The Early Adhesion Effects of Human Gingival Fibroblasts on Bovine Serum Albumin Loaded Hydrogenated Titanium Nanotube Surface
Abstract
:1. Introduction
2. Results
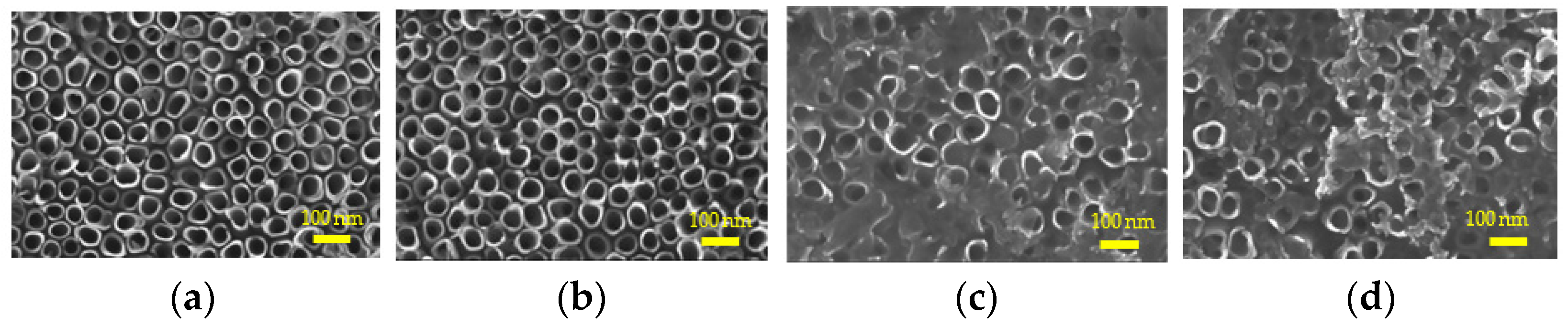
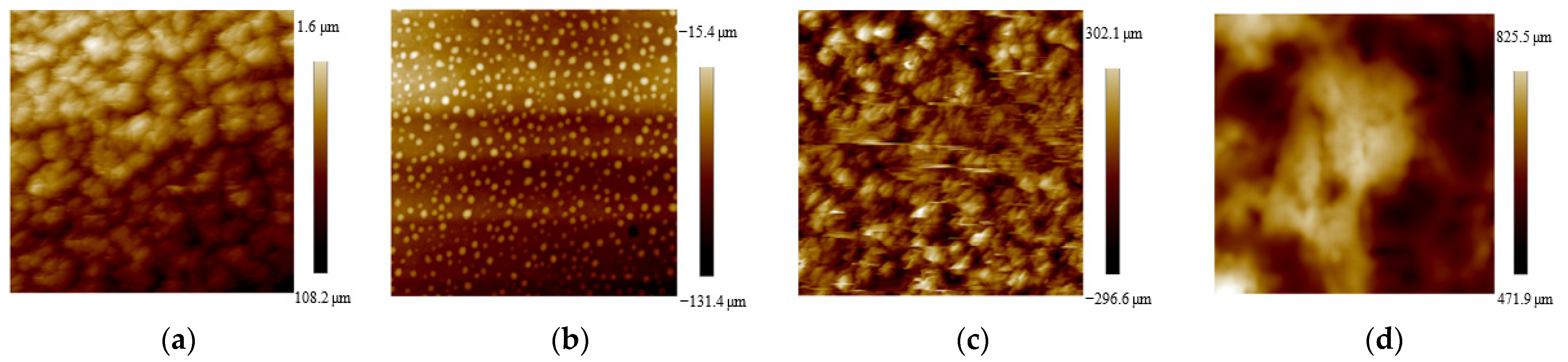
2.1. Sample Characterization
2.2. The Capability of Protein-Loading and Protein-Releasing
2.3. Response of HGFs to the Substrates
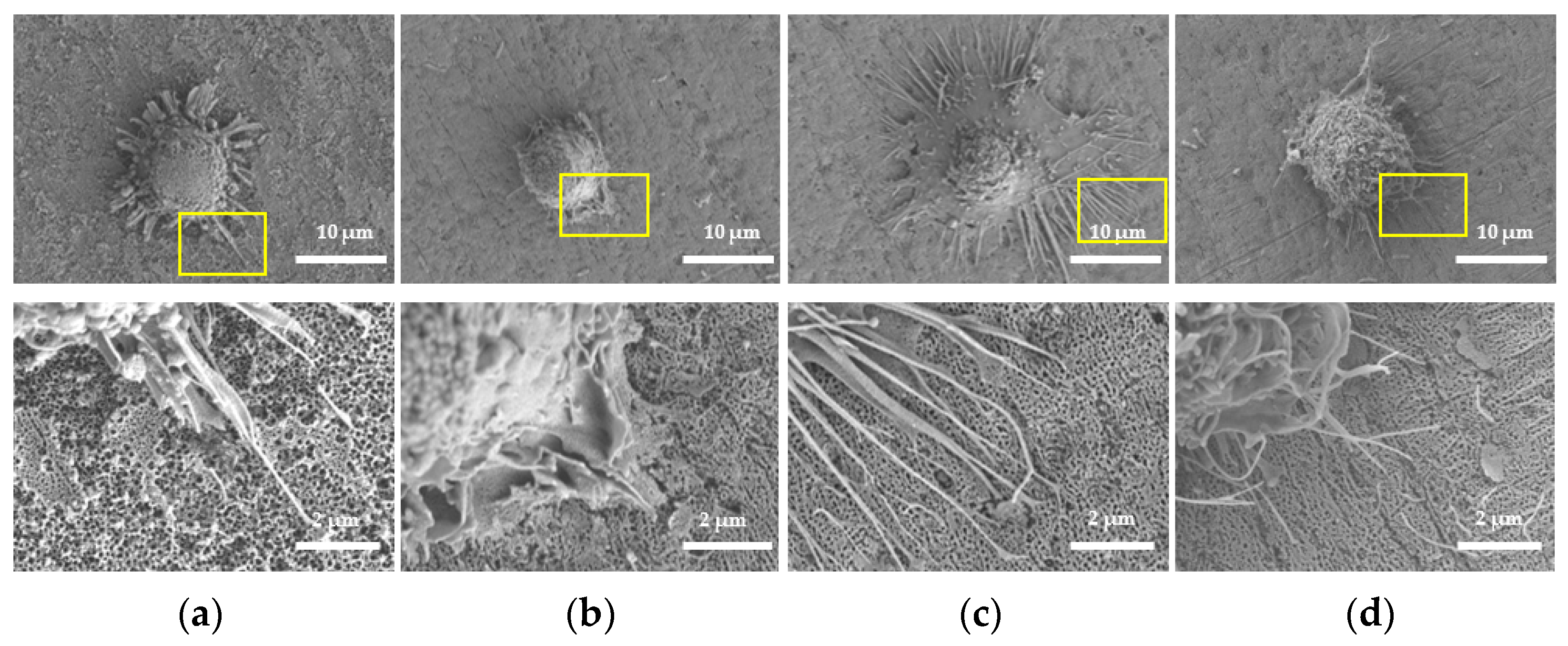
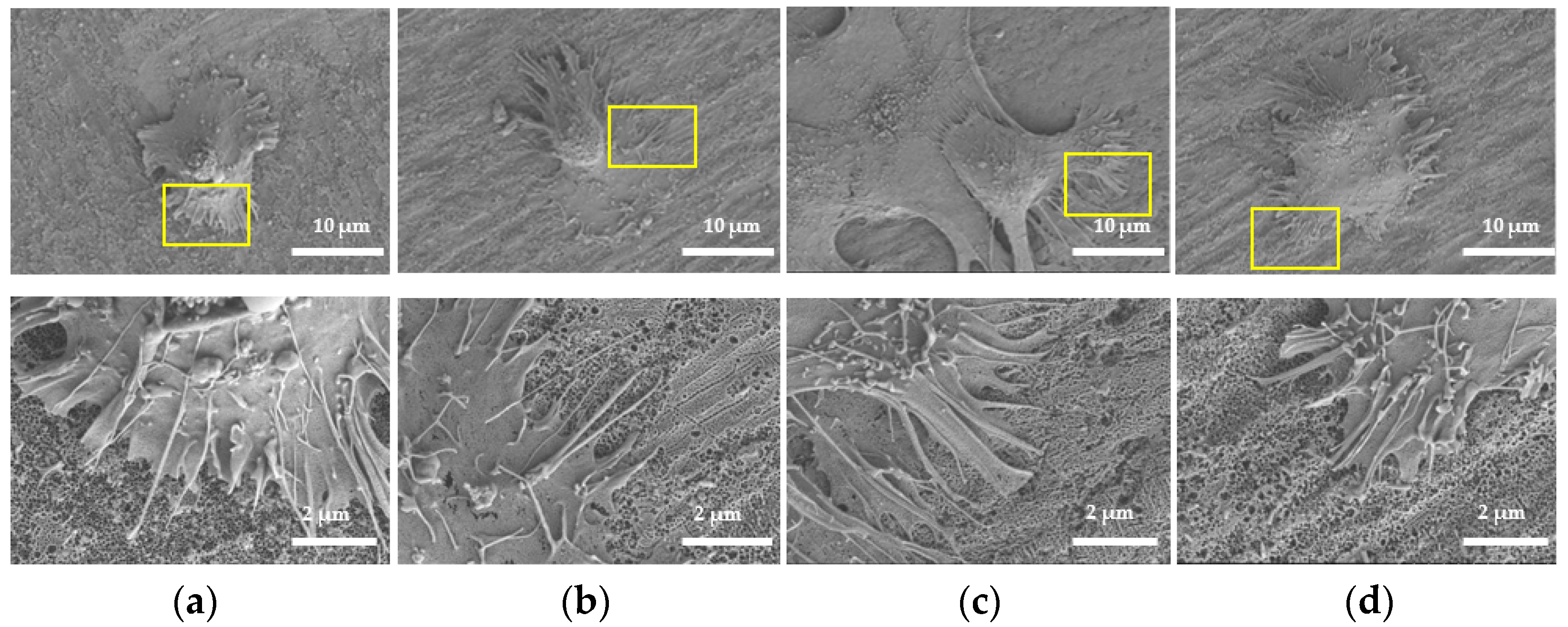
2.3.1. Morphology of HGFs
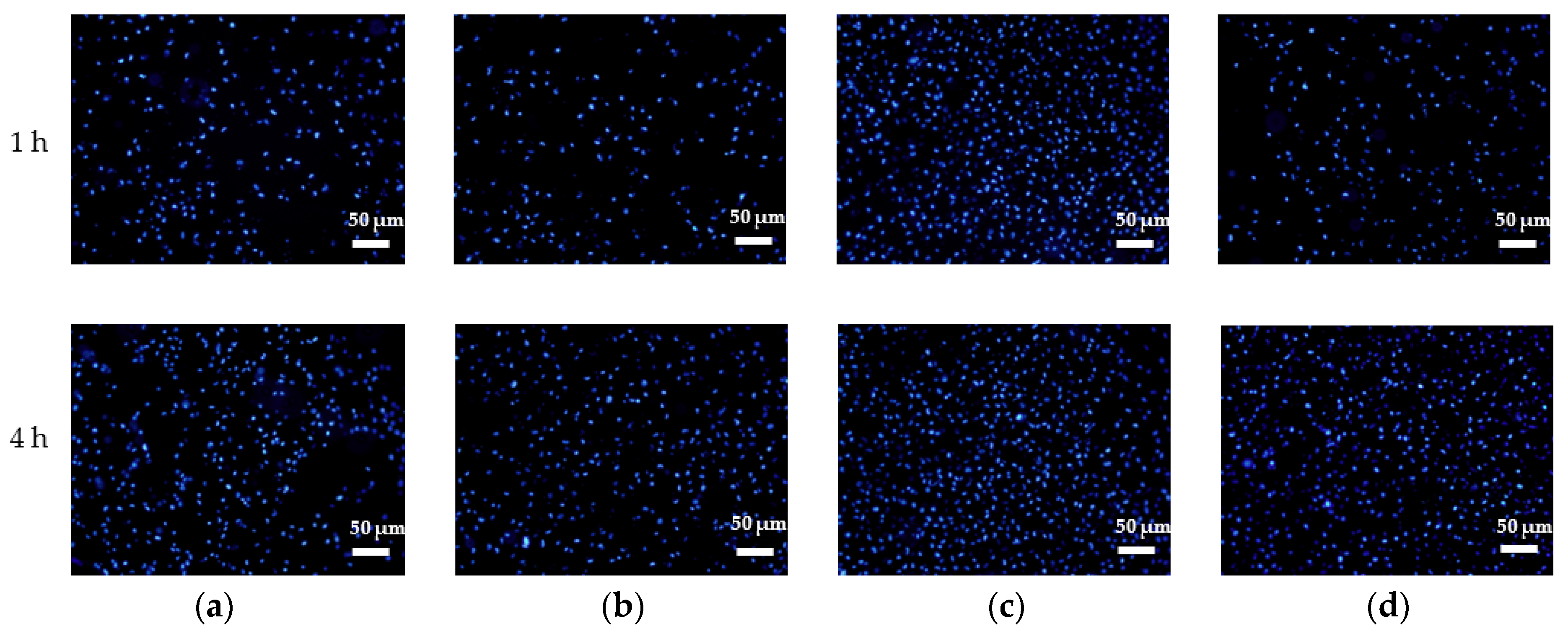
2.3.2. Assay of Adhered HGs
2.3.3. RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Fabrication of the TNTs and H2-TNTs Arrays
4.2. Loading BSA into the TNTs and H2-TNTs
4.3. Characterizations of Samples
4.4. BSA Releasing Assay
4.5. Cell Culture
4.6. Cell Morphology
4.7. Early Adhesion of HGFs
4.8. Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhuang, L.F.; Watt, R.M.; Mattheos, N.; Si, M.-S.; Lai, H.-C.; Lang, N.P. Periodontal and peri-implant microbiota in patients with healthy and inflamed periodontal and periimplant tissues. Clin. Oral Implants Res. 2016, 27, 13–21. [Google Scholar] [CrossRef]
- Takamori, Y.; Atsuta, I.; Nakamura, H.; Sawase, T.; Koyano, K.; Hara, Y. Histopathological comparison of the onset of peri-implantitis and periodontitis in rats. Clin. Oral Implants Res. 2017, 28, 163–170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implants Dent. Relat. Res. 2015, 18, 618–634. [Google Scholar] [CrossRef]
- Igarashi, K.; Nakahara, K.; Kobayashi, E.; Watanabe, F.; Haga-Tsujimura, M. Hard and soft tissue responses to implant made of three different materials with microgrooved collar in a dog model. Dent. Mater. J. 2018, 37, 964–972. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, C.; Gu, Y.; Shi, J.-Y.; Mo, J.-J.; Qian, S.-J.; Qiao, S.-C.; Lai, H.-C. The responses of human gingival fibroblasts to magnesium-doped titanium. J. Biomed. Mater. Res. Part A 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Nevárez-Martínez, M.C.; Mazierski, P.; Kobylański, M.P.; Szczepańska, G.; Trykowski, G.; Malankowska, A.; Kozak, M.; Espinoza-Montero, P.J.; Zaleska-Medynska, A. Growth, Structure, and Photocatalytic Properties of Hierarchical V2O5-TiO2 Nanotube Arrays Obtained from the One-step Anodic Oxidation of Ti-V Alloys. Molecules 2017, 22, 580. [Google Scholar] [CrossRef] [Green Version]
- Shuang, S.; Zhang, Z. The Effect of Annealing Treatment and Atom Layer Deposition to Au/Pt Nanoparticles-Decorated TiO2 Nanorods as Photocatalysts. Molecules 2018, 23, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and develop-mental challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Li, S.; Lai, R.; Zhou, Z.; Zhang, Y.; Zhou, L. Effects of titania nanotubes with or without bovine serum albumin loaded on human gingival fibroblasts. Int. J. Nanomed. 2014, 9, 1185–1198. [Google Scholar] [CrossRef]
- Mei, S.; Wang, H.; Wang, W.; Tong, L.; Pan, H.; Ruan, C.; Ma, Q.; Liu, M.; Yang, H.; Zhang, L.; et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials 2014, 35, 4255–4265. [Google Scholar] [CrossRef]
- Ma, Q.; Mei, S.; Ji, K.; Zhang, Y.; Chu, P.K. Immobilization of Ag nanoparticles/FGF-2 on a modified titanium implant surface and improved human gingival fibroblasts behavior. J. Biomed. Mater. Res. Part A 2011, 98, 274–286. [Google Scholar] [CrossRef]
- Lu, R.; Wang, C.; Wang, X.; Wang, Y.; Wang, N.; Chou, J.; Li, T.; Zhang, Z.; Ling, Y.; Chen, S. Effects of hydrogenated TiO2 nanotube arrays on protein adsorption and compatibility with osteoblast-like cells. Int. J. Nanomed. 2018, 13, 2037–2049. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, X.; Lu, R.; Gao, S.; Ling, Y.; Chen, S. Responses of human gingival fibroblasts to super hydrophilic hydrogenated titanium dioxide nanotubes. Colloids Surf. B Biointerfaces 2021, 198, 111489. [Google Scholar] [CrossRef]
- Klok, O.; Munoz, A.I.; Mischler, S. An Overview of Serum Albumin Interactions with Biomedical Alloys. Materials 2020, 13, 4858. [Google Scholar] [CrossRef]
- Roufegarinejad, L.; Jahanban-Esfahlan, A.; Sajed-Amin, S.; Panahi-Azar, V.; Tabibiazar, M. Molecular interactions of thymol with bovine serum albumin: Spectroscopic and molecular docking studies. J. Mol. Recognit. 2018, 31, e2704. [Google Scholar] [CrossRef]
- Valero, C.V.; Juan, A.O.; Muñoz, A.I. Adsorption of bovine serum albumin on CoCrMo surface: Effect of temperature and protein concentration. Colloids Surf. B Biointerfaces 2010, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Panahi-Azar, V. Interaction of glutathione with bovine serum albumin: Spectroscopy and molecular docking. Food Chem. 2016, 202, 426–431. [Google Scholar] [CrossRef]
- Dee, K.C.; Puleo, D.A.; Bizios, R. An Introduction to Tissue-Biomaterial Interactions; Wiley-Liss, Inc.: Hoboken, NJ, USA, 2002; Chapter 2; pp. 15–35. [Google Scholar]
- Planell, J.A.; Navarro, M.; Altankov, G.; Aparicio, C.; Engel, E.; Gil, J.; Ginebra, M.-P.; Lacroix, D. Materials Surface Effects on Biological Interactions; Springer: Berlin/Heidelberg, Germany, 2010; pp. 233–252. [Google Scholar]
- Kim, D.; Macak, J.M.; Schimidt-Stein, F.; Schmuki, P. Capillary effects, wetting behavior and photo-induced tube filling of TiO2 nanotube layers. Nanotechnology 2008, 19, 305710. [Google Scholar] [CrossRef]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef] [Green Version]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of protein adsorption in the bio corrosion of metallic implants—A review. Colloids Surf. B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef]
- Khosa, M.; Ullah, A. Mechanistic insight into protein supported biosorption complemented by kinetic and thermodynamics perspectives. Adv. Colloid Interface Sci. 2018, 261, 28–40. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, H.; Su, Y.; Qiao, L. Albumin adsorption on CoCrMo alloy surfaces. Sci. Rep. 2015, 5, 18403. [Google Scholar] [CrossRef] [Green Version]
- Silva-Bermudez, P.; Rodil, S. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Pierres, A.; Benoliel, A.-M.; Touchard, D.; Bongrand, P. How Cells Tiptoe on Adhesive Surfaces before Sticking. Biophys. J. 2008, 94, 4114–4122. [Google Scholar] [CrossRef] [Green Version]
- Pierres, A.; Benoliel, A.M.; Bongrand, P. Cell fitting to adhesive surfaces: A prerequisite to firm attachment and subsequent events. Eur. Cells Mater. 2002, 3, 31–45. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J.; Sheykholeslami, S.O.R.; Moosavifar, M. Biocompatibility assessment of graphene oxide-hydroxyapatite coating applied on TiO2 nano-tubes by ultrasound-assisted pulse electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 87, 10–21. [Google Scholar] [CrossRef]
- Beningo, K.A.; Dembo, M.; Kaverina, I.; Small, J.V.; Wang, Y.-L. Nascent Focal Adhesions Are Responsible for the Generation of Strong Propulsive Forces in Migrating Fibroblasts. J. Cell Biol. 2001, 153, 881–888. [Google Scholar] [CrossRef] [Green Version]
- Margel, S.; Vogler, E.A.; Firment, L.; Watt, T.; Haynie, S.; Sogah, D.Y. Peptide, protein, and cellular interactions with self-assembled monolayer model surfaces. J. Biomed. Mater. Res. 1993, 27, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O.; Yamada, K. Fibronectins: Multifunctional modular glycoproteins. J. Cell Biol. 1982, 95, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seong, J.; Tajik, A.; Sun, J.; Guan, J.-L.; Humphries, M.J.; Craig, S.E.; Shekaran, A.; García, A.J.; Lu, S.; Lin, M.; et al. Distinct biophysical mechanisms of focal adhesion kinase mechanoactivation by different extracellular matrix proteins. Proc. Natl. Acad. Sci. USA 2013, 110, 19372–19377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, Y.-R.V.; Tseng, K.-F.; Lai, H.-Y.; Lin, C.-H.; Lee, O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner. Res. 2011, 26, 730–738. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Linthicum, W.; Man, K.; He, X.; Wen, Q.; Rojanasakul, L.W.; Rojanasakul, Y.; Yang, Y. Substrate Stiffness-Dependent Carbon Nanotube-Induced Lung Fibrogenesis. Nano Lett. 2019, 19, 5443–5451. [Google Scholar] [CrossRef]
- Zamir, E.; Geiger, B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 2001, 114, 3583–3590. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef] [Green Version]






| NT | BNT | HNT | BHNT | |
|---|---|---|---|---|
| C1s | 24.05 | 44.72 | 20.7 | 44.72 |
| Ti2p | 23.89 | 10.07 | 25.97 | 10.07 |
| O1s | 52.06 | 31.46 | 53.33 | 30.55 |
| S2p | – | 0.86 | – | 0.67 |
| N1s | – | 12.9 | – | 11.6 |
| Groups | Roughness (nm) | Contact Angles (°) |
|---|---|---|
| NT | 98.0 ± 4.8 | 46.2 ± 3.2 |
| BNT | 122.1 ± 5.2 | 36.4 ± 3.1 a |
| HNT | 98.7 ± 3.4 | 7.5 ± 0.6 ab |
| BHNT | 148.7 ± 1.5 a | 9.4 ± 0.3 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Lu, R.; Liu, J.; Wang, X.; Dong, H.; Chen, S. The Early Adhesion Effects of Human Gingival Fibroblasts on Bovine Serum Albumin Loaded Hydrogenated Titanium Nanotube Surface. Molecules 2021, 26, 5229. https://doi.org/10.3390/molecules26175229
Sun Y, Lu R, Liu J, Wang X, Dong H, Chen S. The Early Adhesion Effects of Human Gingival Fibroblasts on Bovine Serum Albumin Loaded Hydrogenated Titanium Nanotube Surface. Molecules. 2021; 26(17):5229. https://doi.org/10.3390/molecules26175229
Chicago/Turabian StyleSun, Yuchen, Ran Lu, Jingming Liu, Xin Wang, Haitao Dong, and Su Chen. 2021. "The Early Adhesion Effects of Human Gingival Fibroblasts on Bovine Serum Albumin Loaded Hydrogenated Titanium Nanotube Surface" Molecules 26, no. 17: 5229. https://doi.org/10.3390/molecules26175229
APA StyleSun, Y., Lu, R., Liu, J., Wang, X., Dong, H., & Chen, S. (2021). The Early Adhesion Effects of Human Gingival Fibroblasts on Bovine Serum Albumin Loaded Hydrogenated Titanium Nanotube Surface. Molecules, 26(17), 5229. https://doi.org/10.3390/molecules26175229





