disease. Int. J. Mol. Med. 2018, 41, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Angelova, P.R.; Abramov, A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Elkouzi, A.; Vedam-Mai, V.; Eisinger, R.S.; Okun, M.S. Emerging therapies in Parkinson disease-repurposed drugs and new approaches. Nat. Rev. Neurol. 2019, 15, 204–223. [Google Scholar] [CrossRef]
- Nagane, M.; Yamashita, T.; Voros, P.; Kalai, T.; Hideg, K.; Bognar, B. Synthesis and evaluation of paramagnetic caffeic acid phenethyl ester (CAPE) analogs. Monatsh. Chem. 2019, 150, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.F.; Kuo, Y.H.; Yeh, W.L.; Wu, C.Y.; Lin, H.Y.; Lai, S.W.; Liu, Y.S.; Wu, L.H.; Lu, J.K.; Lu, D.Y. Regulatory effects of caffeic acid phenethyl ester on neuroinflammation in microglial cells. Int. J. Mol. Sci. 2015, 16, 5572–5589. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Zhang, Y.; Lu, W.; Gao, X.; Xu, C.; Bao, H. Caffeic acid phenethyl ester attenuates neuropathic pain by suppressing the p38/NF-κB signal pathway in microglia. J. Pain. Res. 2018, 11, 2709–2719. [Google Scholar] [CrossRef] [Green Version]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective effect of caffeic acid phenethyl ester in a mouse model of Alzheimer’s disease involves Nrf2/HO-1 pathway. Aging Dis. 2018, 9, 605–622. [Google Scholar] [CrossRef] [Green Version]
- Ning, X.; Guo, Y.; Wang, X.; Ma, X.; Tian, C.; Shi, X.; Zhu, R.; Cheng, C.; Du, Y.; Ma, Z.; et al. Design, synthesis, and biological evaluation of (e)-3, 4-dihydroxystyryl aralkyl sulfones and sulfoxides as novel multifunctional neuroprotective agents. J. Med. Chem. 2014, 57, 4302–4312. [Google Scholar] [CrossRef]
- Ning, X.; Yuan, M.; Guo, Y.; Tian, C.; Wang, X.; Zhang, Z.; Liu, J. Neuroprotective effects of (E)-3,4-diacetoxystyryl sulfone and sulfoxide derivatives in vitro models of Parkinson’s disease. J. Enzym. Inhib. Med. Chem. 2016, 31, 464–469. [Google Scholar]
- Wen, Z.H.; Chao, C.H.; Wu, M.H.; Sheu, J.H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef]
- Pakavathkumar, P.; Noel, A.; Lecrux, C.; Tubeleviciute-Aydin, A.; Hamel, E.; Ahlfors, J.-E.; LeBlanc, A.C. Caspase vinyl sulfone small molecule inhibitors prevent axonal degeneration in human neurons and reverse cognitive impairment in caspase-6-overexpressing mice. Mol. Neurodegener. 2017, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Kim, S.; Park, J.H.; Kim, H.J.; Shin, S.J.; Kim, J.W.; Woo, S.Y.; Lee, C.; Han, S.M.; Lee, J.; et al. Optimization of vinyl sulfone derivatives as potent nuclear factor erythroid 2-related factor 2 (Nrf2) activators for Parkinson’s disease therapy. J. Med. Chem. 2019, 62, 811–830. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Puspita, L.; Chung, S.Y.; Shim, J.W. Oxidative stress and cellular pathologies in Parkinson’s disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, R. The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: Focus on astrocytes. Mol. Neurobiol. 2014, 49, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, Z.; Gu, J.; Ge, J.; Liang, Z.; Qin, Z. p38(MAPK)/p53-Mediated Bax induction contributes to neurons degeneration in rotenone-induced cellular and rat models of Parkinson’s disease. Neurochem. Int. 2013, 63, 133–140. [Google Scholar] [CrossRef]
- Harper, S.J.; Wilkie, N. MAPKs: New targets for neurodegeneration. Expert. Opin. Ther. Targets 2003, 7, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Himeda, T.; Araki, T. Mechanisms of MPTP toxicity and their implications for therapy of Parkinson’s disease. Med. Sci. Monit. 2005, 11, RA17–RA23. [Google Scholar]
- Luo, D.; Zhao, J.; Cheng, Y.; Lee, S.; Rong, J. N-propargyl caffeamide (PACA) ameliorates dopaminergic neuronal loss and motor dysfunctions in MPTP mouse model of Parkinson’s disease and in MPP+-induced neurons via promoting the conversion of proNGF to NGF. Mol. Neurobiol. 2018, 55, 2258–2267. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Mielke, K.; Herdegen, T. JNK and p38 stresskinases-degenerative effectors of signal-transduction-cascades in the nervous system. Prog. Neurobiol. 2000, 61, 45–60. [Google Scholar] [CrossRef]
- Kyosseva, S.V. Targeting MAPK signaling in age-related macular fegeneration. Ophthalmol. Eye Dis. 2016, 8, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Chen, L.; Yu, H.; Sun, Q.; Kou, J.; Yu, B. Diosgenin down-regulates NF-κB p65/p50 and p38MAPK pathways and attenuates acute lung injury induced by lipopolysaccharide in mice. Int. Immunopharmacol. 2013, 15, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, J.; Wang, Y.; Tan, R. Anti-neuroinflammatory effect of alantolactone through the suppression of the NF-κB and MAPK signaling pathways. Cells 2019, 8, 739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Han, C.; Ma, K.; Xia, Y.; Wan, F.; Yin, S.; Kou, L.; Sun, Y.; Wu, J.; Hu, J.; et al. Hydralazine protects nigrostriatal dopaminergic neurons from MPP+ and MPTP induced neurotoxicity: Roles of Nrf2-ARE signaling pathway. Front. Neurol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, N.J. Recent advances in the inhibition of p38 MAPK as a potential strategy for the treatment of Alzheimer’s disease. Molecules 2017, 22, 1287. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Ma, Z.; Fontanilla, C.V.; Zhao, L.; Xu, Z.C.; Taggliabraci, V.; Johnstone, B.H.; Dodel, R.C.; Farlow, M.R.; Du, Y. Caffeic acid phenethyl ester prevents cerebellar granule neurons (CGNs) against glutamate-induced neurotoxicity. Neuroscience 2008, 155, 1098–1105. [Google Scholar] [CrossRef]
- Fontanilla, C.V.; Wei, X.; Zhao, L.; Johnstone, B.; Pascuzzi, R.M.; Farlow, M.R.; Du, Y. Caffeic acid phenethyl ester extends survival of a mouse model of amyotrophic lateral sclerosis. Neuroscience 2012, 205, 185–193. [Google Scholar] [CrossRef]
- Karunakaran, S.; Ravindranath, V. Activation of p38 MAPK in the substantia nigra leads to nuclear translocation of NF-κappaB in MPTP-treated mice: Implication in Parkinson’s disease. J. Neurochem. 2009, 109, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, I.; Miao, E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol. Rev. 2015, 265, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redo. Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todorovic, M.; Wood, S.A.; Mellick, G.D. Nrf2: A modulator of Parkinson’s disease? J. Neural. Transm. (Vienna) 2016, 123, 611–619. [Google Scholar] [CrossRef]
- Tong, H.; Zhang, X.; Meng, X.; Lu, L.; Mai, D.; Qu, S. Simvastatin inhibits activation of NADPH oxidase/p38 MAPK pathway and enhances expression of antioxidant protein in Parkinson disease models. Front. Mol. Neurosci. 2018, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Mandlekar, S.; Lei, W.; Fahl, W.E.; Tan, T.H.; Kong, A.N. p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J. Biol. Chem. 2000, 275, 2322–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontanilla, C.V.; Ma, Z.; Wei, X.; Klotsche, J.; Zhao, L.; Wisniowski, P.; Dodel, R.C.; Farlow, M.R.; Oertel, W.H.; Du, Y. Caffeic acid phenethyl ester prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration. Neuroscience 2011, 188, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, Z.; Lin, S.; Dodel, R.C.; Gao, F.; Bales, K.R.; Triarhou, L.C.; Chernet, E.; Perry, K.W.; Nelson, D.L.; et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2001, 98, 14669–14674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomide, V.G.; Bibancos, T.; Chadi, G. Dopamine cell morphology and glial cell hypertrophy and process branching in the nigrostriatal system after striatal 6-OHDA analyzed by specific sterological tools. Int. J. Neurosci. 2005, 115, 557–582. [Google Scholar] [CrossRef]
- Deguil, J.; Chavant, F.; Lafay-Chebassier, C.; Pérault-Pochat, M.C.; Fauconneau, B.; Pain, S. Neuroprotective effect of PACAP on translational control alteration and cognitive decline in MPTP parkinsonian mice. Neurotox. Res. 2010, 17, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Sun, M.; Jia, X.; Li, Y.; Zhang, B.; Zhao, L.; Shi, Y.; Zhou, Z.; Zhu, Y.; Cui, C.; et al. Sodium butyrate exacerbates Parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem. Res. 2020, 45, 2128–2142. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Ma, Z.; Ning, X.; Chen, Y.; Tian, C.; Wang, X.; Zhang, Z.; Liu, J. A Novel Synthetic Precursor of Styryl Sulfone Neuroprotective Agents Inhibits Neuroinflammatory Responses and Oxidative Stress Damage through the P38 Signaling Pathway in the Cell and Animal Model of Parkinson’s Disease. Molecules 2021, 26, 5371. https://doi.org/10.3390/molecules26175371
Guo Y, Ma Z, Ning X, Chen Y, Tian C, Wang X, Zhang Z, Liu J. A Novel Synthetic Precursor of Styryl Sulfone Neuroprotective Agents Inhibits Neuroinflammatory Responses and Oxidative Stress Damage through the P38 Signaling Pathway in the Cell and Animal Model of Parkinson’s Disease. Molecules. 2021; 26(17):5371. https://doi.org/10.3390/molecules26175371
Chicago/Turabian StyleGuo, Ying, Zhizhong Ma, Xianling Ning, Ying Chen, Chao Tian, Xiaowei Wang, Zhili Zhang, and Junyi Liu. 2021. "A Novel Synthetic Precursor of Styryl Sulfone Neuroprotective Agents Inhibits Neuroinflammatory Responses and Oxidative Stress Damage through the P38 Signaling Pathway in the Cell and Animal Model of Parkinson’s Disease" Molecules 26, no. 17: 5371. https://doi.org/10.3390/molecules26175371
APA StyleGuo, Y., Ma, Z., Ning, X., Chen, Y., Tian, C., Wang, X., Zhang, Z., & Liu, J. (2021). A Novel Synthetic Precursor of Styryl Sulfone Neuroprotective Agents Inhibits Neuroinflammatory Responses and Oxidative Stress Damage through the P38 Signaling Pathway in the Cell and Animal Model of Parkinson’s Disease. Molecules, 26(17), 5371. https://doi.org/10.3390/molecules26175371





