Synthesis and Biological Activity of a Cytostatic Inhibitor of MLLr Leukemia Targeting the DOT1L Protein
Abstract
:1. Introduction
2. Results
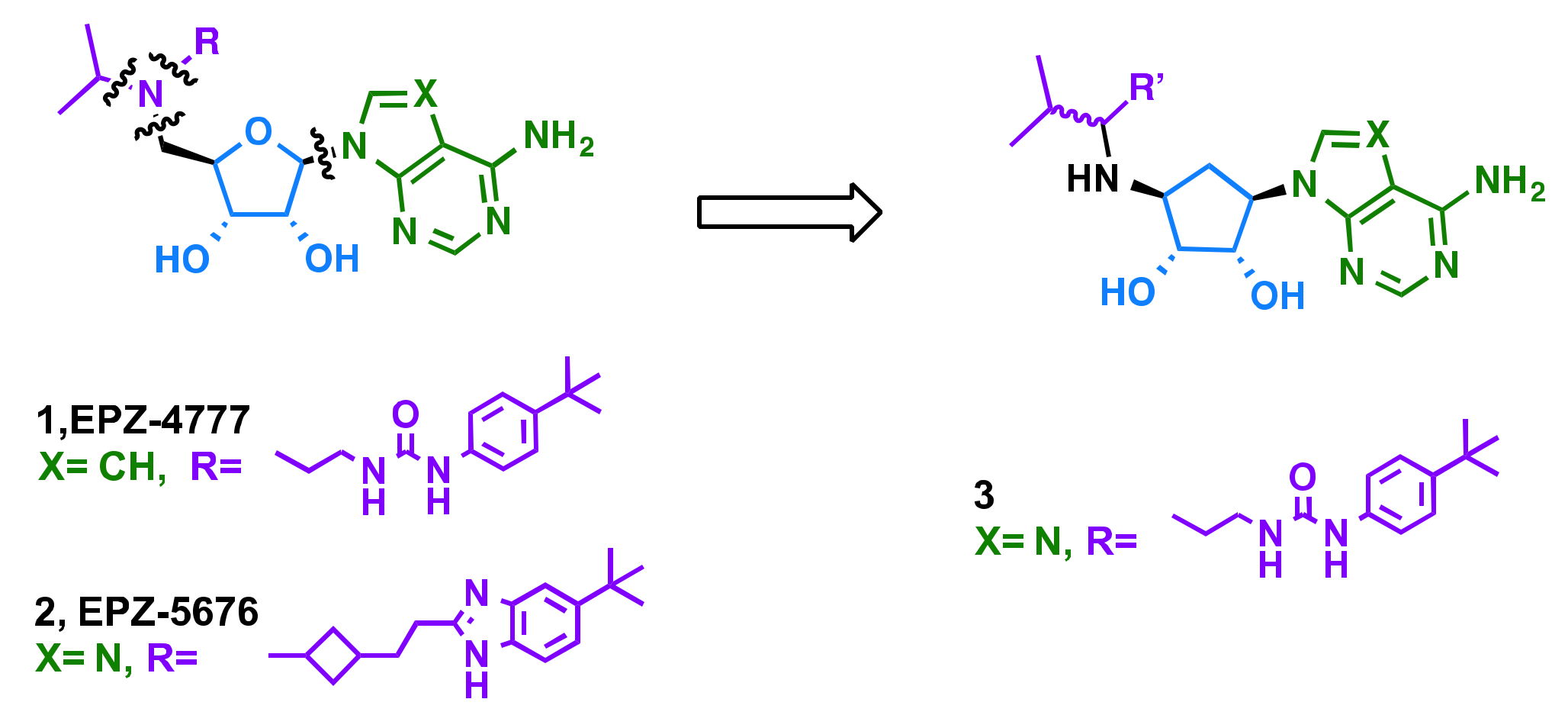
2.1. Rational Design
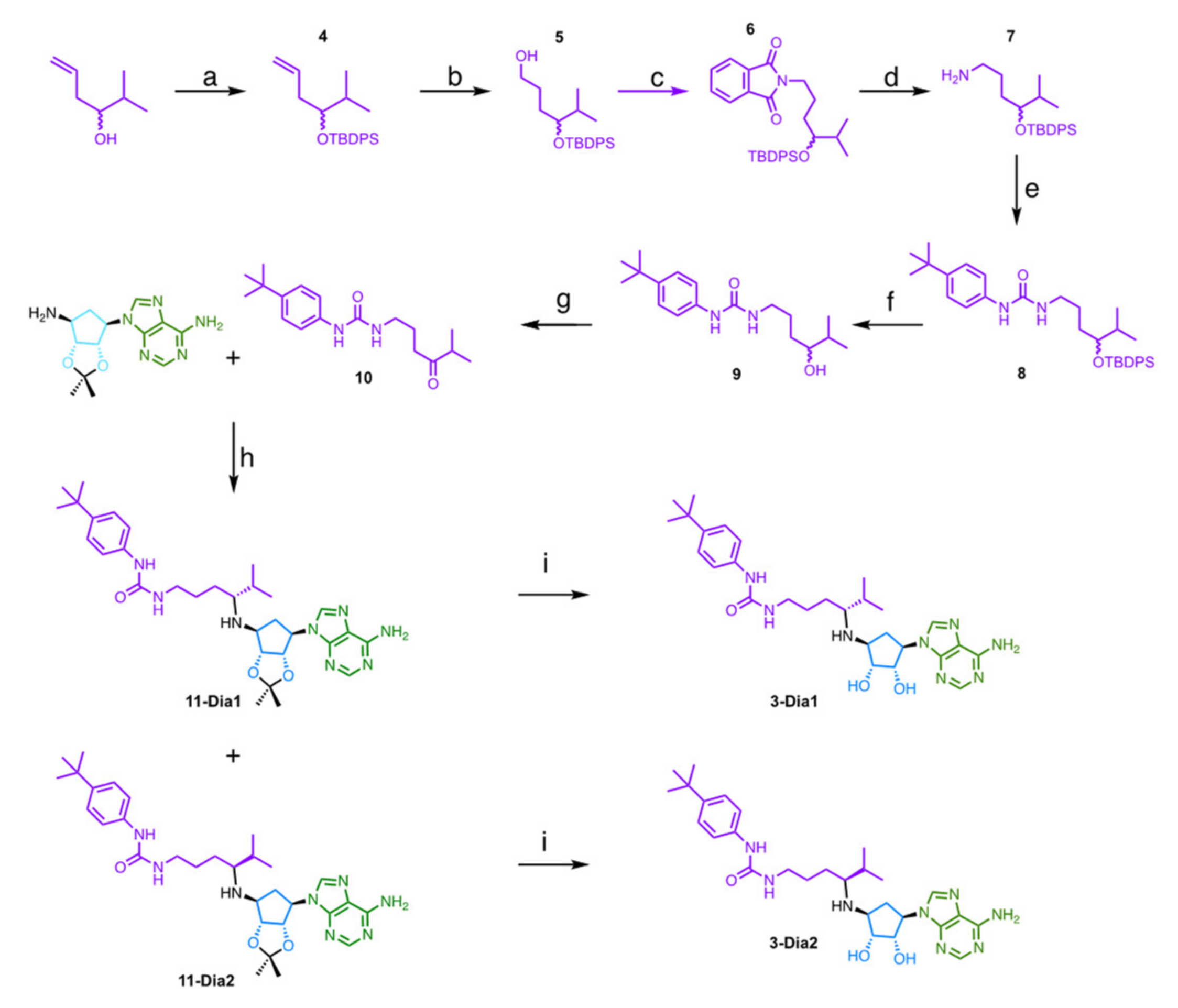
2.2. Chemistry
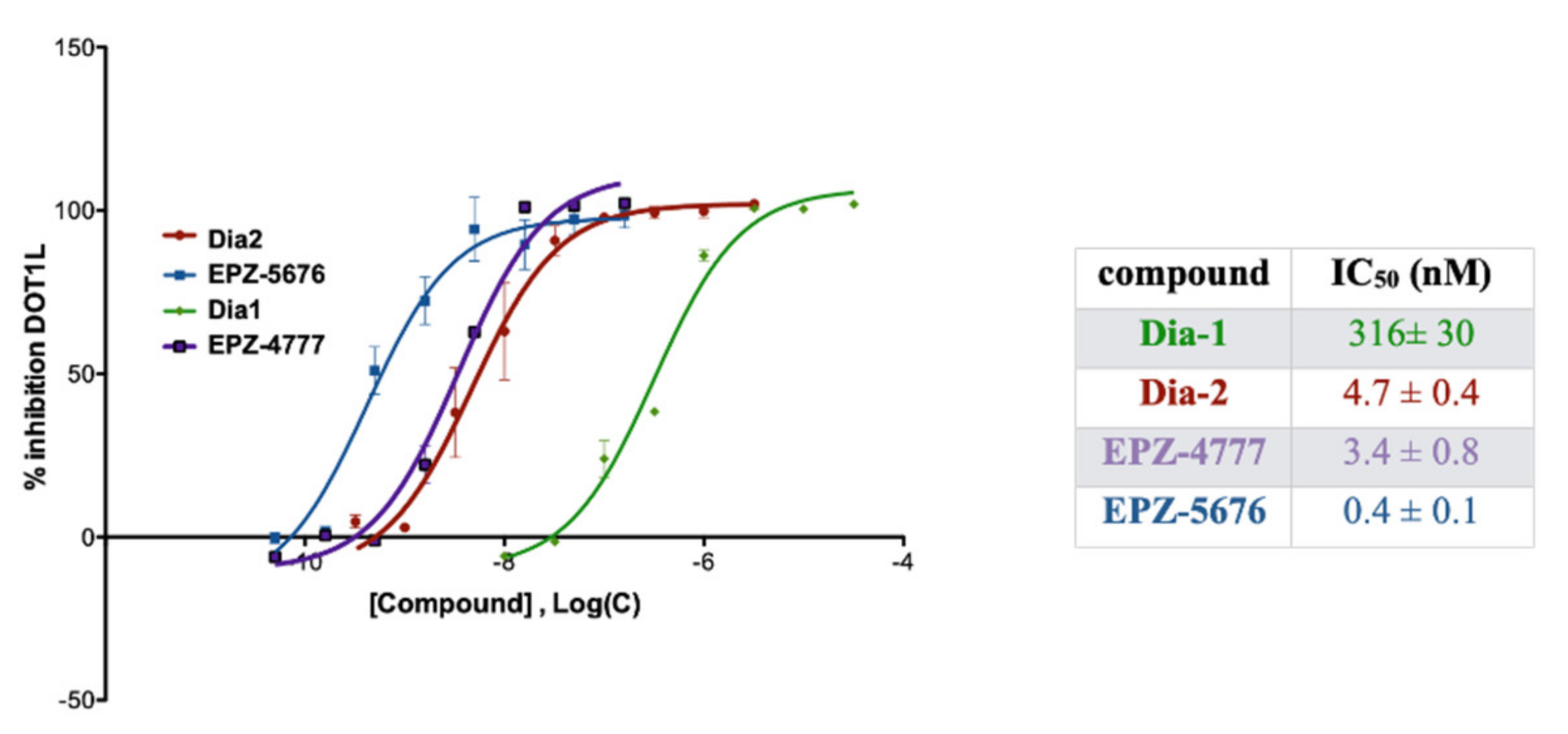
2.3. DOTL1 Inhibition
2.4. Microsomal Stability
2.5. Molecular Docking
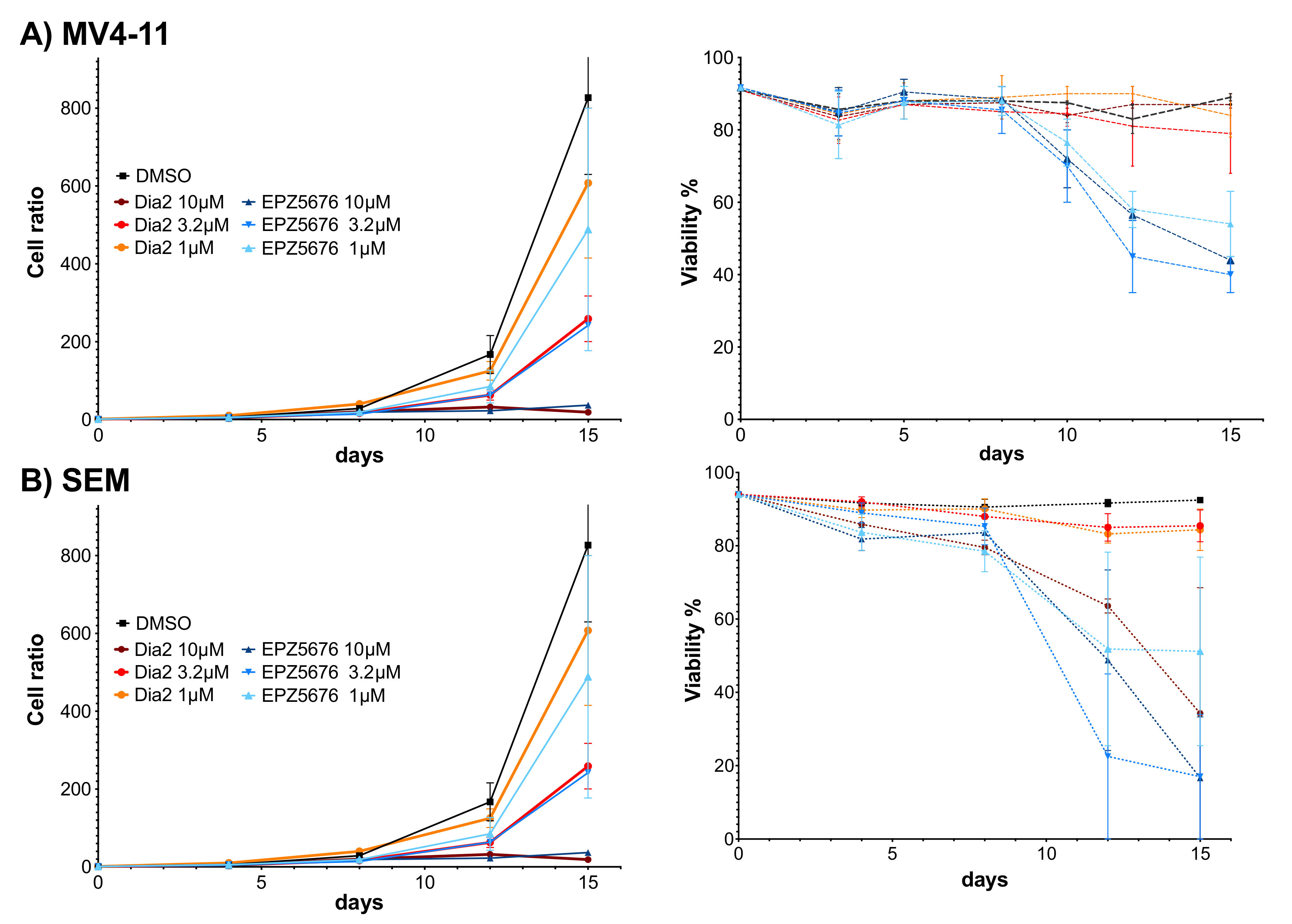
2.6. Dia2 Inhibits Cell Proliferation by a Cytostatic Effect
2.7. Dia2 Inhibits Cellular H3K79 Dimethylation
2.8. Dia2 Inhibits Cellular MLL Target Genes and Causes Cell Cycle Change and Apoptosis in MLL-Rearranged Cells
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis
4.2. Biological Assays
DOT1L Enzymatic Inhibition Assays
4.3. Cell Culture
4.4. Histone Extraction
4.5. Western Blotting
4.6. Flow Cytometric Analysis of Cell Cycle and Apoptosis
4.7. Quantitative PCR Real Time
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DIAD | Diisopropyl azodicarboxylate |
| DOTIL | Disruptor of telomeric silencing 1-like H3K79: 79th lysine of the histone 3 MLL: Mixed Lineage Leukemia |
| MLLr | Mixed-lineage rearranged leukemia |
| PCC | Pyridinium chlorochromate |
| SAM | S-Adenosyl-L-methionine |
| TBAF | Tetrabutylammonium fluoride |
| TBDPSCl | tert-Butyldiphenylchlorosilane |
References
- Choudhuri, S. From Waddington’s epigenetic landscape to small noncoding RNA: Some important milestones in the history of epigenetics research. Toxicol. Mech. Methods 2011, 21, 252–274. [Google Scholar] [CrossRef]
- Neganova, M.E.; Klochkov, S.G.; Aleksandrova, Y.R.; Aliev, G. Histone modifications in epigenetic regulation of cancer: Perspectives and achieved progress. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.; Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 2005, 6, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 2002, 12, 198–209. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal. Transduct. Target. Ther. 2019, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhu, W.-G. Biological function and regulation of histone and non-histone lysine methylation in response to DNA damage. Acta Biochim. Biophys. Sin. 2016, 48, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Krivtsov, A.V.; Armstrong, S.A. MLL translocations, histone modifications and leukaemia stem-cell development. Nat. Rev. Cancer 2007, 7, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Feng, Q.; Lin, Y.; Jiang, Q.; Li, Y.; Coffield, V.M.; Su, L.; Xu, G.; Zhang, Y. hDOT1L links histone methylation to leukemogenesis. Cell 2005, 121, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Drexler, H.G.; Quentmeier, H.; MacLeod, R.A.F. Malignant hematopoietic cell lines: In vitro models for the study of MLL gene alterations. Leukemia 2004, 18, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Ayton, P.M.; Cleary, M.L. Transformation of myeloid progenitors by MLL oncoproteins is dependent on Hoxa7 and Hoxa9. Genes Dev. 2003, 17, 2298–2307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bei, L.; Shah, C.; Wang, H.; Huang, W.; Platanias, L.C.; Eklund, E.A. Regulation of CDX4 gene transcription by HoxA9, HoxA10, the MLL-ELL oncogene and Shp2 during leukemogenesis. Oncogenesis 2014, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winters, A.C.; Bernt, K.M. MLL-Rearranged Leukemias-An Update on Science and Clinical Approaches. Front. Pediatr. 2017, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Sarno, F.; Nebbioso, A.; Altucci, L. DOT1L: A key target in normal chromatin remodelling and in mixed-lineage leukaemia treatment. Epigenetics 2020, 15, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Daigle, S.R.; Olhava, E.J.; Therkelsen, C.A.; Majer, C.R.; Sneeringer, C.J.; Song, J.; Johnson, L.D.; Scott, M.P.; Smith, J.J.; Xiao, Y.; et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell 2011, 20, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.W.; Chory, E.J.; Wernimont, A.K.; Tempel, W.; Scopton, A.; Federation, A.; Marineau, J.J.; Qi, J.; Barsyte-Lovejoy, D.; Yi, J.; et al. Catalytic site remodelling of the DOT1L methyltransferase by selective inhibitors. Nat. Commun. 2012, 3, 1. [Google Scholar] [CrossRef]
- Daigle, S.R.; Olhava, E.J.; Therkelsen, C.A.; Basavapathruni, A.; Jin, L.; Boriak-Sjodin, P.A.; Allain, C.J.; Klaus, C.R.; Raimondi, A.; Scott, M.P.; et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood 2013, 122, 1017–1025. [Google Scholar] [CrossRef] [Green Version]
- Shukla, N.; Wetmore, C.; O’Brien, M.M.; Silverman, L.B.; Brown, P.; Cooper, T.M.; Thomson, B.; Blakemore, S.J.; Daigle, S.; Suttle, B.; et al. Final Report of Phase 1 Study of the DOT1L Inhibitor, Pinometostat (EPZ-5676), in Children with Relapsed or Refractory MLL-r Acute Leukemia. Blood 2016, 128, 2780. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, H.; Stauffer, F.; Caravatti, G.; Vollmer, S.; Machauer, R.; Holzer, P.; Möbitz, H.; Scheufler, C.; Klumpp, M.; et al. Discovery of Novel Dot1L Inhibitors through a Structure-Based Fragmentation Approach. ACS Med. Chem. Lett. 2016, 7, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Möbitz, H.; Machauer, R.; Holzer, P.; Vaupel, A.; Stauffer, F.; Ragot, C.; Caravatti, G.; Scheufler, C.; Fernández, C.; Hommel, U.; et al. Discovery of Potent, Selective, and Structurally Novel Dot1L Inhibitors by a Fragment Linking Approach. ACS Med. Chem. Lett. 2017, 8, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Scheufler, C.; Möbitz, H.; Gaul, C.; Ragot, C.; Be, C.; Fernández, C.; Beyer, K.S.; Tiedt, R.; Stauffer, F. Optimization of a Fragment-Based Screening Hit toward Potent DOT1L Inhibitors Interacting in an Induced Binding Pocket. ACS Med. Chem. Lett. 2016, 7, 730–734. [Google Scholar] [CrossRef] [Green Version]
- Stauffer, F.; Weiss, A.; Scheufler, C.; Möbitz, H.; Ragot, C.; Beyer, K.S.; Calkins, K.; Guthy, D.; Kiffe, M.; van Eerdenbrugh, B.; et al. New Potent DOT1L Inhibitors for in Vivo Evaluation in Mouse. ACS Med. Chem. Lett. 2019, 10, 1655–1660. [Google Scholar] [CrossRef]
- Basavapathruni, A.; Olhava, E.J.; Daigle, S.R.; Therkelsen, C.A.; Jin, L.; Boriak-Sjodin, P.A.; Allain, C.J.; Klaus, C.R.; Raimondi, A.; Scott, M.P.; et al. Nonclinical pharmacokinetics and metabolism of EPZ-5676, a novel DOT1L histone methyltransferase inhibitor. Biopharm. Drug Dispos. 2014, 35, 237–252. [Google Scholar] [CrossRef]
- Bon, C.; Halby, L.; Arimondo, P.B. Bisubstrate inhibitors: The promise of a selective and potent chemical inhibition of epigenetic “writers”. Epigenomics 2020, 12, 1479–1482. [Google Scholar] [CrossRef]
- Yang, M.; Schneller, S.W. Amino substituted derivatives of 5′-amino-5′-deoxy-5′-noraristeromycin. Bioorg. Med. Chem. 2005, 13, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Bernt, K.M.; Zhu, N.; Sinha, A.U.; Vempati, S.; Faber, J.; Krivtsov, A.V.; Feng, Z.; Punt, N.; Daigle, A.; Bullinger, L.; et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell 2011, 20, 66–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, E.R.; Krueger, W.; Jakuba, C.M.; Veilleux, E.; Ambrosi, D.J.; Nelson, C.E.; Rasmussen, T.P. ES cell cycle progression and differentiation require the action of the histone methyltransferase Dot1L. Stem Cells 2009, 27, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Prinz, A.; Steger, J.; Garcia-Cuéllar, M.-P.; Radsak, M.; Bentaher, A.; Slany, R.K. HoxA9 transforms murine myeloid cells by a feedback loop driving expression of key oncogenes and cell cycle control genes. Blood Adv. 2018, 2, 3137–3148. [Google Scholar] [CrossRef]
- Zeisig, B.B.; Milne, T.; Garcia-Cuéllar, M.-P.; Schreiner, S.; Martin, M.-E.; Fuchs, U.; Borkhardt, A.; Chanda, S.K.; Walker, J.; Soden, R.; et al. Hoxa9 and Meis1 Are Key Targets for MLL-ENL-Mediated Cellular Immortalization. Mol. Cell. Biol. 2004, 24, 617–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.T.; Zhang, Y. The diverse functions of DOT1 and H3K79 methylation. Genes Dev. 2011, 25, 1345–1358. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Szabó, P.E. Maternal DOT1L is dispensable for mouse development. Sci. Rep. 2020, 10, 20636. [Google Scholar] [CrossRef]
- Chory, E.J.; Calarco, J.P.; Hathaway, N.A.; Bell, O.; Nell, D.S.; Crabtree, G.R. Nucleosome Turnover Regulates Histone Methylation Patterns over the Genome. Mol. Cell 2019, 73, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Bon, C.; Si, Y.; Arimondo, P.B. Chapter 4—Targeting DOT1L for mixed-lineage rearranged leukemia. In Histone Modifications in Therapy; Castelo-Branco, P., Jeronimo, C., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 20, pp. 81–99. [Google Scholar]
- Dafflon, C.; Gaulis, S.; Barys, L.; Kapur, K.; Cornacchione, V.; Schukur, L.; Bergling, S.; Traggiai, E.; Jansky, S.; Hellmann, L.; et al. DOT1L inhibition is lethal for multiple myeloma due to perturbation of the endoplasmic reticulum stress pathway. Oncotarget 2020, 11, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wei, Z.; Wang, Q.; Zhang, X.; Tao, X.; Wu, N.; Liu, X.; Qian, J. The role of DOT1L in the proliferation and prognosis of gastric cancer. Biosci. Rep. 2020, 41, BSR20193515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobl, J.; Sinha, D.K.; Sima, R.; Perner, J.; Kopáček, P.; Valdés, J.J.; Rego, R.O.M.; Cabezas-Cruz, A. Histone Methyltransferase DOT1L Is Involved in Larval Molting and Second Stage Nymphal Feeding in Ornithodoros moubata. Vaccines 2020, 8, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soria-Valles, C.; Osorio, F.G.; López-Otín, C. Reprogramming aging through DOT1L inhibition. Cell Cycle 2015, 14, 3345–3346. [Google Scholar] [CrossRef] [Green Version]
- Ceccaldi, A.; Rajavelu, A.; Champion, C.; Rampon, C.; Jurkowska, R.; Jankevicius, G.; Sénamaud-Beaufort, C.; Ponger, L.; Gagey, N.; Ali, H.D.; et al. C5-DNA Methyltransferase Inhibitors: From Screening toEffects on Zebrafish Embryo Development. ChemBioChem 2011, 12, 1337–1345. [Google Scholar] [CrossRef] [PubMed]

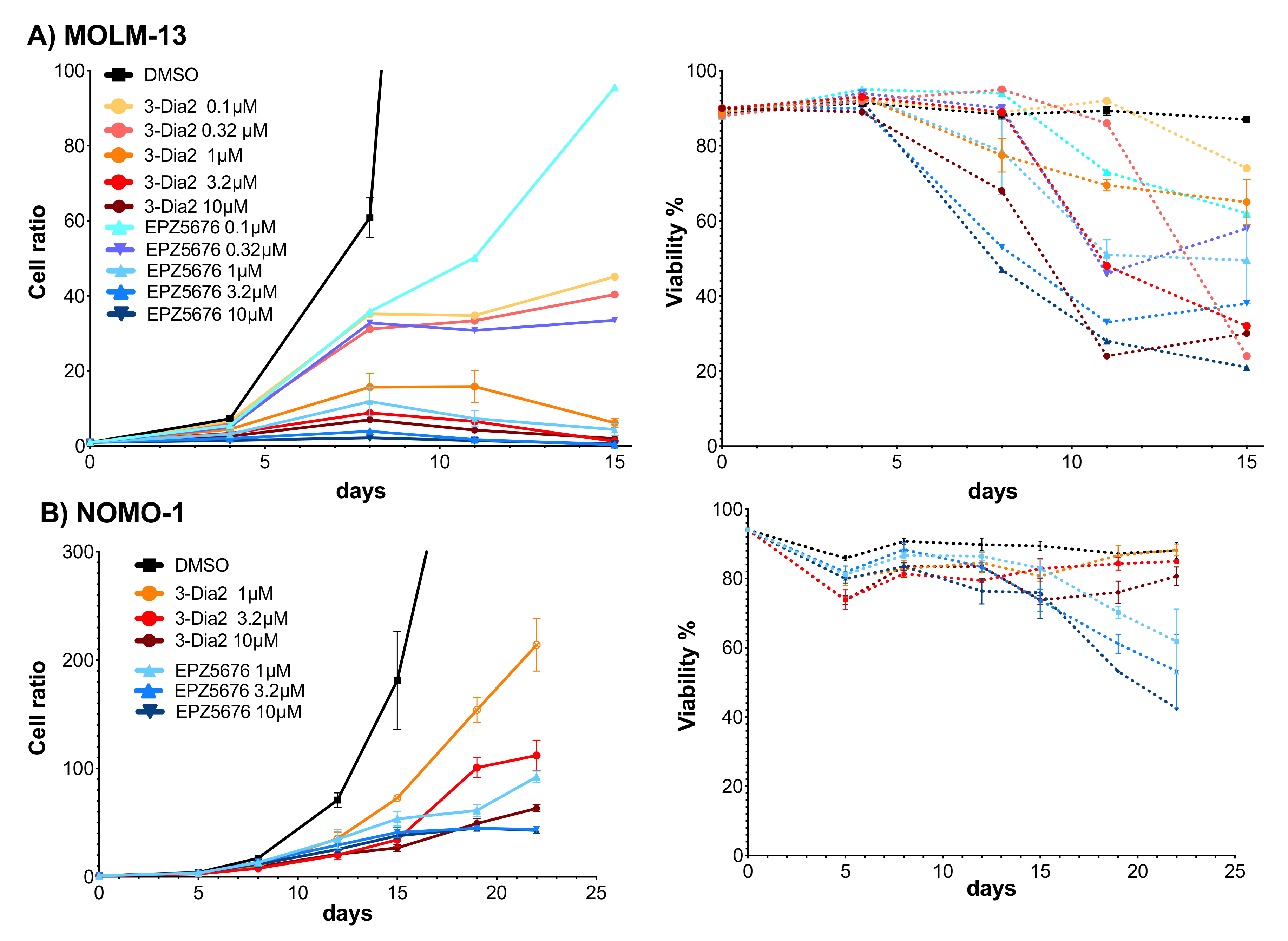
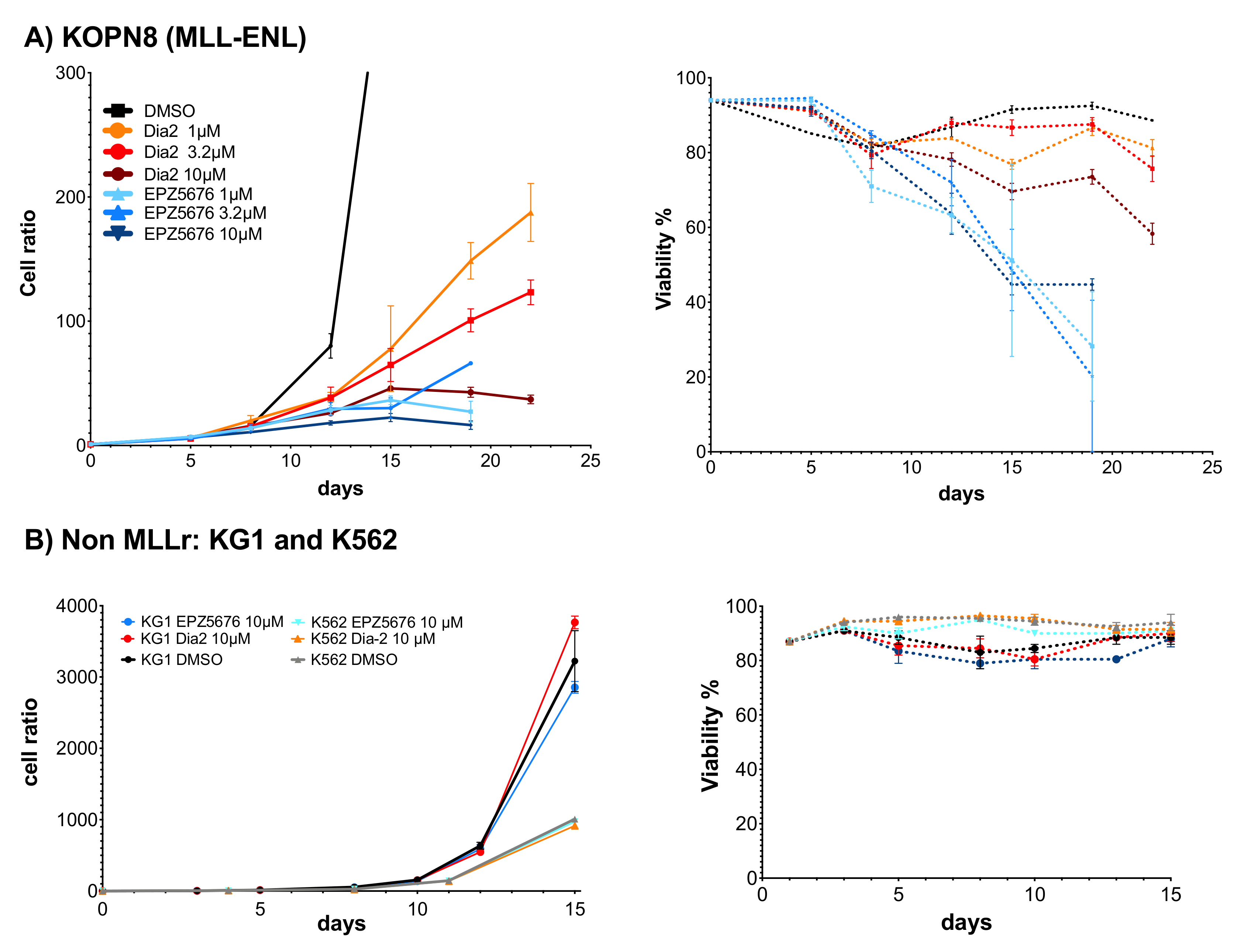

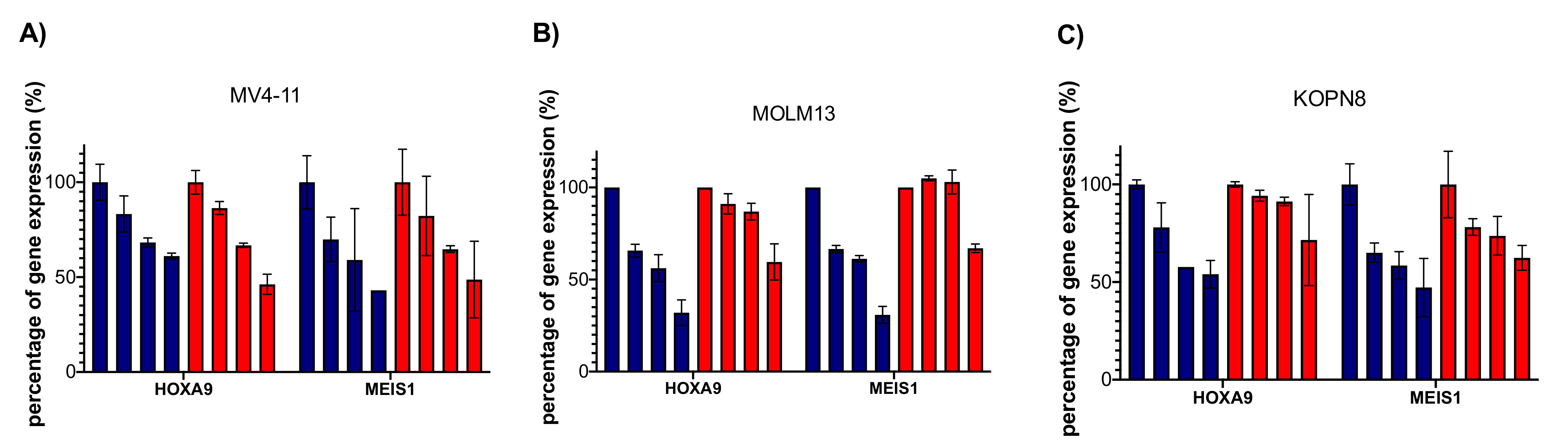
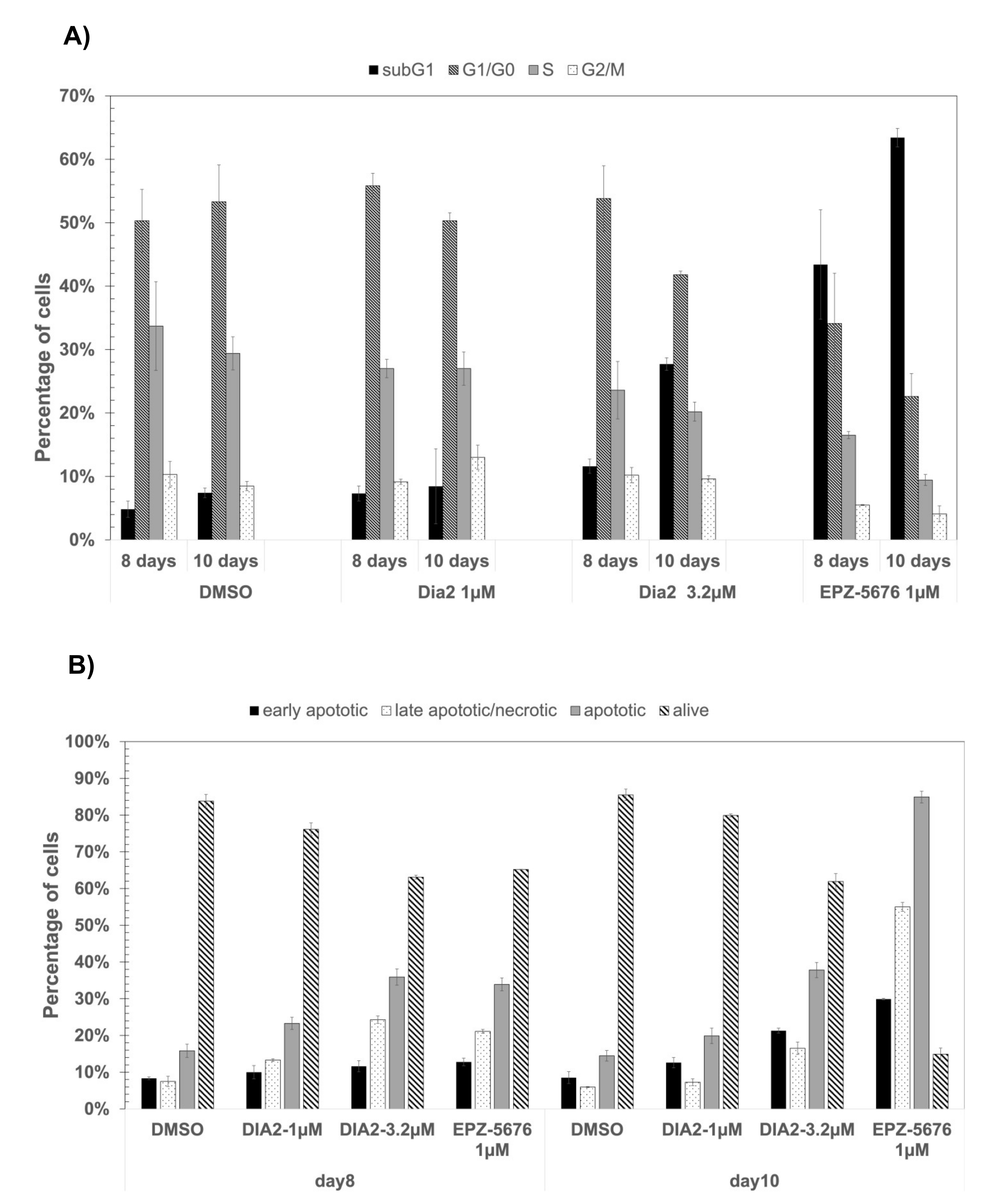
| Cell Line | Dia2 | EPZ-5676 | Doubling Time |
|---|---|---|---|
| MV4-11 (AF4) | 93 ± 4 | 21 ± 1 | 12 h |
| KOPN8 (ENL) | 63 ± 2 | 86 ± 4 | 48 h |
| NOMO-1 (AF9) | 660 ± 20 | 562 ± 15 | 30 h |
| MOLM-13 (AF9) | 120 ± 10 | 92 ± 2 | 22 h |
| HOXA9-F2 | CCCCCATCGATCCCAATAACCC |
| HOXA9-R2 | CCAGTTCCAGGGTCTGGTGTT |
| MEIS1-F1 | CGATGGCGCAAAGGTACGAC |
| MEIS-1-R2 | ATGCGGGTCCCCATACATCG |
| B2M-F | GAGATGTCTCGCTCCGTGGC |
| B2M-R | CCTGAATCTTTGGAGTACGCTGG |
| TBP | TTACCGCAGCAAACCGCTTG |
| TBP | TTGACCTAAAGACCATTGCACTCCGT |
| YWHAZ (U/L) | CCCTCAAACCTTGCTTCTAGGAGA |
| YWHAZ (U/L) | TCATATCGCTCAGCCTGCTCG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bon, C.; Si, Y.; Pernak, M.; Barbachowska, M.; Levi-Acobas, E.; Cadet Daniel, V.; Jallet, C.; Ruzic, D.; Djokovic, N.; Djikić, T.; et al. Synthesis and Biological Activity of a Cytostatic Inhibitor of MLLr Leukemia Targeting the DOT1L Protein. Molecules 2021, 26, 5300. https://doi.org/10.3390/molecules26175300
Bon C, Si Y, Pernak M, Barbachowska M, Levi-Acobas E, Cadet Daniel V, Jallet C, Ruzic D, Djokovic N, Djikić T, et al. Synthesis and Biological Activity of a Cytostatic Inhibitor of MLLr Leukemia Targeting the DOT1L Protein. Molecules. 2021; 26(17):5300. https://doi.org/10.3390/molecules26175300
Chicago/Turabian StyleBon, Corentin, Yang Si, Melanie Pernak, Magdalena Barbachowska, Eva Levi-Acobas, Veronique Cadet Daniel, Corinne Jallet, Dusan Ruzic, Nemanja Djokovic, Teodora Djikić, and et al. 2021. "Synthesis and Biological Activity of a Cytostatic Inhibitor of MLLr Leukemia Targeting the DOT1L Protein" Molecules 26, no. 17: 5300. https://doi.org/10.3390/molecules26175300
APA StyleBon, C., Si, Y., Pernak, M., Barbachowska, M., Levi-Acobas, E., Cadet Daniel, V., Jallet, C., Ruzic, D., Djokovic, N., Djikić, T., Nikolic, K., Halby, L., & Arimondo, P. B. (2021). Synthesis and Biological Activity of a Cytostatic Inhibitor of MLLr Leukemia Targeting the DOT1L Protein. Molecules, 26(17), 5300. https://doi.org/10.3390/molecules26175300








