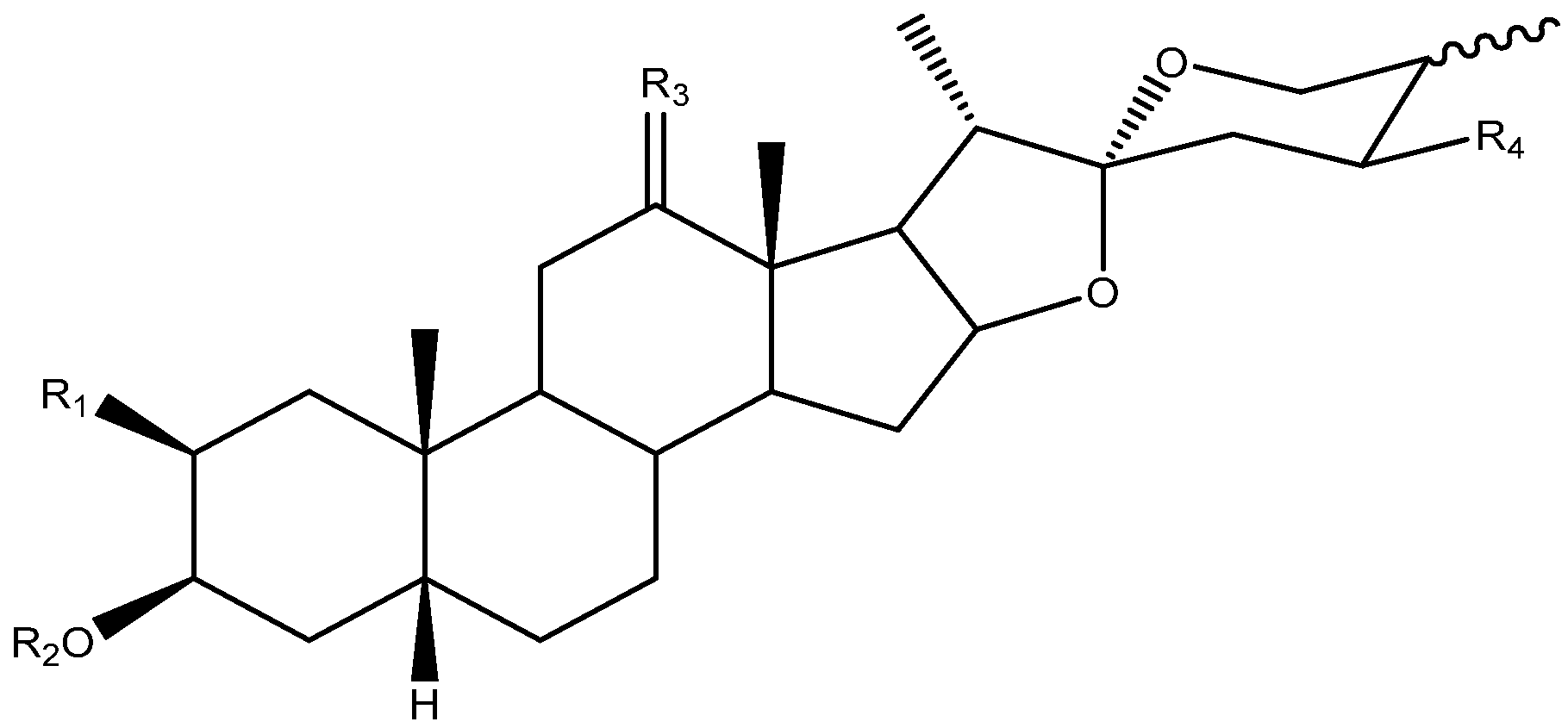
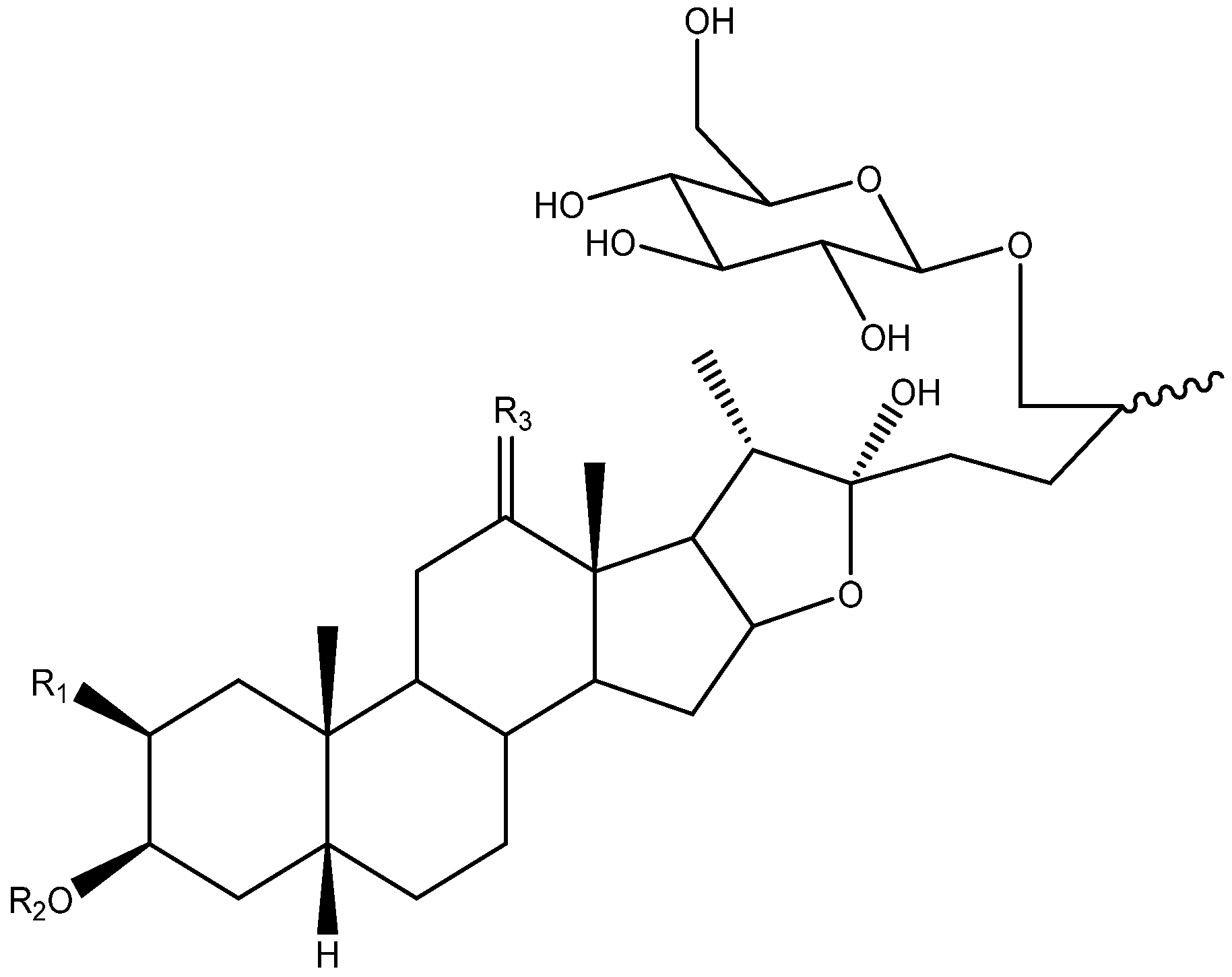
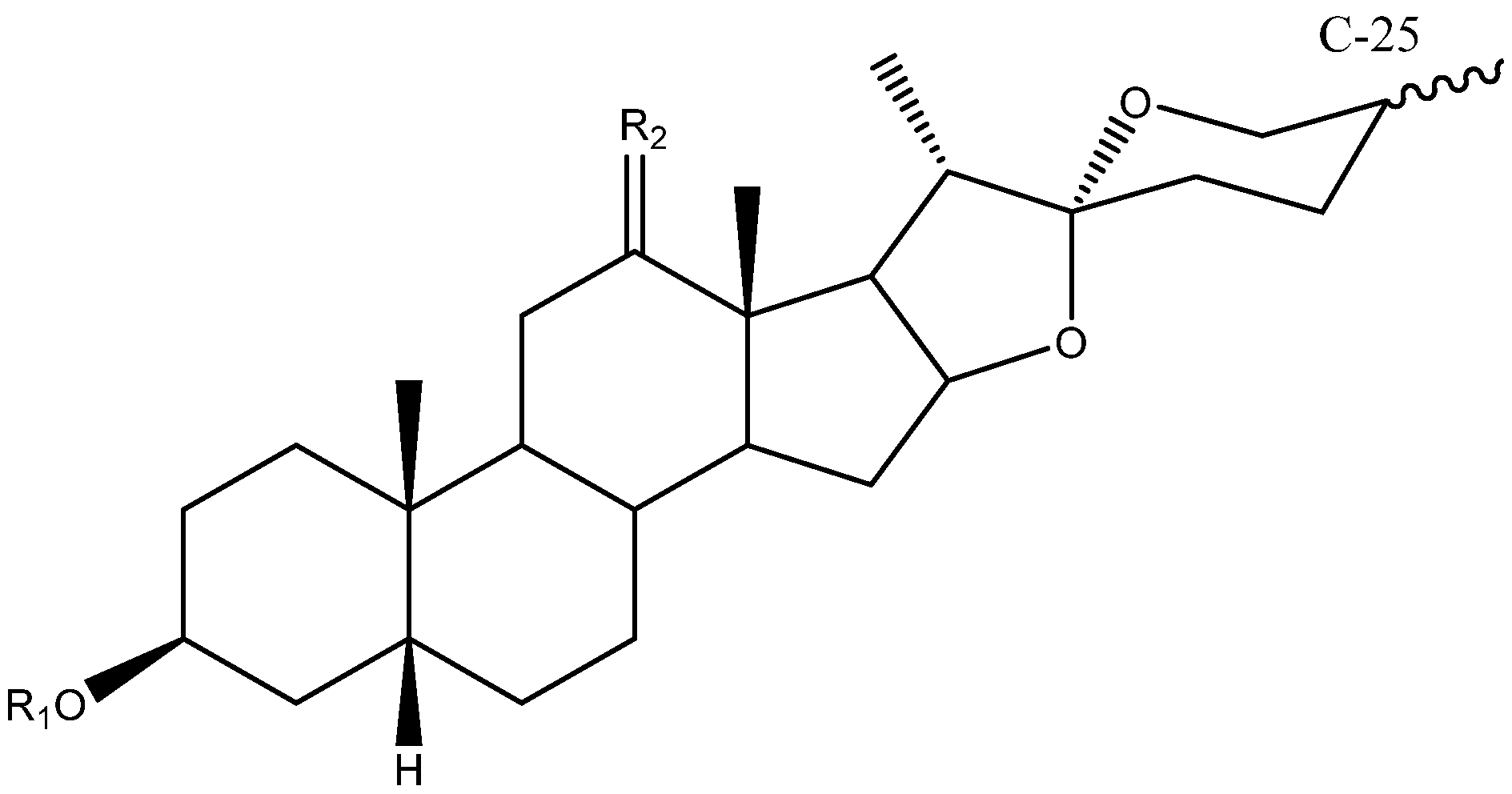
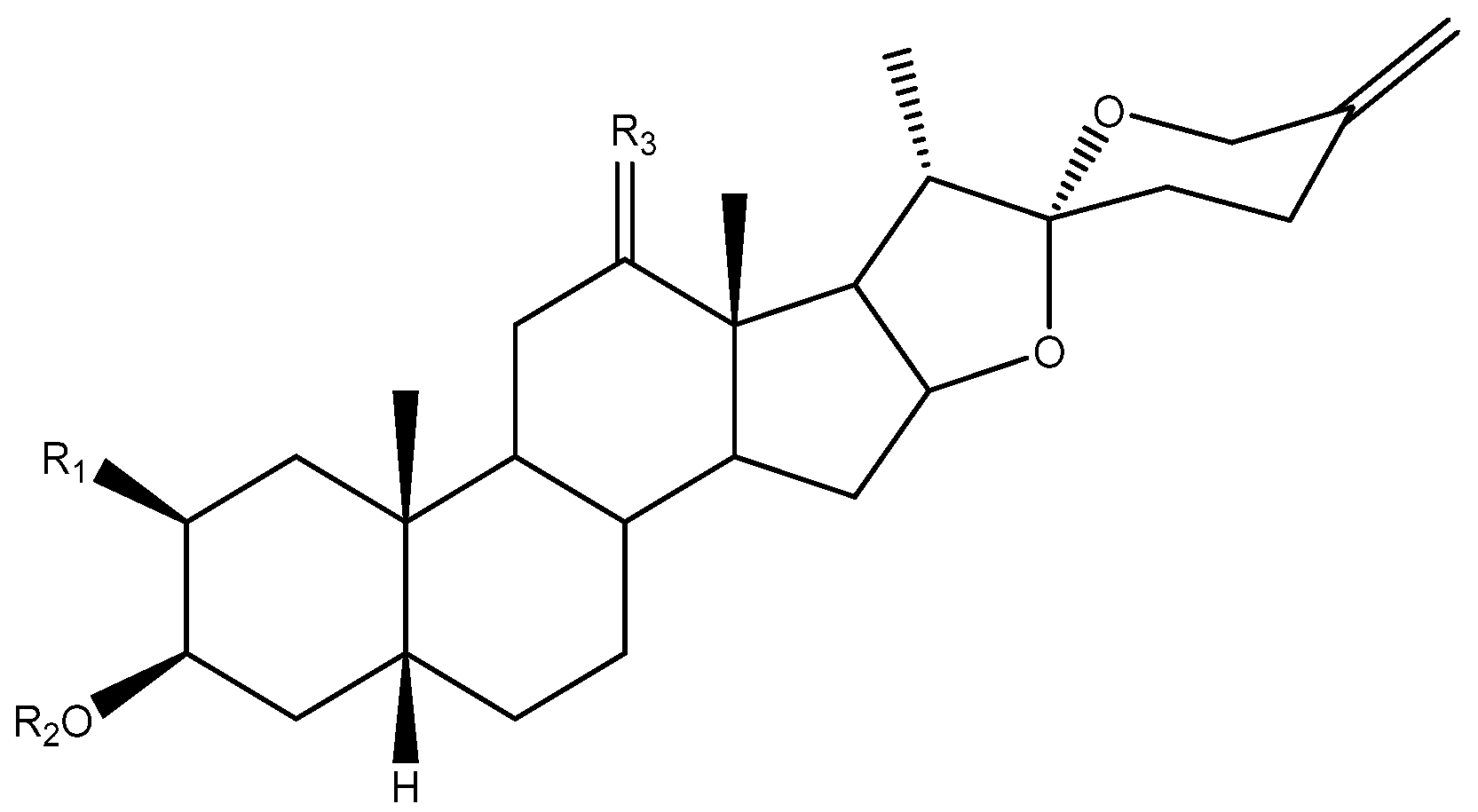
Abstract
Yucca is one of the main sources of steroidal saponins, hence different extracts are commercialized for use as surfactant additives by beverage, animal feed, cosmetics or agricultural products. For a deeper understanding of the potential of the saponins that can be found in this genus, an exhaustive review of the structural characteristics, bioactivities and analytical methods that can be used with these compounds has been carried out, since there are no recent reviews on the matter. Thus, a total of 108 saponins from eight species of the genus Yucca have been described. Out of these, the bioactivity of 68 saponins derived from the isolation of Yucca or other genera has been evaluated. Regarding the evaluation and quality control of the saponins from this genus LC-MS technique is the most often used. Nevertheless, the development of methods for their routine analysis in commercial preparations are needed. Moreover, most of the studies found in the literature have been carried out on Y. schidigera extract, since is the most often used for commercial purposes. Only eight of the 50 species that belong to this genus have been studied, which clearly indicates that the identification of saponins present in Yucca genus is still an unresolved question.
1. Introduction
The Yucca genus comprises around 50 plant species of the Agavaceae family from the American continent, but mainly from the United States, Mexico and Central America [1,2]. The species that belong to the Yucca genus have been used for years in traditional medicinal practices all over the world, but especially by Native Americans [1]. Subsequently, the research on this genus has made clear that they are plants with a high content of bioactive steroidal saponins [3].
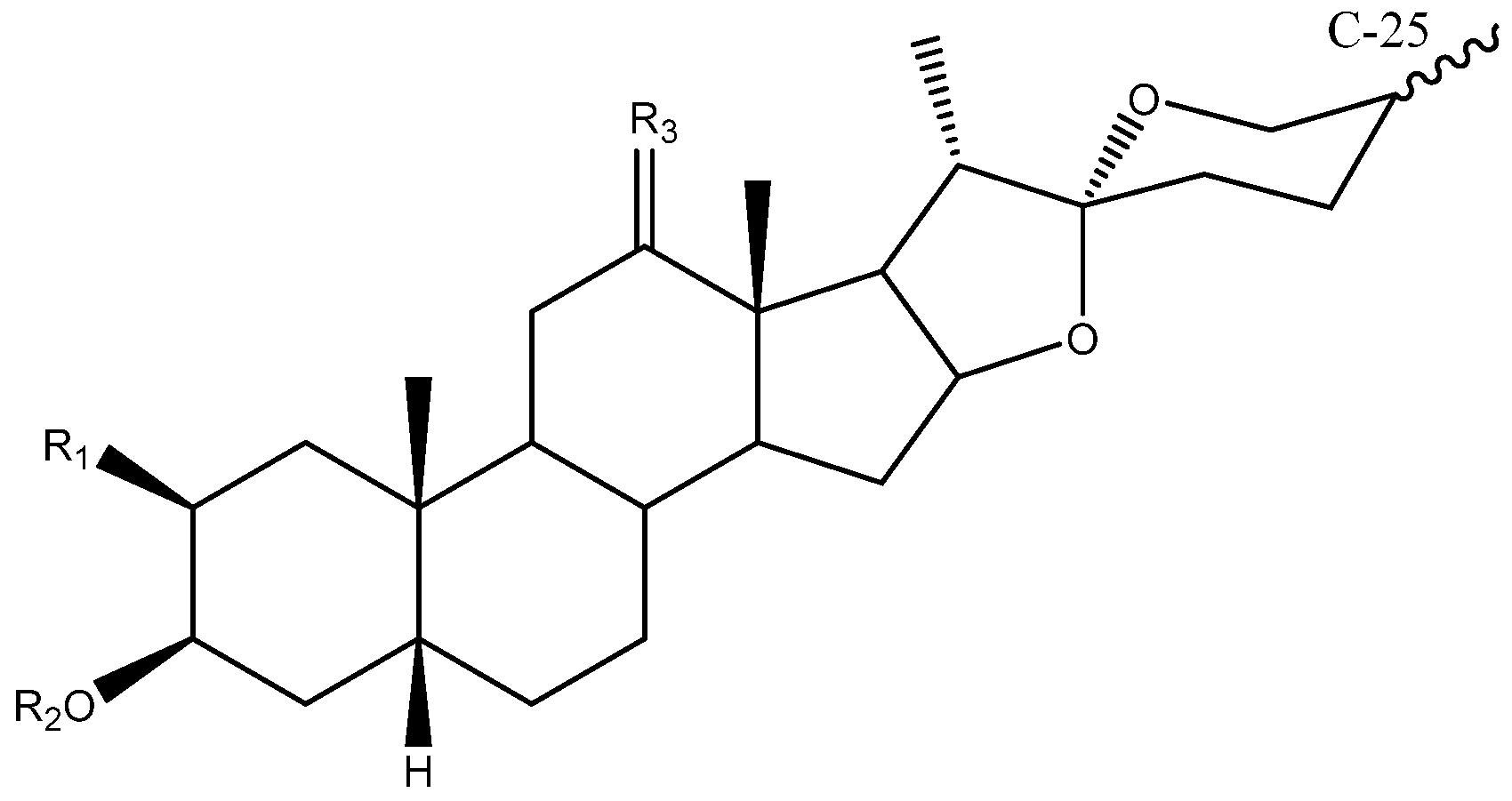
Steroidal saponins are secondary metabolites of plant origin, with a high molecular weight and a complex two-part structure. One of these parts, known as the sapogenin or aglycone, is hydrophobic and consists of 27 carbon atoms arranged in five or six rings (named A–F). Depending on the nature of the aglycone attachment at C-22, they can be classified as either spirostane or furostane (Figure 1). The other moiety, the hydrophilic one, is usually formed by sugar residues with different monosaccharides linked by O-glycosidic bonds (Figure 1) [4,5]. In addition, two cholestane-type saponins have been uniquely described in the Yucca genus [6].
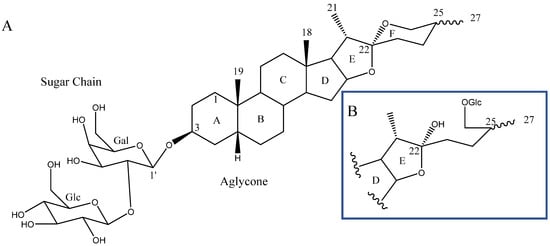
Figure 1.
(A) Structure of a spirostanic saponin. (B) C-22 to C-27 carbons of a furostanic saponin.
At present, some species of the Yucca genus and their saponins have been classified as Generally Recognized As Safe (GRAS) by the Food and Drug Administration of the United States (FDA) [1,7,8,9]. Therefore, several extracts and products derived from these plants have been commercialized as food supplements, parapharmaceutical complements, moisturizing agents, soil conditioners, etc. There are several producers of Y. schidigera products in the world such as Naturex, BAJA Yucca and American Extracts [10]. The saponins present in these plants have also been tested in separate studies, showing properties such as cytotoxic, phytotoxic, antifungal, molluscicidal, anti-inflammatory [11,12,13,14,15].
There are several reviews on the steroidal saponins from the Agavaceae family, including the Yucca genus, however, no work has been published to address this genus exclusively. The data that are usually collected in these studies are related to the structure and bioactivities of the saponins. An example of such work is that by Simmons-Boyce in 2007, where a total of 44 saponins found in the Yucca genus and their bioactivities were described [3].
Other papers have reviewed saponins from a specific species, such as Y. schidigera [9,16]. This species is the most widely marketed and the one with the largest number of studies. Even some very active saponins such as the so-called timosaponin AIII, present in Y. macrocarpa and Y. gloriosa and their bioactivities have been the subject of a number of reviews [17].
2. Structure of Yucca Saponins
2.1. Aglycone
To date, 33 aglycones have been described as part of the saponins found in the Yucca genus [6,7,11,12,14,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], including spirostanic and furostanic saponins (Figure 2).
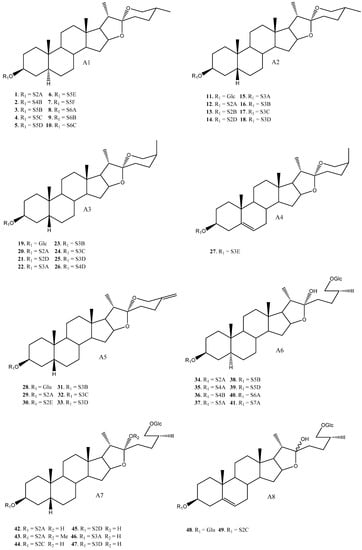
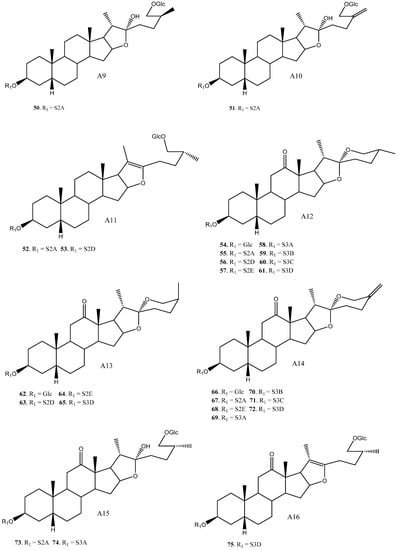
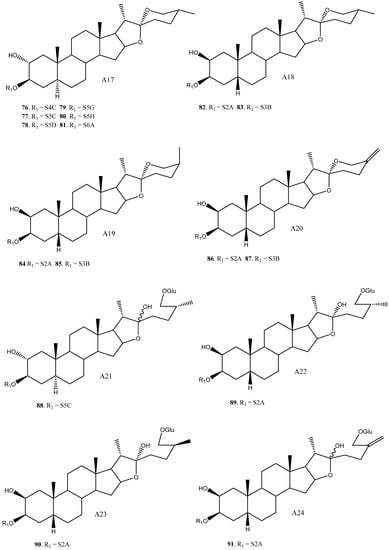
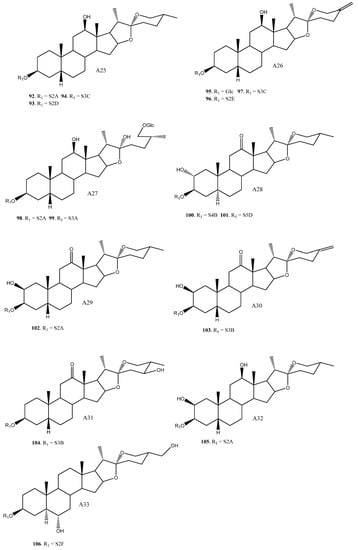
Figure 2.
Structure of the spirostanic and furostanic saponins described in Yucca (1–106). The codes used for the aglycones (A1–A33) and the sugar chains (S#A-S#H) are included. Structures of sugars chains can be found further on Figures 6–8.
The B to D rings are always fused with a trans arrangement, however, the A and B rings can also appear with a cis fusion as well as a double bond between the C-5 and C-6 carbons (Figure 3). Most common in Yucca is the cis fusion, which implies a β-arrangement of the H-5. However, depending on the species, one arrangement may be predominant over the other. For example, in Y. desmetiana the most abundant aglycone has a trans fusion of the A and B rings with H-5α [12,34], while in other species such as Y. glauca [11] or Y. schidigera [14,16,20] the H-5β form is the most abundant.
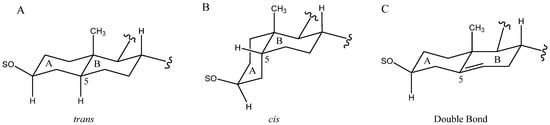
Figure 3.
Structure of rings A and B with trans fusion (A), cis fusion (B) or double bonds between C-5 and C-6 (C).
The C-22 configuration determines the spirostanic or furostanic nature of a saponin. In the former, it is a spirostanic carbon, through which the E and F rings are linked, while in the furostanic ones C-22 is part of a hemiketal or enolic carbon with an open chain [6,16] (Figure 4). The most abundant aglycones in Yucca are the spirostanic ones, but this varies depending on the species. Thus, in the case of Y. elephantipes [19,36] or Y. desmetiana [12,34] furostanic aglycones are mostly isolated. It should be mentioned that C-22 is a chiral centre that always presents an R configuration in the case of the spirostanic derivatives, and it is also R in the case of the furostanic derivatives where the configuration has been described.
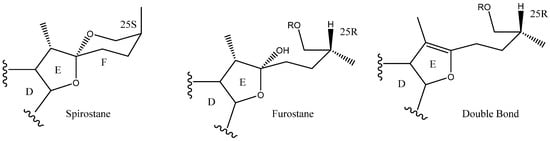
Figure 4.
Structure of steroidal aglycones on the C-22.
C-25 is a chiral carbon which can have either R or S configuration, and a significant number of aglycones contain a double bond between C-25 and C-27 (Figure 5).
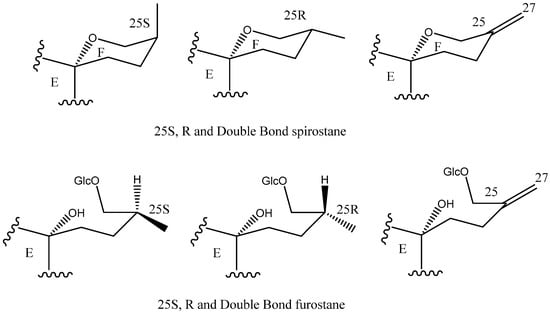
Figure 5.
Structure of steroidal aglycones on the C-25.
Regarding the oxygenation in Yucca aglycones, we find them at carbons 2, 12, 24 and 27. On position 2, hydroxyl groups appear in α and β arrangements, and their configuration always coincides with that of H-5, which allows us to suggest that this structural outcome results from their biosynthesis pathway. On position 12, a carbonyl group and, in some cases, a hydroxyl group with a β-arrangement are most frequently observed. Moreover, only one spirostanic saponin (104), described in Y. glauca [11], is hydroxylated at C-24 and another found in Y. smalliana (106) is hydroxylated at C-27 [33]. It is interesting to note that all of the hydroxyl groups that substitute the Yucca aglycones present an equatorial arrangement within the rings that contain them.
2.2. Sugar Chains
Saponins can be classified according to the number of sugar chains that they contain. Those which have only one chain are called monodesmosidic, and with respect to the saponins in the Yucca genus, this is located on position C-3. The saponins with two sugar chains are called bidesmosidic, and in the genus Yucca coincide with the furostanic saponins, which have a glucose unit on C-26 in addition to the sugar chain on C-3 [5].
In addition to glucose, 22 different sugar chains have been described in Yucca. These are linked to aglycones on the C-3 position [6,7,11,12,14,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The chains described in this genus possess up to seven monosaccharides.
Disaccharides and trisaccharides exhibit a wide structural diversity (Figure 6). The monomer that bonds directly to the C-3 of the aglycone can be either β-d-galactopyranose or β-d-glucopyranose. These monosaccharides are linked by glycosidic bonding to β-d-glucopyranoses or β-d-xylopyranoses on the positions 2’, 3’ or 4’, in the case of galactose, and 2’ or 3’, in the case of glucose. The most common disaccharide in Yucca is glucopyranosyloxy (1→2) galactopyranoside (S2A).
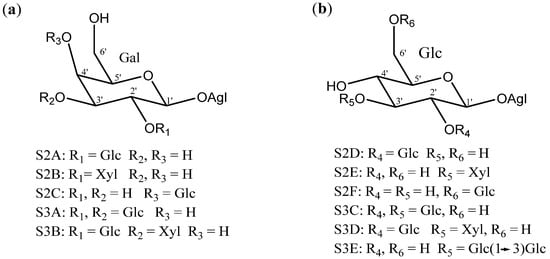
Figure 6.
Structure of the disaccharides (S2A to S2F) and trisaccharides (S3A to S3E) present in Yucca (a) when β-d-galactopyranose is linked at C-3 of the aglycone and (b) when is a β-d-glucopyranose. Gal = galactose; Glc = glucose and Xyl = xylose.
Sugar chains of four or more units have also been described in Yucca, although they are less abundant than the above described (Figure 7). Ten of them have the same core, which consists of a β-d-galactopyranose and two β-d-glucopyranoses, to which β-d-glucopyranoses, β-d-xylopyranoses or α-l-ramnopyranoses are linked at different positions to form the actual polysaccharide.
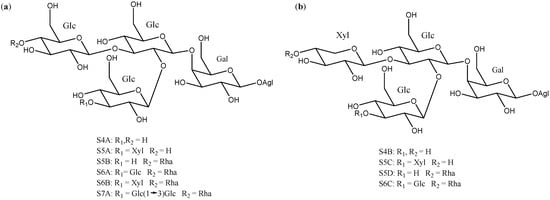
Figure 7.
Structure of Yucca polysaccharides S4A to S7A (a) with a β-d-galactopyranose and three β-d-glucopyranoses and (b) with a β-d-galactopyranose, two β-d-glucopyranoses and a β-d-xylopyranose core. Gal = galactose; Glc = glucose; Xyl = xylose and Rha = rhamnose.
On the other hand, other polysaccharides, such as S4C [25], S4D [18], S5G [24] and S5H [26], which present structures that do not follow this pattern have also been described (Figure 8), however their characterization was carried out, in some cases, using the non- fully developed structural elucidation techniques that were available for saponins before the early 1990s. Hence, the sugar chains S4C, S5G and S5H were reported in different publications on the saponins present in Y. aloifolia [24,25,26]. The structures of these saponins are very different from each other, which leads us to suspect errors in their identification. In fact, subsequent investigations that used mono- and bidimensional NMR techniques showed that, the larger sugar chains were derived from the simpler ones in saponins isolated from the same species. Thus, the larger sugar chains are formed by addition of monosaccharides to the simpler core [11,16,29]. This reinforces the idea that previous determinations of these structures are rather questionable.
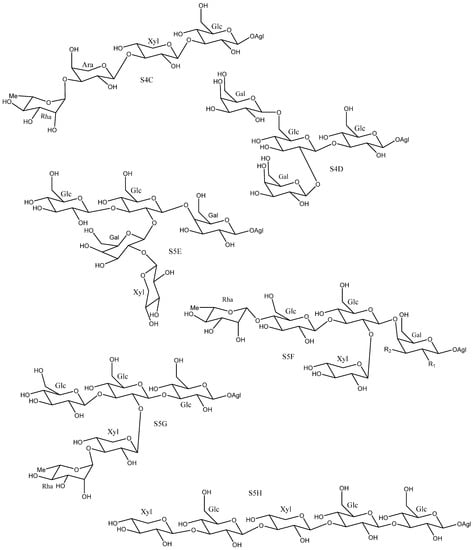
Figure 8.
Yucca polysaccharides with unusual structures.
2.3. Saponins in the Yucca Genus
106 spirostanic and furostanic saponins (Table 1) have been reported in the species of the Yucca genus that have been studied, namely Y. gloriosa, Y. glauca, Y. schidigera, Y. aloifolia, Y. desmetiana, Y. elephantipes Y. macrocarpa, and Y. smalliana [6,7,11,12,14,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Only a few of these have been studied in depth, which lead us to conclude that the saponins from the Yucca genus remain a rather underexplored field.

Table 1.
Spirostanic and furostanic saponins from Yucca, their origin and bioactivities.
Overall, based on the data available, it is observed that some saponins such as 12, 16, 42, 55 and 82 are described in three or four different species, however, the general trend is that most of the saponins are found in only one or two species.
The saponins with a H-5β aglycone arrangement have a sugar chain of three or fewer units attached to C-3. For example, in Y. schidigera [14,16,20], the majority of the saponins are 5β-spirostanic and all of them have chains formed by two or three monosaccharides. In other species, such as Y. aloifolia [24,25,26], the isolated saponins are 5α-spirostanic with sugar chains of four and five units. In the case of Y. gloriosa, a wide variety of spirostanic and furostanic saponins with sugar chains ranging from two to seven units have been isolated. In particular, it is in this species that the largest sugar chains have been described [29]. The same trend is also true for Y. gloriosa, where the saponins with a β-arrangement at H-5 have chains formed by less than three polysaccharides, while those with an α-arrangement at H-5 present the longest chains.
This trend has also been observed in the saponins from the Agave genus [91] and there seems to be a correlation between the fusion of the A and B rings and the size of the sugar chain in the saponins, which seems to be related to some spatial effect that occurs between the cis fusion aglycones and the more voluminous chains.
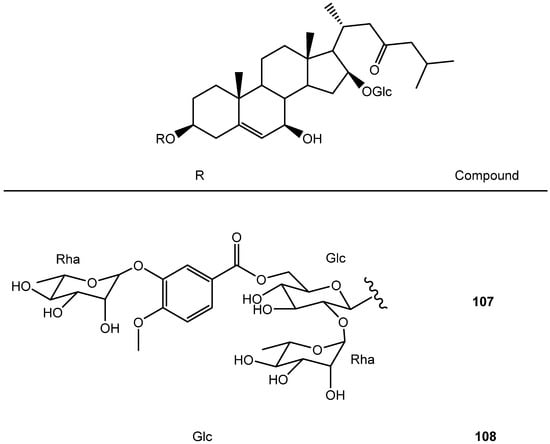
Figure 9.
Structure of cholestane-type saponins from Yucca.
3. Bioactivities by Yucca Saponins
In addition to compiling the activities shown by extracts from species of the genus Yucca, a bibliographic search has been carried out on the bioactivity of the 108 saponins described in the genus. The most significant results are shown in Table 1. Only 69 of these saponins have been tested and their biological activities that have been most studied are cytotoxic, antifungal, anti-inflammatory and effects on platelet aggregation.
Some extracts such as those from Y. gloriosa flowers or dried leaves [31,32], as well as Y. schidigera stems [14] showed antifungal activity against a large number of yeasts and fungi, which makes of these extracts suitable candidates to be used as food additives or preservatives [92]. In fact, Y. schidigera extract is commercially available and has been classified as GRAS by the FDA (USA) [9] and it appears on the “List of Existing Food Additives” in Japan, so that it can be used there as a human dietary additive [92].
In addition to this, certain saponins and extracts from Y. schidigera have been proposed to improve the health conditions of aquatic organisms [93,94] as well as the immune response of broilers [95]. Moreover, when Y. schidigera extract is administered to ruminants, or horses, there is a reduction in the growth of protozoa and Gram-positive bacteria which are associated in some cases to colitis, and other processes leading to inflammatory diseases [96]. In addition, some studies have associated the regular ingestion of Yucca extracts with a reduction in cholesterol levels in hypercholesterolemic humans [97].
The saponins isolated from different extracts of the genus Yucca have also been studied. Interesting cytotoxic activities against various cancer cell lines have been described for saponins from Y. glauca [11], Y. desmetiana [34] and Y. schidigera [28]. Antifungal activity has also been tested on pure saponins and epimers mixtures on the C-25 position in Y. gloriosa [31], Y. elephantipes [36] and Y. schidigera [14], showing very promising activity values. These two activities have been the most frequently tested on the saponins from Yucca, including studies on structure-activity relationships (SAR). Apart from these activities, two saponins from Y. desmetiana [12] and one from Y. aloifolia [25] presented molluscidal activity.
Some of the saponins described in the genus Yucca have been tested for bioactivities such as anti-inflammatory after being isolated from other species. Inflammation [98], a defensive physiological response to noxious stimuli, is a highly complex process involving multiple cells, signaling pathways and molecules. Although inflammation is a natural and beneficial process, an excessive inflammatory response may lead to chronic damage and adverse reactions. Some saponins have been tested against certain processes involved in the inflammatory response, such as the release of nitric oxide (NO) [72,86], production of inflammatory interleukins [72,86], recruitment of immune system cells [59] or inhibition of specific metabolic pathways [15,78,86] and in some cases, they exhibited rather promising results. Some of the chronic conditions that may result from inflammatory processes are osteoporosis [64] or cardiovascular damages due to high blood glucose levels [99]. Additionally, neurodegenerative diseases such as Alzheimer’s or Parkinson’s diseases are among other possible undesirable conditions [63,75,90]. Three of the saponins present in Yucca, namely timosaponin AIII (20), timosaponin BII (50), and degalactotigonin (2), have been tested more extensively and have been distinguished by their intense and varied activity.
3.1. Cytotoxicity: Study of the Structure-Activity Relationships (SAR)
Cytotoxicity is one of the activities that has been most frequently tested in natural products and is often described in Yucca saponins. In most cases, these molecules are evaluated for their ability to be toxic by producing visible damage to various cancer cell lines [100]. Such damage may occur through various pathways or mechanisms of action, being rather frequent the regulation of cellular apoptotic pathways [11,71].
In some cytotoxicity studies, inhibitory concentration (IC50) values are used as a benchmark to assess the cytotoxic effect of the active substance. IC50 is the concentration level of a compound that is required to inhibit growth by 50% in comparison to untreated cells [11,34].
Yokosuka et al. [11] tested 20 spirostanic and furostanic saponins extracted from Y. glauca (Table 2 and Table 3) against human acute myelogenous leukemia (HL-60) and human lung adenocarcinome (A549) cell lines. A connection between the structure of these 20 saponins and their activity could be identified [11].

Table 2.
Bioactivity of spirostanic saponins by Y. glauca and by cisplatin and etoposide as control substances [11].

Table 3.
Bioactivity of furostanic saponins by Y. glauca and by Cisplatin and Etoposide as control substances [11].
As a bioguided isolation method, all the saponins were first tested against HL-60 cells and only the active ones were tested against the A549 line. Cisplatin and Etoposide were used as the control drugs. All the tested saponins from Y. glauca present a cis fusion of the A and B rings (H-5β) with residues of three or less sugar units attached to C-3. Moreover, they differ regarding the oxygenation of the aglycone, by the nature of the C-25, their furostanic or spirostanic structure, and in the sugar chains.
The saponins that presented IC50 values lower than 20 µM against both cell lines (12, 16, 29, 31, 42, 50 and 51), had in common the absence of oxygenation at the aglycone, while those with a hydroxyl group on C-2 (82, 86, 89, 90 and 91), were only active against HL-60 [11]. Finally, oxygenation on the C-12 position, did not favor cytotoxicity, since all the saponins of this type presented IC50 values above 20 µM against the HL-60 cell line.
If the influence attributable to their spirostanic or furostanic nature is examined, the comparison of some pairs of compounds such as 29 vs. 51, 12 vs. 42, 86 vs. 91 and 82 vs. 89 indicate that the spirostanic derivatives were more active in all the cases [11].
The influence of the R or S configuration of C-25 and even the presence of a double bond between this carbon and C-27, does not appear to be significant, since only slight variations of the IC50 values and no definite trend were observed.
Regarding the type of sugar chain of the tested saponins, the vast majority of active saponins presented a glucopyranosyl (1–2) galactopyranoside (S2A) chain, or a derivative of it such as the S3B chain, which presents a xylopyranosyl unit on the C-3 of the galactose (31). On the other hand, β-d-glucopyranosyloxy (1→4) β-d-galactopyranoside (S2C) chains give rise to a less active saponin (42 vs. 44), especially against the A549 cell line.
The most active saponin 29 and its furostane derivative 51 were assayed to determine their ability to induce apoptosis of the HL-60 cells. The assay was based on measuring the activity of caspase-3 [11]. Thus, it could be determined that spirostanic saponin 29 was effective to significantly produce apoptosis in a shorter period of time (6 h) than saponin 51 (16 h) and with higher caspase-3 activity values, which were close to those presented by the control substance (etoposide) [11].
In a different study by Qu et al. [28], the cytotoxic activity of 12 saponins isolated from Y. schidigera (Table 4) was analyzed against human colon adenocarcinoma cell line (SW620).

Table 4.
Cytotoxic activity of the saponins in Y. schidigera [28].
It can be observed that the saponins with carbonyl substituents on C-12 (54, 56, 57, 62, 63 and 64) are generally less active than those without such functionalization (11, 14, 18, 19, 21 and 25), which is in agreement with the reports from previous studies. On the other hand, it is noteworthy that in the saponins with a non-functionalized C-12, the S epimers on C-25 are more active. The difference in activity between R and S epimers, which in some cases is rather noticeable, had not been observed in previous studies. The size of the sugar chain has an influence on the activity displayed, being compounds with three-unit chains the most active ones (25, 12.02 µM) [28].
Sun et al. [65], demonstrated with saponins from Anemarrhena asphodeloides, which included some of the saponins contained in Yucca, that glycosylation is considered essential regarding cytotoxic activity, since when the aglycones were tested separately, they did not exhibit any activity.
3.2. Antifungal: Study of the Structure-Activity Relationships (SAR)
Antifungal or antimycotic activity is the effect that certain substances have on fungi, preventing their proliferation, development and, in some cases, causing their death. The activity is represented by IC50 values, or Minimum Inhibitory Concentration (MIC) values, i.e., the minimum amount of compound at which the organism does not appear to grow [14].
Miyakoshi et al. [14], tested a collection of saponins, which included six pure saponins and eight mixtures of C-25 epimers, all of them extracted from Y. schidigera (Table 5 and Table 6) against a number of selected organisms: Saccharomyces cerevisiae IFO 203 (Sc), Candida albicans TIMM 0134 (Ca), Hansenula anomala HUT 7083 (Ha), Pichia nakazawae HUT 1688 (Pn), Kloeckera apiculata IFO 154 (Ka) and Debaryomyces hansenii IFO18 (Dh).

Table 5.
MIC (µM) values calculated for the individual saponins from Y. schidigera [14].

Table 6.
Calculated MIC (µM) values for the epimer pairs on the C-25 position in Y. schidigera [14].
The isolation process of the saponins was conducted following a bioguided procedure, on which as the fraction containing furostanic saponins did not show activity, therefore, the study was focused on the spirostanic saponins [14]. On the other hand, the spirostanic saponins were hydrolyzed and the aglycones were tested and found to be inactive. It has been determined that the participation of both parts of the saponin, the sugar chain and the aglycone, is required to present any relevant activity.
The results from the tests with spirostanic saponins and their mixtures showed that the presence of oxidations on the C-2 and C-12 positions (hydroxyl and carbonyl groups) resulted in a drastic drop or even the absence of activity. On the other hand, those without such oxidations present better MIC values [14].
If the saponins are compared, in the ones that only differ in the presence or absence of a double bond between C-25 and C-27, no general trends can be observed for all the tested organisms, except for Kloeckera apiculata IFO 154 (Ka) and Candida albicans TIMM 0134 (Ca) where the absence of such a double bond results in a sharp decrease in activity (cf. 33 vs. 18 + 25).
With regard to the nature of the sugar chain, four types of trisaccharides and one disaccharide have been tested for saponins without oxygenation in the aglycone. In the case of saponins 31, 32 and 33, with a double bond between carbons C-25 and C-27 and whose only difference is the nature of the trisaccharide, we observed that saponin 31 is the most active, with particular relevance for Kloeckera apiculata IFO 154 (Ka) and Debaryomyces hansenii IFO18 (Dh). However, their saponin analogues without a double bond, do not present such a marked difference, with the 18 + 25 saponin mixture showing the highest activity, especially on Candida albicans TIMM 0134 (Ca). The sugar chain of saponins 33, 18 and 25 is a β-d-xylpyranosyl(1→3)[β-d-glucopyranosyl(1→2) β-d-glucopyranoside] (S3D) which differs from the S3B chain (31, 16, 23) where the innermost monosaccharide is a β-d-galactopyranose. On the other hand, the S3C chain (32, 17, 24) has a β-d-glucopyranose unit instead of the β-d-xylopyranose that is found in S3B. Finally, the 22 + 15 saponin mixture with the S3A chain, which is similar to S3C although with a β-d-galactopyranose as the innermost monosaccharide, exhibited activity values against all of the organisms tested. Although it could be concluded that the nature of the sugar chain affects the activity values reached by the tested spirostanic saponins, no clear trends were observed in relation to the nature of the constituent monosaccharides.
3.3. Selection of Active Saponins
3.3.1. Degalactotigonin (2)
Degalactotigonin (2, Figure 10) has been isolated from Y. gloriosa flowers [6,7], but also from other species or genera [39,40,42,43,44,45,46,47,48].
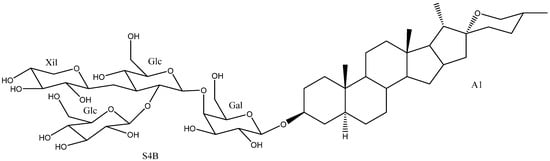
Figure 10.
Degalactotigonin (2).
Degalactotigonin (2) from different species has been tested for cytotoxic, antifungal, spermicidal and platelet aggregation effects. Regarding its antifungal activity, it has been tested against several fungal strains, reaching an IC50 value of 0.40 µM for Candida neoformans, which exceeds the expected performance by the control fungicide amphotericin B (0.50 µM) [39]. In addition, it was found to be effective for the control of black spot disease caused by Alternaria brassicicola that affects cabbage leaves, as it inhibits spore germination [40].
Other studies [39,44,45,46,47,48], have evaluated its cytotoxic capacity. It has been tested against 26 cell lines, being active in 21 of them with IC50 values ranging from 0.03 to 31.46 µM. The best results were obtained against human lung cancer (PC-12), and human colon cancer (HCT-116) cell lines with IC50 values of 0.03 and 0.22 µM respectively. These values were 10 times higher than those reported for the control substance, cisplatin, with 0.33 and 2.25 µM, respectively [46].
Its spermicidal capacity has been confirmed, presenting an EC50 value of 29.8 µM and being much more effective, selective and safe than the control substance, nonoxynol-9 (78.34 µM) [43]. In addition, the mixture of saponin 2 with gitonin, which differs from the first one in a hydroxyl group on the C-2 position of the aglycone, has shown a moderate capacity as a platelet aggregator, with 74% maximum induction percentage at a concentration of 250 µM [42]. Finally, a pharmaceutical preparation to prevent and relieve flu symptoms containing degalactotigonin (2) has been patented [41].
3.3.2. Timosaponin AIII (20)
Timosaponin AIII (20, Figure 11) has been identified in Y. gloriosa [6] and in Y. macrocarpa [37], as well as in other genera, and a considerable number of studies on this saponin have described five bioactivities (Table 7).
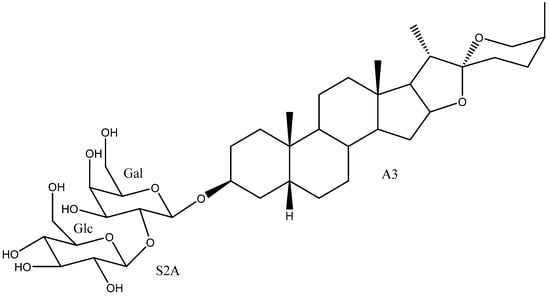
Figure 11.
Timosaponin AIII (20).

Table 7.
Timosaponin AIII (20) bioactivities.
Although timosaponin AIII (20) was not included in previous studies on the structure-activity relationship (SAR), this saponin and its epimer on C-25 (12) were synthesized [38] and tested on human epithelial cervical cancer cell (HeLa) and showed similar activity values. Timosaponin AIII (20) is notable for its exceptional cytotoxic and anti-inflammatory properties. In particular, it showed cytotoxic activity against the Hep3B liver cancer cell line (0.35 µM), where its IC50 was almost 100 times lower than that of the control substance 5-fluorouracil (22.99 µM) [65]. On the other hand, its anti-inflammatory capacity is quite potent too, being able to reduce the total number of inflammatory cells in the broncho-alveolar lavage fluid (BALF) by up to 64%, which is similar to that of the control compound, dexomethasone [59].
Timosaponin AIII (20) is included in a patent where it is proposed for cosmetic use as an active ingredient in a moisturizing cream [101] and in another patent where it is proposed to be included together with timosaponins BI and AI in a drug against cognitive disorders [102].
3.3.3. Timosaponin BII (50)
Timosaponin BII (50) (Figure 12) has been isolated from Y. glauca [11] and, it is the furostanic derivative of timosaponin AIII (20). Seven activities have been tested and are shown in Table 8.
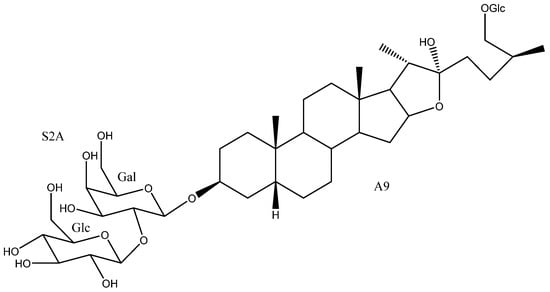
Figure 12.
Timosaponin BII (50).

Table 8.
Timosaponin BII (50) bioactivities.
Among the activities tested for this saponin, the most notable results were obtained with regard to its cytotoxic and anti-inflammatory capacities. Timosaponin BII (50) was tested against the HL-60 and A549 cell lines together with other furostanic saponins, showing IC50 values of 3.3 and 9.3 µM, respectively. These values are among the best in the study and are comparable to those of the control drug, cisplatin (1.7 and 2.1 µM) [11]. On the other hand, it has been demonstrated that timosaponin BII (50)-loaded nanofibers are capable of inhibiting human hepatocellular carcinoma SMMC-7721 cells, both in vivo and in vitro. More specifically, 10–15% volume weight percentages of timosaponin BII nanofibers can generate a potent growth inhibition against SMMC-7721 cells [76].
Timosaponins AIII (20) and BII (50) [15] were tested for their ability to selectively inhibit two molecules closely related to inflammatory metabolic pathways (2-COX and 5-LOX). Timosaponin BII (50) showed a better IC50 value for 2-COX inhibition (0.77 vs. 1.82 µM), whereas timosaponin AIII (20) proved to be more effective to inhibit 5-LOX (1.21 vs. 1.57 µM).
Timosaponin BII (50) has been patented as one of the main active compounds in a vaginal antifungal cream [105]. Moreover, together with other timosaponins including AIII (20) and N (90) it has been proposed as a preparation for the treatment of viral myocarditis [62].
4. Analytical Methods for the Steroidal Saponins in Yucca
As it was previously mentioned, Yucca saponins are of particular interest to the cosmetic, pharmaceutical and beverage industries as well as for the animal feed industry, being a highlighted feature their foaming activity. Consequently, Yucca extracts have been the focus of attention for numerous researchers. One of the main sources of saponins for commercial purposes and also one of the best known species is Y. schidigera [9,106]. Saponins present a rather complex structure, generally appearing as a mixture of isomers whose separation and quantification still represent a considerable challenge. Environmental conditions and plant development stage result in varying metabolite content [107]. These variations represent an additional difficulty when trying to determine quantity and quality of these compounds for commercial use. Conventional analytical methods used for this purpose include foam height measurements, spectrophotometric and gravimetric methods. Foam properties (a highly significant feature required in some chemical, foods, cosmetic and pharmaceutical processes) can be directly related to its saponin content [21]. These compounds are capable of reducing surface tension and improve the foamability of some aqueous solutions, even when added at low concentrations [108,109]. However, classifying saponins into ionic or non-ionic surfactants as the basis to determine their foaming properties does not seem to be the appropriate procedure, since these compounds are not necessarily sensitive to changes in ionic strength [110]. Besides, the volume and quality of foam produced by any method depends on several and complex factors [111]. Moreover, gravimetric methods base their measurement on butanol fractions, which presents a double-edged difficulty. On the one hand, butanol has no affinity with polar saponins and, on the other, this solvent may extract other kinds of compounds such as polyphenols or organic acids. This would result in an inaccurate determination of the saponin content [8,106].
Spectrophotometric methods, such as UV, have also failed to prove accurate, since saponins do not have a strong UV chromophore that can be detected by UV analysis [6]. In order to improve results, saponins have been derivatized to form colored substances. Thus, using this method, a spectrophotometric assay claimed to have determined the deglycosylation of steroidal saponins into sapogenin within the ruminal fluid of cattle that had been fed with Y. schidigera saponins. This assay. which was a modified version of the method described by Baccou et al. [112] showed that rumen bacteria are capable of degrading saponin into sapogenin or aglycone but cannot degrade the aglycone core. This colorimetric method is based on the reactions that take place with anisaldehyde, sulphuric acid and ethyl acetate to form chromophores [113].
Other chromatographic methods have been widely used for the analysis of saponins. Thus, a low-cost method that provides a preliminary assessment and verification of the saponins found in some commercial products containing Y. schidigera has been recently developed. This method uses TLC silica gel 60 F254, CHCl3:CH3OH:H2O (35:14:1) and 10% sulphuric acid-ethanol solution to improve plate visualization. The saponins became apparent under these chromatographic conditions when the retention factor was 0.38 or lower [114]. In another study, despite the difficulty to separate isomers, six pairs of 25 (R/S)-spirostanol saponin diastereomers from Y. schidigera were successfully separated through HPLC using a C30 column and their structure was unequivocally confirmed by NMR analysis [28]. On the other hand, 25 (R/S)-spirostanol saponin diastereomers have been successfully separated using supercritical fluid chromatography [115].
Mass spectrometry represents an effective detection method where selectivity and specificity can be improved by tandem mass spectrometry. Thus, in the last few years, different methods using tandem mass spectrometry have been developed for a quick and reliable determination of saponins [8]. It should also be mentioned that the use of mass spectrometry as a single technique for the determination of saponins presents some drawbacks, since it does not allow to differentiate isomers, and neither the position of the sugar chain link on the aglycone, nor the connectivity between sugar residues can be determined. Mass spectrometry and chromatographic methods are not often used as a single technique for the determination of these compounds, but rather in combination or coupled to other techniques. In this way, the detection and quantification of the nineteen steroidal saponins contained in a commercial syrup and in a bark’s extract from Y. schidigera have been carried out by LC-MS. Thus, twelve saponins were confirmed by comparing their fragmentation patterns against their corresponding standards and their spectrometric data, while the rest of the saponins were structurally proposed [8]. Furthermore, Montoro et al. developed a method for the quantitative analysis of the steroidal saponins in Y. gloriosa flowers real samples using HPLC coupled to tandem mass spectrometry [7]. LC-ESI-MS analysis has also been applied to the detection of the saponins contained in different Yucca aloifolia varieties imported into Egypt [116]. HPLC/ELSD is another accurate analysis and quantification method that has been applied to determine the total steroidal saponin content in Y. schidigera extracts. This is a fast and reliable technique that allows the quantification of molecules that do not contain a chromophore group, like in the case of saponins [106]. In the same way, Sastre et al. described a method for the analysis of the four major saponins in certain commercial samples of Y. schidigera by means of HPLC coupled to an evaporative light scattering detector (ELSD) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry [117]. Recently, Ruan and co-workers have identified 110 spirostanol saponins by multi-phase liquid chromatography (MPLC) combined with MS/MS. This approach improves chromatographic peak capacity and became the first method to provide a comprehensive characterization of spirostanol saponins from Y. schidigera [118].
NMR-based metabolomics is another reliable tool, which in combination with certain conventional quality control methods, can perform an effective initial screening of the raw materials in Yucca extracts [107]. For instance, a general approach to the identification and structural elucidation of the steroidal saponins in Y. filamentosa has been performed successfully. No full signal assignments were required to identify the compounds, since MS analyses were carried out firstly and then their results were supplemented with NMR data [27].
Yucca has proven to be a valuable source of steroidal saponins and its commercial extracts have been classified as GRAS by the US FDA. It is, therefore, widely used as a surfactant additive for the manufacturing of beverages, animal feed, cosmetics and some agricultural products. Despite its increasing applications, a method to evaluate and control the quality of the saponins contained in this plant genus and often incorporated to commercial products is still an unresolved issue. These metabolites are usually found in complex mixtures, structurally related and their separation is still a task [119]. Thereby, the recent methods described for their identification are based in different techniques coupled between them, being the most used, LC-MS technique. Moreover, the most of the studies have been carried out with extracts of Y. schidigera, being this species one of the major commercial sources of saponins.
5. Materials and Methods
The bibliographic searches were carried out mainly using the SciFinder database. The first search used the keywords ”Yucca”, “saponin”, “steroidal”, “spirostanic glycoside”. A second search using the respective Chemical Abstracts Service (CAS) numbers was carried out for the bioactivities of the 108 saponins described in the genus Yucca. The filter “biological study” was used. In order to retrieve information on the analytical methods used for the analysis of saponins, a third bibliographic search was carried out using the keywords “analysis”, “analytical methods”, “saponins” and “Yucca”.
6. Conclusions
The plants from the Yucca genus are generally recognized as a valuable source of steroidal saponins. This explains why their commercial extracts have been classified by the United States Food and Drug Administration as GRAS. Consequently, they have been widely used as surfactant additives by different industries, such as beverage, animal feed, cosmetics or agriculture.
A total of 108 saponins have been described in eight species of this genus. The most common structural features of Yucca saponins are a spirostanic aglycone with cis fusion of the A and B rings, and sugar chains of three or less sugar residues. This type of structure is associated to potent cytotoxic and antifungal activity as long as the aglycone is free from oxygenated functional groups.
Despite the increasing number of saponin applications, an accurate and reliable method to assess and control their quality is still to be fully developed. This is probably due to the difficulties to carry out their separation, since they are usually found in complex mixtures that are structurally related [119].
If we are to describe the state of things at present, we should mention that Y. schidigera is the species most often used for commercial extracts and that LC-MS is the most widely used technique for the identification, quantification and quality control of saponins in Yucca or Yucca extracts. The identification of saponins in Yucca genus is still a relatively unexplored field and further investigations would allow to know new structures and potential bioactivities. Furthermore, there is the need to develop methods that allow a reliable and suitable analysis of the saponins for quality control of commercial Yucca samples.
Author Contributions
Conceptualization, A.M.S.; methodology, A.G.D. and A.M.S.; resources, G.G.J. and A.G.D.; data curation, G.G.J., A.G.D. and A.M.S.; writing—original draft preparation, G.G.J. and A.G.D.; writing—review and editing, A.M.S. and A.G.D.; visualization, A.M.S.; supervision, A.M.S. and F.A.M.; project administration, F.A.M.; funding acquisition, F.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the ‘Ministerio de Economía y Competitividad’ (Project AGL2017-88083-R), Spain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Patel, S. Yucca: A medicinally significant genus with manifold therapeutic attributes. Nat. Prod. Bioprospect. 2012, 2, 231–234. [Google Scholar] [CrossRef]
- Clary, K.H.; Simpson, B.B. Sistemática y evolución del género Yucca (Agavaceae): Evidencias de análisis nunfológicos y moleculares. Bot. Sci. 1995, 56, 77–88. [Google Scholar] [CrossRef][Green Version]
- Simmons-Boyce, J.L.; Tinto, W.F. Steroidal Saponins and Sapogenins from the Agavaceae Family. Nat. Prod. Commun. 2007, 2, 99–114. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Skhirtladze, A.; Perrone, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Steroidal saponins from Yucca gloriosa L. rhizomes: LC-MS profiling, isolation and quantitative determination. Phytochemistry 2011, 72, 126–135. [Google Scholar] [CrossRef]
- Montoro, P.; Skhirtladze, A.; Perrone, A.; Benidze, M.; Kemertelidze, E.; Piacente, S. Determination of steroidal glycosides in Yucca gloriosa flowers by LC/MS/MS. J. Pharm. Biomed. Anal. 2010, 52, 791–795. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Pecio, Ł.; Stochmal, A.; Oleszek, W. Qualitative and quantitative analysis of steroidal saponins in crude extract and bark powder of Yucca schidigera Roezl. J. Agric. Food Chem. 2011, 59, 8058–8064. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; Oleszek, W. Saponins and phenolics of Yucca schidigera Roezl: Chemistry and bioactivity. Phytochem. Rev. 2005, 4, 177–190. [Google Scholar] [CrossRef]
- Report Insights. Yucca Schidigera Plant Extract Market 2020 by Manufacturers, Regions Analysis(Europe, Asia Pacific, North America and South America), Type and Application, Forecast to 2025; Report Insights: Maharashtra, India, 2020. [Google Scholar]
- Yokosuka, A.; Suzuki, T.; Tatsuno, S.; Mimaki, Y. Steroidal glycosides from the underground parts of Yucca glauca and their cytotoxic activities. Phytochemistry 2014, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Diab, Y.; Ioannou, E.; Emam, A.; Vagias, C.; Roussis, V. Desmettianosides A and B, bisdesmosidic furostanol saponins with molluscicidal activity from Yucca desmettiana. Steroids 2012, 77, 686–690. [Google Scholar] [CrossRef]
- Pérez, A.J.; Calle, J.M.; Simonet, A.M.; Guerra, J.O.; Stochmal, A.; Macías, F.A. Bioactive steroidal saponins from Agave offoyana flowers. Phytochemistry 2013, 95, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Miyakoshi, M.; Tamura, Y.; Masuda, H.; Mizutani, K.; Tanaka, O.; Ikeda, T.; Ohtani, K.; Kasai, R.; Yamasaki, K. Antiyeast steroidal saponins from Yucca schidigera (Mohave Yucca), a new anti-food-deteriorating agent. J. Nat. Prod. 2000, 63, 332–338. [Google Scholar] [CrossRef]
- Xie, L.; Lee, D.Y.-W.; Shang, Y.; Cao, X.; Wang, S.; Liao, J.; Zhang, T.; Dai, R. Characterization of spirostanol glycosides and furostanol glycosides from anemarrhenae rhizoma as dual targeted inhibitors of 5-lipoxygenase and Cyclooxygenase-2 by employing a combination of affinity ultrafiltration and HPLC/MS. Phytomedicine 2020, 77, 153284. [Google Scholar] [CrossRef]
- Oleszek, W.; Sitek, M.; Stochmal, A.; Piacente, S.; Pizza, C.; Cheeke, P. Steroidal saponins of Yucca schidigera Roezl. J. Agric. Food Chem. 2001, 49, 4392–4396. [Google Scholar] [CrossRef]
- Han, F.Y.; Song, X.Y.; Chen, J.J.; Yao, G.D.; Song, S.J. Timosaponin AIII: A novel potential anti-tumor compound from Anemarrhena asphodeloides. Steroids 2018, 140, 125–130. [Google Scholar] [CrossRef]
- Dragalin, I.P.; Gulya, A.P.; Krokhmalyuk, V.V.; Kintya, P.K. The structure of yuccoside E from Yucca filamentosa. Chem. Nat. Compd. 1975, 11, 772–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.R.; Zhang, Y.J. New Steroidal Saponins from the Leaves of Yucca elephantipes. Helv. Chim. Acta 2013, 96, 1807–1813. [Google Scholar] [CrossRef]
- Qu, L.; Wang, J.; Ruan, J.; Yao, X.; Huang, P.; Wang, Y.; Yu, H.; Han, L.; Zhang, Y.; Wang, T. Spirostane-type saponins obtained from Yucca schidigera. Molecules 2018, 23, 167. [Google Scholar] [CrossRef]
- Skhirtladze, A.; Plaza, A.; Montoro, P.; Benidze, M.; Kemertelidze, E.; Pizza, C.; Piacente, S. Furostanol saponins from Yucca gloriosa L. rhizomes. Biochem. Syst. Ecol. 2006, 34, 809–814. [Google Scholar] [CrossRef]
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Hydroxy steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 1993–1995. [Google Scholar] [CrossRef]
- Nakano, K.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T.; Midzuta, Y.; Hara, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. 12-Keto steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1991, 30, 633–636. [Google Scholar] [CrossRef]
- Kishor, N.; Sati, P.; Sakakibarat, J.; Kaiya, T. A spirostanol glycoside from Yucca aloifolia. Phytochemistry 1992, 31, 706–707. [Google Scholar] [CrossRef]
- Kishor, N. A new molluscicidal spirostanol glycoside of Yucca aloifolia. J. Nat. Prod. 1990, 53, 1557–1559. [Google Scholar] [CrossRef]
- Bahuguna, S.; Kishor, N.; Sati, O.P.; Sati, S.P.; Sakakibara, J.; Kaiya, T. A new spirostanol glycoside from Yucca aloifolia. J. Nat. Prod. 1991, 54, 863–865. [Google Scholar] [CrossRef]
- Plock, A.; Beyer, G.; Hiller, K.; Gründemann, E.; Krause, E.; Nimtz, M.; Wray, V. Application of MS and NMR to the structure elucidation of complex sugar moieties of natural products: Exemplified by the steroidal saponin from Yucca filamentosa L. Phytochemistry 2001, 57, 489–496. [Google Scholar] [CrossRef]
- Qu, L.; Ruan, J.; Wu, S.; Huang, P.; Yan, J.; Yu, H.; Zhang, Y.; Wang, T. Separation and bioactive assay of 25R/S-spirostanol saponin diastereomers from Yucca schidigera roezl (Mojave) stems. Molecules 2018, 23, 2562. [Google Scholar] [CrossRef] [PubMed]
- Kemertelidze, E.; Benidze, M.; Skhirtladze, A. Steroidal glycosides from the leaves of Yucca gloriosa L. Bull. Georg. Natl. Acad. Sci. 2011, 5, 158–163. [Google Scholar]
- Nakano, K.; Matsuda, E.; Tsurumi, K.; Yamasaki, T.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides of the flowers of Yucca gloriosa. Phytochemistry 1988, 27, 3235–3239. [Google Scholar] [CrossRef]
- Favel, A.; Kemertelidze, E.; Benidze, M.; Fallague, K.; Regli, P. Antifungal activity of steroidal glycosides from Yucca gloriosa L. Phyther. Res. 2005, 19, 158–161. [Google Scholar] [CrossRef]
- Kemertelidze, É.P.; Benidze, M.M.; Skhirtladze, A.V. Steroid compounds from Yucca gloriosa L. introduced into Georgia and their applications. Pharm. Chem. J. 2009, 43, 45–47. [Google Scholar] [CrossRef]
- Jin, Y.-L.; Kuk, J.-H.; Oh, K.-T.; Kim, Y.-J.; Piao, X.-L.; Park, R.-D. A new steroidal saponin, yuccalan, from the leaves of Yucca smalliana. Arch. Pharm. Res. 2007, 30, 543–546. [Google Scholar] [CrossRef]
- Eskander, J.; Sakka, O.K.; Harakat, D.; Lavaud, C. Steroidal saponins from the leaves of Yucca de-smetiana and their in vitro antitumor activity: Structure activity relationships through a molecular modeling approach. Med. Chem. Res. 2013, 22, 4877–4885. [Google Scholar] [CrossRef]
- Nakano, K.; Yamasaki, T.; Imamura, Y.; Murakami, K.; Takaishi, Y.; Tomimatsu, T. The steroidal glycosides from the caudex of Yucca gloriosa. Phytochemistry 1989, 28, 1215–1217. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.J.; Jacob, M.R.; Li, X.C.; Yang, C.R. Steroidal saponins from the stem of Yucca elephantipes. Phytochemistry 2008, 69, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Grishkovets, V.L.; Karpov, A.; Iksanovr, S.V.; Chirva, V.Y. A steroid glycoside from the seeds of Yucca macrocarpa. Chem. Nat. Compd. 1998, 34, 738–740. [Google Scholar] [CrossRef]
- Fang, M.; Gu, L.; Gu, G.; Fang, J. Facile synthesis and antitumor activities of timosaponin AIII and its analogs. J. Carbohydr. Chem. 2012, 31, 187–202. [Google Scholar] [CrossRef]
- Yang, C.R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.J.; Li, X.C. Antifungal activity of C-27 steroidal saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef]
- Lin, T.C.; Fan, M.C.; Wang, S.Y.; Huang, J.W. Identification of the Solanum nigrum extract component involved in controlling cabbage black leaf spot disease. J. Agric. Food Chem. 2011, 59, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Jiang, J.; Zhang, T.; Chen, F.; Zheng, Y.; Han, M.; Hu, Y. Application of Degalactotigonin in Preparing Pharmaceutical for Preventing and Treating Influenza Virus Infection 2018. Patent CN 107595868 A, 19 January 2018. [Google Scholar]
- Kang, L.-P.; Wu, K.-L.; Yu, H.-S.; Pang, X.; Liu, J.; Han, L.-F.; Zhang, J.; Zhao, Y.; Xiong, C.-Q.; Song, X.-B.; et al. Steroidal saponins from Tribulus terrestris. Phytochemistry 2014, 107, 182–189. [Google Scholar] [CrossRef]
- Chakraborty, D.; Maity, A.; Jha, T.; Mondal, N.B. Spermicidal and contraceptive potential of Desgalactotigonin: A prospective alternative of Nonoxynol-9. PLoS ONE 2014, 9, e107164. [Google Scholar] [CrossRef]
- Chakraborty, D.; Jain, C.K.; Maity, A.; Ghosh, S.; Choudhury, S.R.; Jha, T.; Majumder, H.K.; Mondal, N.B. Chenopodium album metabolites act as dual topoisomerase inhibitors and induce apoptosis in the MCF7 cell line. Medchemcomm 2016, 7, 837–844. [Google Scholar] [CrossRef]
- Le Tuan Anh, H.; Tran, P.T.; Thao, D.T.; Trang, D.T.; Dang, N.H.; Van Cuong, P.; Van Kiem, P.; Van Minh, C.; Lee, J.-H. Degalactotigonin, a steroidal glycoside from Solanum nigrum, induces apoptosis and cell cycle arrest via inhibiting the EGFR signaling pathways in pancreatic cancer cells. Biomed. Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsumagari, H.; Honbu, T.; Nohara, T. Cytotoxic activity of steroidal glycosides from solanum plants. Biol. Pharm. Bull. 2003, 26, 1198–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mimaki, Y.; Yokosuka, A.; Kuroda, M.; Sashida, Y. Cytotoxic activities and structure-cytotoxic relationships of steroidal saponins. Biol. Pharm. Bull. 2001, 24, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jia, Q.; Wu, M.S.; Xie, X.; Wang, Y.; Song, G.; Zou, C.Y.; Tang, Q.; Lu, J.; Huang, G.; et al. Degalactotigonin, a natural compound from Solanum nigrum L., inhibits growth and metastasis of osteosarcoma through GSK3β inactivation-mediated repression of the Hedgehog/gli1 pathway. Clin. Cancer Res. 2018, 24, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Mimaki, Y.; Hasegawa, F.; Yokosuka, A.; Sashida, Y.; Sakagami, H. Steroidal glycosides from the bulbs of Camassia leichtlinii and their cytotoxic activities. Chem. Pharm. Bull. 2001, 49, 726–731. [Google Scholar] [CrossRef]
- Jin, J.M.; Zhang, Y.J.; Yang, C.R. Spirostanol and furostanol glycosides from the fresh tubers of Polianthes tuberosa. J. Nat. Prod. 2004, 67, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, T.; Koyano, T.; Kowithayakorn, T.; Sakai, S.; Kawahara, N.; Goda, Y.; Yamaguchi, N.; Ishibashi, M. New chlorogenin hexasaccharide isolated from Agave fourcroydes with cytotoxic and cell cycle inhibitory activities. Bioorganic Med. Chem. 2004, 12, 3841–3845. [Google Scholar] [CrossRef]
- Candra, E.; Matsunaga, K.; Fujiwara, H.; Mimaki, Y.; Sashida, Y.; Yamakuni, T.; Ohizumi, Y. Two steroidal saponins from Camassia cusickii induce L1210 cell death through the apoptotic mechanism. Can. J. Physiol. Pharmacol. 2001, 79, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; Lin, Y.Y.; Kong, L.Y. Steroids from the roots of Asparagus officinalis and their cytotoxic activity. J. Integr. Plant Biol. 2008, 50, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Miyamoto, T.; Lacaille-Dubois, M.A. Bioactive steroidal saponins from Smilax medica. Planta Med. 2006, 72, 667–670. [Google Scholar] [CrossRef]
- Sy, L.K.; Lok, C.N.; Wang, J.Y.; Liu, Y.; Cheng, L.; Wan, P.K.; Leung, C.T.; Cao, B.; Kwong, W.L.; Chang, R.C.C.; et al. Identification of “sarsasapogenin-aglyconed” timosaponins as novel Aβ-lowering modulators of amyloid precursor protein processing. Chem. Sci. 2016, 7, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, J.; Wang, M.; Xia, X.; Dai, R.; Zhao, Y. Steroidal saponins and sapogenins from fenugreek and their inhibitory activity against α-glucosidase. Steroids 2020, 161, 108690. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Ha, H.; Lee, H.Y.; Kim, C.; Lee, J.H.; Son, K.H. Rat growth-hormone release stimulators from fenugreek seeds. Chem. Biodivers. 2008, 5, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, K.-H.; Lee, I.-S.; Park, J.Y.; Na, Y.-C.; Chung, W.-S.; Jang, H.-J. Apoptosis and G2/M cell cycle arrest induced by a timosaponin A3 from Anemarrhena asphodeloides Bunge on AsPC-1 pancreatic cancer cells. Phytomedicine 2019, 56, 48–56. [Google Scholar] [CrossRef]
- Park, B.K.; So, K.S.; Ko, H.J.; Kim, H.J.; Kwon, K.S.; Kwon, Y.S.; Son, K.H.; Kwon, S.Y.; Kim, H.P. Therapeutic Potential of the Rhizomes of Anemarrhena asphodeloides and Timosaponin A-III in an Animal Model of Lipopolysaccharide-Induced Lung Inflammation. Biomol. Ther. 2018, 26, 553–559. [Google Scholar] [CrossRef]
- Cong, Y.; Wang, L.; Peng, R.; Zhao, Y.; Bai, F.; Yang, C.; Liu, X.; Wang, D.; Ma, B.; Cong, Y. Timosaponin AIII induces antiplatelet and antithrombotic activity via Gq-mediated signaling by the thromboxane A2 receptor. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Kang, L.-P.; Zhang, J.; Cong, Y.; Li, B.; Xiong, C.-Q.; Zhao, Y.; Tan, D.-W.; Yu, H.; Yu, Z.-Y.; Cong, Y.-W.; et al. Steroidal glycosides from the rhizomes of Anemarrhena asphodeloides and their antiplatelet aggregation activity. Planta Med. 2012, 78, 611–616. [Google Scholar] [CrossRef]
- Xu, Y.; Xue, J.; Huang, R. Timosaponin Composition, and Preparation Method and Application Thereof in Treating Viral Miocarditis 2020. Patent CN 110882266 A, 17 March 2020. [Google Scholar]
- Wang, H.Q.; Liu, M.; Wang, L.; Lan, F.; Zhang, Y.H.; Xia, J.E.; Xu, Z.D.; Zhang, H. Identification of a novel BACE1 inhibitor, timosaponin A-III, for treatment of Alzheimer’s disease by a cell extraction and chemogenomics target knowledgebase-guided method. Phytomedicine 2020, 75, 153244. [Google Scholar] [CrossRef]
- Wang, N.; Xu, P.; Wang, X.; Yao, W.; Wang, B.; Wu, Y.; Shou, D. Timosaponin AIII attenuates inflammatory injury in AGEs-induced osteoblast and alloxan-induced diabetic osteoporosis zebrafish by modulating the RAGE/MAPK signaling pathways. Phytomedicine 2020, 75, 153247. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, J.; Sun, X.; Huang, X.; Li, L.; Liu, Q.; Song, S. Steroids from the rhizome of Anemarrhena asphodeloides and their cytotoxic activities. Bioorg. Med. Chem. Lett. 2016, 26, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-F.; Zhou, J.; Zhang, M.-J.; Zhang, M.; Huang, X.-F. Cytotoxic steroidal saponins from the rhizome of Anemarrhena asphodeloides. Steroids 2020, 155, 108557–108561. [Google Scholar] [CrossRef]
- Carpinteyro Díaz, A.E.; Herfindal, L.; Rathe, B.A.; Sletta, K.Y.; Vedeler, A.; Haavik, S.; Fossen, T. Cytotoxic saponins and other natural products from flowering tops of Narthecium ossifragum L. Phytochemistry 2019, 164, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Han, F.Y.; Chen, J.J.; Wang, W.; Zhang, Y.; Yao, G.D.; Song, S.J. Timosaponin AIII, a steroidal saponin, exhibits anti-tumor effect on taxol-resistant cells in vitro and in vivo. Steroids 2019, 146, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Zhao, W.R.; Xiao, Y.; Zhou, X.M.; Huang, C.; Shi, W.T.; Zhang, J.; Ye, Q.; Chen, X.L.; Tang, J.Y. Antiangiogenesis effect of timosaponin AIII on HUVECs in vitro and zebrafish embryos in vivo. Acta Pharmacol. Sin. 2020, 41, 260–269. [Google Scholar] [CrossRef]
- Nho, K.J.; Chun, J.M.; Kim, H.K. Induction of mitochondria-dependent apoptosis in HepG2 human hepatocellular carcinoma cells by timosaponin A-III. Environ. Toxicol. Pharmacol. 2016, 45, 295–301. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, X.; Xiang, L.; Huang, Y.; Wang, Z.; He, X. Furostanol saponins from Chinese onion induce G2/M cell-cycle arrest and apoptosis through mitochondria-mediate pathway in HepG2 cells. Steroids 2019, 148, 11–18. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Yi, X.; He, X. Potential anti-inflammatory steroidal saponins from the berries of Solanum nigrum L. (European Black Nightshade). J. Agric. Food Chem. 2017, 65, 4262–4272. [Google Scholar] [CrossRef]
- Nath, L.R.; Gorantla, J.N.; Thulasidasan, A.K.T.; Vijayakurup, V.; Shah, S.; Anwer, S.; Joseph, S.M.; Antony, J.; Veena, K.S.; Sundaram, S.; et al. Evaluation of uttroside B, a saponin from Solanum nigrum Linn, as a promising chemotherapeutic agent against hepatocellular carcinoma. Sci. Rep. 2016, 6, 36318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Li, X.C.; Yang, H.Y.; Wang, J.J.; Yang, C.R. Inhibitory effects of some steroidal saponins on human spermatozoa in vitro. Planta Med. 1996, 62, 130–132. [Google Scholar] [CrossRef]
- Chen, W.; Li, R.; Zhu, S.; Ma, J.; Pang, L.; Ma, B.; Du, L.; Jin, Y. Nasal timosaponin BII dually sensitive in situ hydrogels for the prevention of Alzheimer’s disease induced by lipopolysaccharides. Int. J. Pharm. 2020, 578, 119115. [Google Scholar] [CrossRef]
- Huo, Z.; Qiu, Y.; Chu, Z.; Yin, P.; Lu, W.; Yan, Y.; Wan, N.; Chen, Z. Electrospinning preparation of Timosaponin B-II-loaded PLLA nanofibers and their antitumor recurrence activities in vivo. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Ji, K.-Y.; Kim, K.M.; Kim, Y.H.; Im, A.-R.; Lee, J.Y.; Park, B.; Na, M.; Chae, S. The enhancing immune response and anti-inflammatory effects of Anemarrhena asphodeloides extract in RAW 264.7 cells. Phytomedicine 2019, 59, 152789–152797. [Google Scholar] [CrossRef]
- Yuan, Y.L.; Lin, B.Q.; Zhang, C.F.; Cui, L.L.; Ruan, S.X.; Yang, Z.L.; Li, F.; Ji, D. Timosaponin B-II ameliorates palmitate-induced insulin resistance and inflammation via IRS-1/PI3K/Akt and IKK/NF- κ B pathways. Am. J. Chin. Med. 2016, 44, 755–769. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Li, X.; Ma, Y.; Liu, L.; Guo, L.; Liu, Q.; Sun, Y. Optimization of extraction for Anemarrhena asphodeloides Bge. using silica gel-based vortex-homogenized matrix solid-phase dispersion and rapid identification of antioxidant substances. J. Sep. Sci. 2020, 43, 2180–2192. [Google Scholar] [CrossRef] [PubMed]
- Du, M.J.; Chen, J.P.; Lian, B.W.; Mei, W.J.; Yu, J.X.; Chen, L.S.; Deng, S.B.; Zhong, Y.M.; Yu, C.Q. Cardioprotective effects of timosaponin B-II isolated from Anemarrhena rhizome in a zebrafish model. Pharmazie 2020, 75, 201–204. [Google Scholar] [CrossRef]
- Zhang, W.Y.; Yu, Y.; Yan, L.L.; Li, C.; Han, J.Y.; Qin, Z.F.; Dai, Y.; Yao, Z.H.; Zhou, H.; Yao, X.S. Discovery of cardio-protective constituents of Gualou Xiebai Decoction, a classical traditional Chinese medicinal formula. Phytomedicine 2019, 54, 318–327. [Google Scholar] [CrossRef]
- Deng, X.Y.; Chen, J.J.; Li, H.Y.; Ma, Z.Q.; Ma, S.P.; Fu, Q. Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem. Biol. Interact. 2015, 240, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xue, J.; Huang, R. Timosaponin Composition and Application Thereof in Treating Viral Myocarditis 2019. Patent CN 110623965 A, 31 December 2019. [Google Scholar]
- Kim, Y.S.; Suh, W.S.; Park, K.J.; Choi, S.U.; Lee, K.R. Allimacrosides A–E, new steroidal glycosides from Allium macrostemon Bunge. Steroids 2017, 118, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, G.; Wang, N.; Yang, M.; Wang, Z.; Wang, X.; Yao, X. New furostanol saponins from the bulbs of Allium macrostemon Bunge and their cytotoxic activity. Pharmazie 2007, 62, 544–548. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, J.; Fu, Q.; Cheng, L.; Wu, L.; Zhang, W.; Zhang, Y.; Jin, Y.; Zhang, C. Anti-inflammatory activities of compounds isolated from the rhizome of Anemarrhena asphodeloides. Molecules 2018, 23, 2631. [Google Scholar] [CrossRef]
- Yao, X.; Peng, J. Identification of New Constituents in Allium macrostemon Bunge and A. chinense G. Don Bulbs and Their Anti-Blood Platelet Agglutination Activities 1995. Patent CN 1102186A, 3 May 1995. [Google Scholar]
- Mimaki, Y.; Kameyama, A.; Kuroda, M.; Sashida, Y.; Hirano, T.; Oka, K.; Koike, K.; Nikaido, T. Steroidal glycosides from the underground parts of Hosta plantaginea var. japonica and their cytostatic activity on leukaemia HL-60 cells. Phytochemistry 1997, 44, 305–310. [Google Scholar] [CrossRef]
- Mimaki, Y.; Kuroda, M.; Kameyama, A.; Yokosuka, A.; Sashida, Y. Steroidal saponins from the rhizomes of Hosta sieboldii and their cytostatic activity on HL-60 cells. Phytochemistry 1998, 48, 1361–1369. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, S.Y.; Moon, E.; Lee, M.K.; Lee, K.R. Steroidal constituents from the leaves of Hosta longipes and their inhibitory effects on nitric oxide production. Bioorganic Med. Chem. Lett. 2013, 23, 1771–1775. [Google Scholar] [CrossRef]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef]
- Uematsu, Y.; Hirata, K.; Saito, K.; Kudo, I. Spectrophotometric determination of saponin in Yucca extract used as food additive. J. AOAC Int. 2000, 83, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Paray, B.A.; El-Basuini, M.F.; Alagawany, M.; Albeshr, M.F.; Farah, M.A.; Dawood, M.A.O. Yucca schidigera usage for healthy aquatic animals: Potential roles for sustainability. Animals 2021, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Fayed, W.M.A.; Khalil, R.H.; Sallam, G.R.; Mansour, A.T.; Elkhayat, B.K.; Omar, E.A. Estimating the effective level of Yucca schidigera extract for improvement of the survival, haematological parameters, immunological responses and Water quality of European seabass juveniles (dicentrarchus labrax). Aquac. Rep. 2019, 15, 100208–100214. [Google Scholar] [CrossRef]
- Sun, D.; Shi, B.; Tong, M.; Yan, S. Improved performance and immunological responses as a result of dietary Yucca schidigera extract supplementation in broilers. Ital. J. Anim. Sci. 2018, 17, 511–517. [Google Scholar] [CrossRef]
- Cheeke, P.R. Actual and Potential Applications of Yucca Schidigera and Quillaja Saponaria Saponins in Human and Animal Nutrition. In Saponins in Food, Feedstuffs and Medicinal Plants; Springer: Dordrecht, The Netherlands, 2000; pp. 241–254. [Google Scholar]
- Cheeke, P.R.; Piacente, S.; Oleszek, W. Anti-inflammatory and anti-arthritic effects of Yucca schidigera: A review. J. Inflamm. 2006, 3, 1–7. [Google Scholar] [CrossRef]
- Franco Ospina, L.A.; Castro Guerrero, J.P.; Ocampo Buendía, Y.C.; Pájaro Bolívar, I.B.; Díaz Castillo, F. Actividad antiinflamatoria, antioxidante y antibacteriana de dos especies del género Tabebuia. Rev. Cuba Plantas Med. 2013, 18, 34–46. [Google Scholar]
- Guo, C.; Li, L.; Yang, X.; Meng, Z.; Li, F.; Zhang, C.; Yang, Z. Protective effects of timosaponin B-II on high glucose-induced apoptosis in human umbilical vein endothelial cells. Environ. Toxicol. Pharmacol. 2014, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Arencibia, D.F.; Rosario, L.A.; Curveco, D.L. Principal assays that to determine the cytotoxicity of a substance, some considerations and their utility. Retell Rev. Toxicol. Linea 2009, 42–52. [Google Scholar]
- Chae, S.U.; Lee, M.Y. Cosmetics Containing Anemarrhena asphodeloides Extract 2014. Patent WO 2014092325 A1, 19 June 2014. [Google Scholar]
- Liu, M.; Bi, X.; Hong, Z.; Fan, C.; Wang, Z.; Xu, X.; Gao, S. Application of Timosaponin BI, Timosaponin IA, and Timosaponin AIII in the Preparation of Anti Cognitive Disorder Drugs 2019. Patent CN 110585224 A, 20 December 2019. [Google Scholar]
- Olas, B.; Urbańska, K.; Bryś, M. Saponins as modulators of the blood coagulation system and perspectives regarding their use in the prevention of venous thromboembolic incidents. Molecules 2020, 25, 5171. [Google Scholar] [CrossRef]
- Nian, S.-H.; Li, H.-J.; Liu, E.-H.; Li, P. Comparison of α-glucosidase inhibitory effect and bioactive constituents of Anemarrhenae Rhizoma and Fibrous Roots. J. Pharm. Biomed. Anal. 2017, 145, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Lian, B.; Liu, Y. Gynecological Compound Composition Based on Clotrimazole and Timosaponin B2 2019. Patent CN 109431968 A, 8 March 2019. [Google Scholar]
- Tenon, M.; Feuillère, N.; Roller, M.; Birtić, S. Rapid, cost-effective and accurate quantification of Yucca schidigera Roezl. steroidal saponins using HPLC-ELSD method. Food Chem. 2017, 221, 1245–1252. [Google Scholar] [CrossRef]
- Suzuki, R.; Ohno, H.; Murakami, T.; Shirataki, Y. Improving quality control of yucca extracts used as food additives by screening antimicrobial activity using NMR metabolomics. J. Nat. Med. 2020, 74, 306–310. [Google Scholar] [CrossRef]
- Do Canto, G.S.; Treter, J.; Yang, S.; Borré, G.L.; Peixoto, M.P.G.; Ortega, G.G. Evaluation of foam properties of saponin from Ilex paraguariensis A. St. Hil. (Aquifoliaceae) fruits. Braz. J. Pharm. Sci. 2010, 46, 237–243. [Google Scholar] [CrossRef][Green Version]
- San Martín, R.; Briones, R. Quality control of commercial quillaja (Quillaja saponaria Molina) extracts by reverse phase HPLC. J. Sci. Food Agric. 2000, 80, 2063–2068. [Google Scholar] [CrossRef]
- Böttcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Ross, J.; Miles, G.D. An apparatus for comparison of foaming properties of soaps and detergents. Oil Soap 1941, 18, 99–102. [Google Scholar] [CrossRef]
- Baccou, J.C.; Lambert, F.; Sauvaire, Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst 1977, 102, 458–465. [Google Scholar] [CrossRef]
- Wang, Y.; McAllister, T.A. A modified spectrophotometric assay to estimate deglycosylation of steroidal saponin to sapogenin by mixed ruminal microbes. J. Sci. Food Agric. 2010, 90, 1811–1818. [Google Scholar] [CrossRef]
- Sastre, F.; Ferreira, F.; Pedreschi, F. TLC fingerprint of phenolics and saponins in commercial extracts of Yucca schidigera Roezl. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 698–701. [Google Scholar] [CrossRef]
- Zhao, Y.; McCauley, J.; Pang, X.; Kang, L.; Yu, H.; Zhang, J.; Xiong, C.; Chen, R.; Ma, B. Analytical and semipreparative separation of 25 (R/S)-spirostanol saponin diastereomers using supercritical fluid chromatography. J. Sep. Sci. 2013, 36, 3270–3276. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Basam, S.M.; El-Naggar, E.-M.B.A.; Marzouk, H.S.; El-Hawary, S. LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci. Rep. 2020, 10, 17778–17792. [Google Scholar] [CrossRef]
- Sastre, F.; Ferreira, F.; Pedreschi, F. MALDI-TOF mass spectrometry and reversed-phase HPLC-ELSD chromatography for structural and quantitative studies of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Pharm. Biomed. Anal. 2016, 120, 270–282. [Google Scholar] [CrossRef]
- Ruan, J.; Qu, L.; Zhao, W.; Gao, C.; Huang, P.; Zheng, D.; Han, L.; Yu, H.; Zhang, Z.; Zhang, Y.; et al. Identification and Structural Analysis of Spirostanol Saponin from Yucca schidigera by Integrating Silica Gel Column Chromatography and Liquid Chromatography/Mass Spectrometry Analysis. Molecules 2020, 25, 3848. [Google Scholar] [CrossRef] [PubMed]
- Sastre, F.; Ferreira, F.; Pedreschi, F. A systematic approach for the chromatographic fractionation and purification of major steroid saponins in commercial extracts of Yucca schidigera Roezl. J. Chromatogr. B 2017, 1046, 235–242. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).