Davidone C Induces the Death of Hepatocellular Carcinoma Cells by Promoting Apoptosis and Autophagy
Abstract
:1. Introduction
2. Results
2.1. Effects of Davidone C on Cell Viability in HepG2 and Bel-7402 Cells
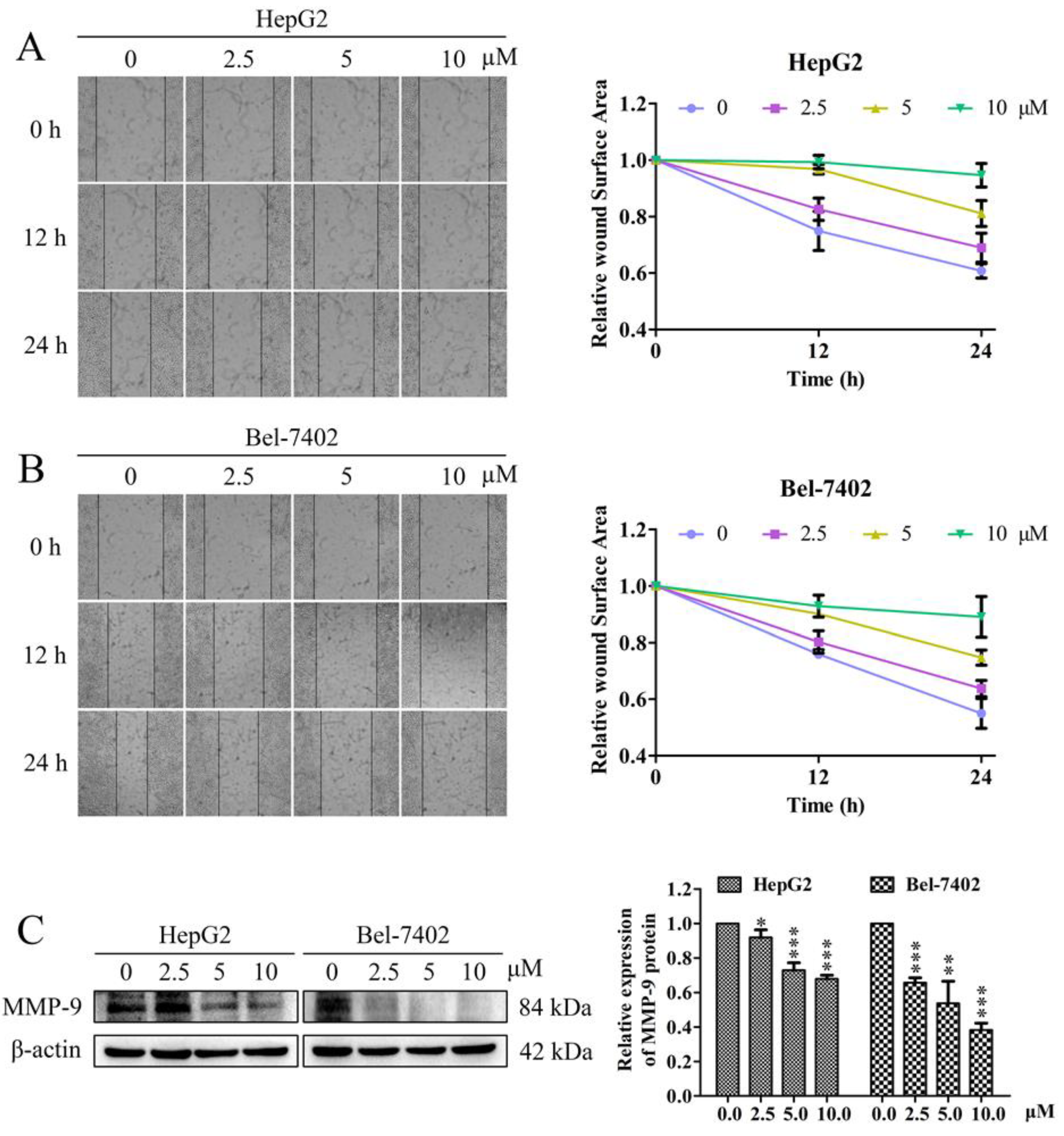
2.2. Effects of Davidone C on Cell Migration and Related Protein Expression in HepG2 and Bel-7402 Cells
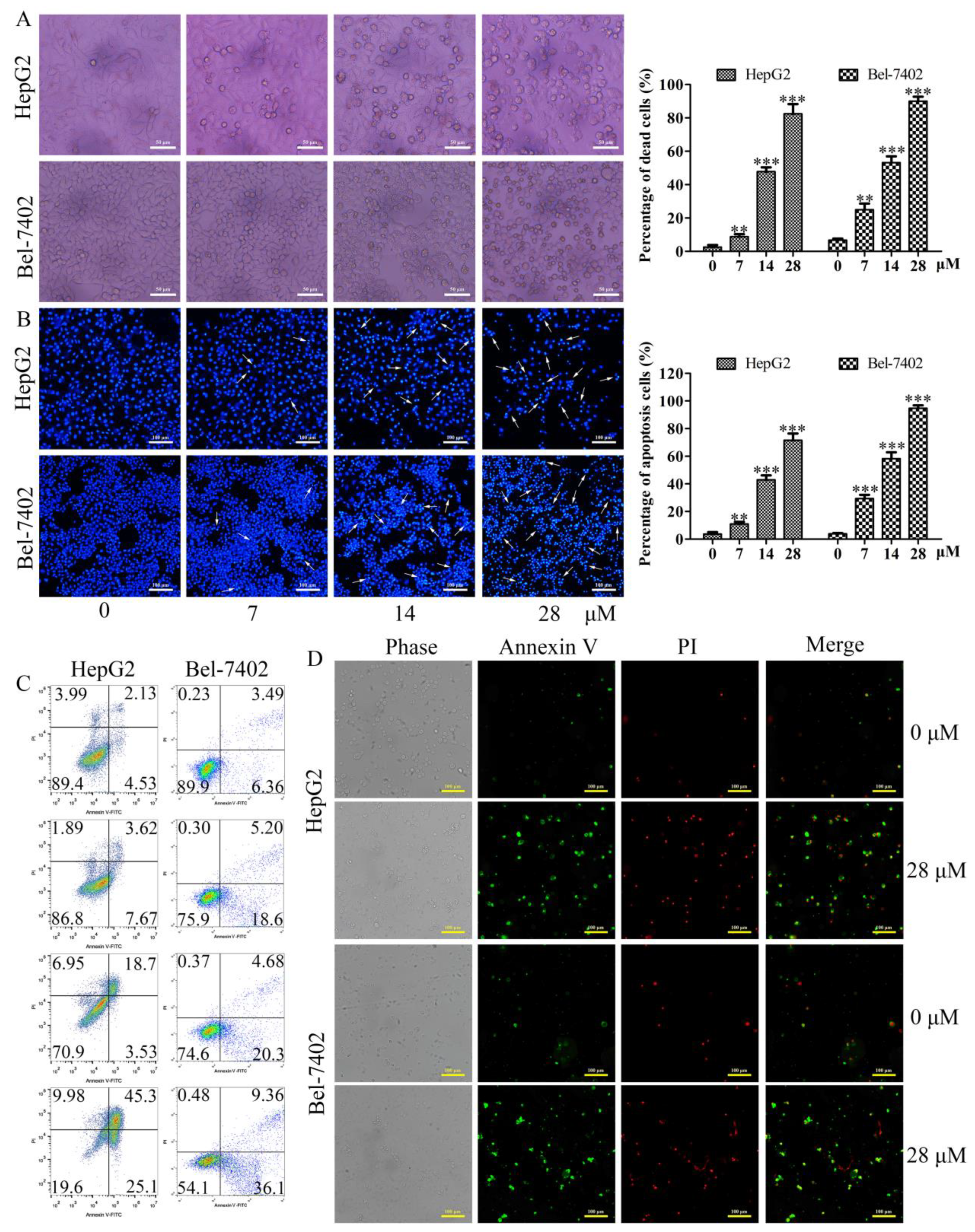
2.3. Effects of Davidone C on Morphological Changes and Apoptosis in HepG2 and Bel-7402 Cells
2.4. Effects of Davidone C on Mitochondrial Apoptotic Pathway in HepG2 and Bel-7402 Cells
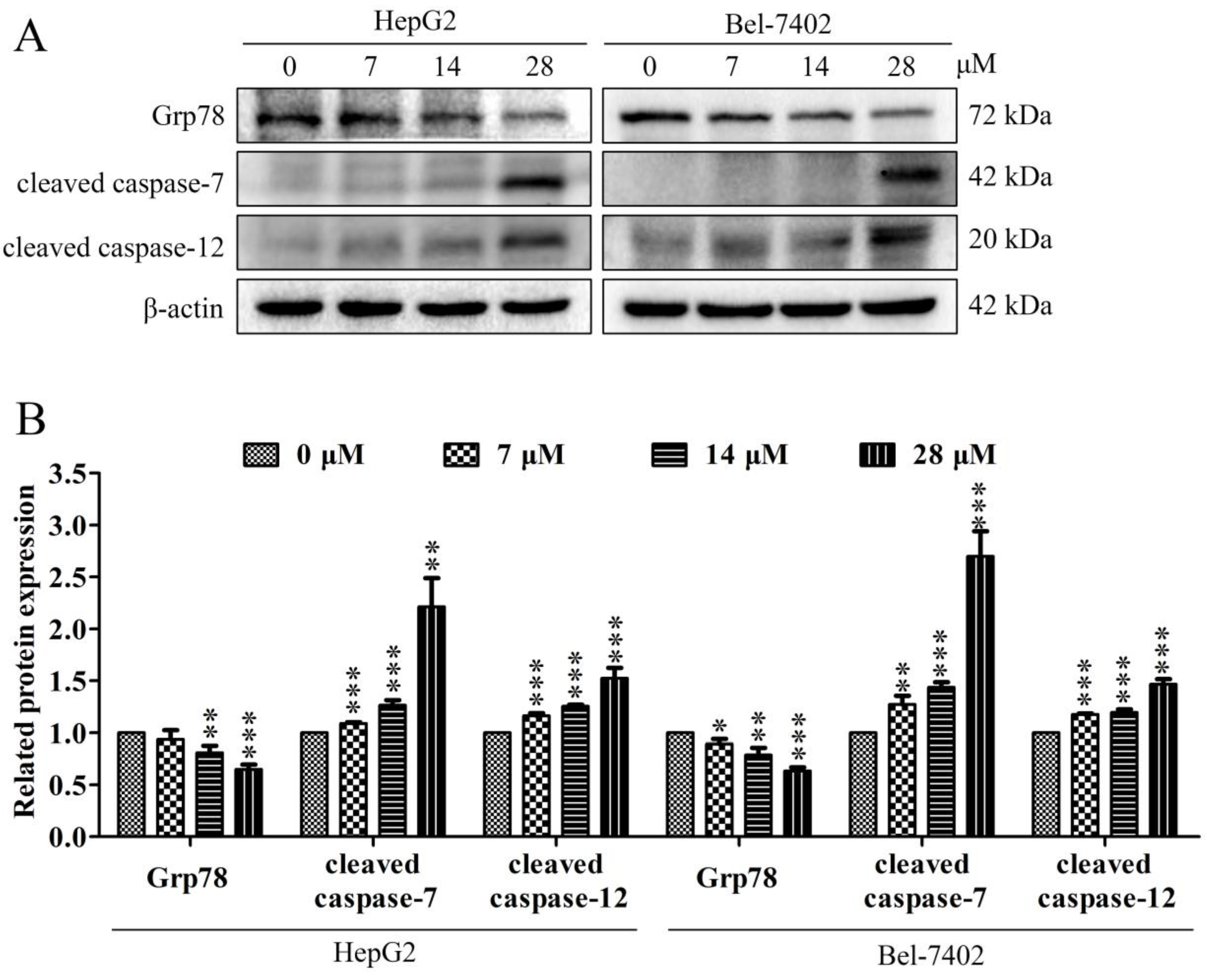
2.5. Effect of Davidone C on Endoplasmic Reticulum Stress (ERS) Pathway in HepG2 and Bel-7402 Cells
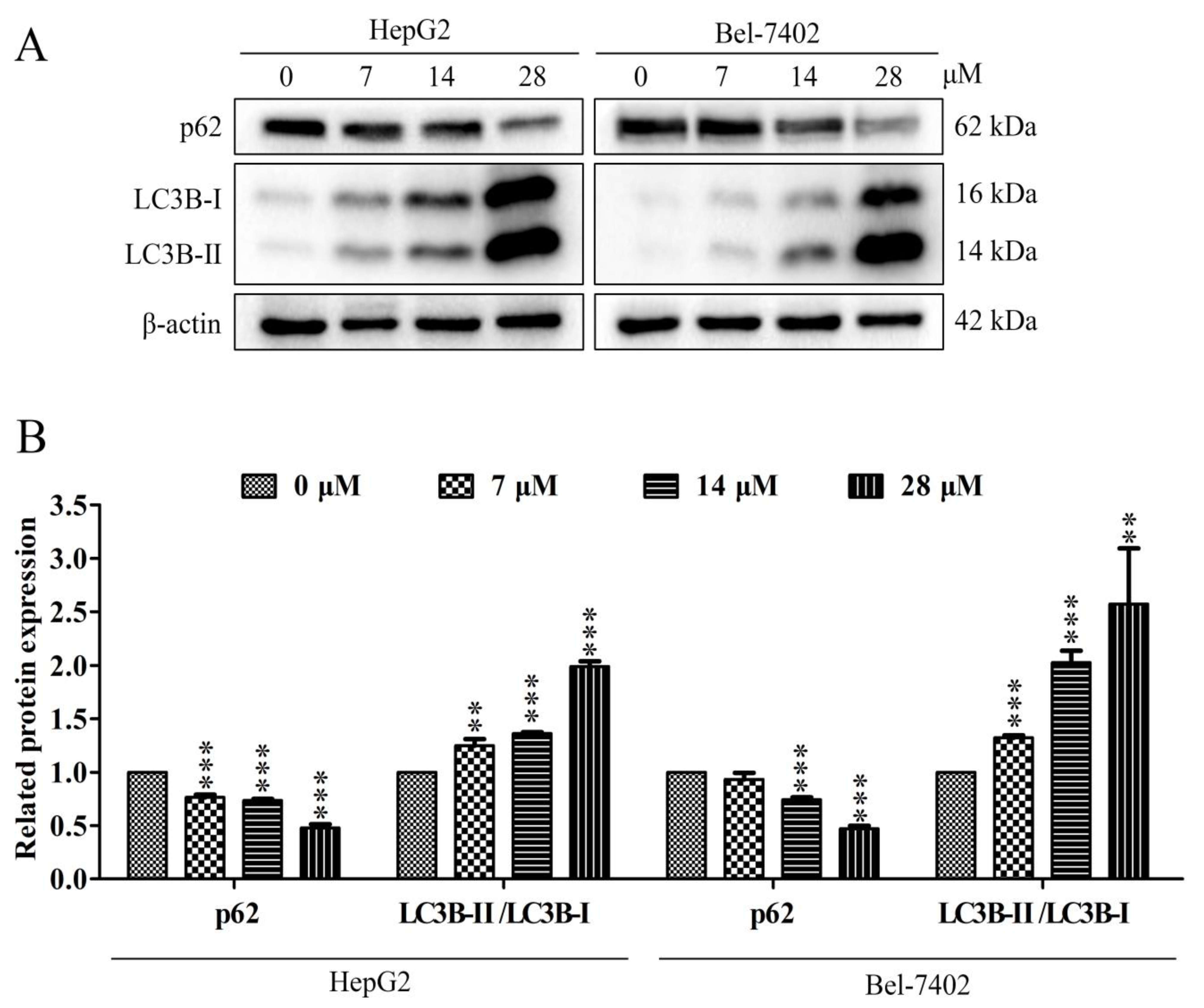
2.6. Effect of Davidone C on Autophagy Pathway in HepG2 and Bel-7402 Cells
2.7. Effect of Chloroquine on Autophagy and Apoptosis Pathway in HepG2 and Bel-7402 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. General Experimental Procedures
4.3. Source and Isolation of Sophora Davidii (Franch.) Skeels
4.4. Extraction and Isolation
4.5. Cell Culture
4.6. MTT Assay
4.7. Scratch Assay
4.8. Hoechst 33258 Stain
4.9. Annexin V- FITC Double Staining
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016, 379, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.A. Modern therapeutic approach-es for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard, J.W., Jr.; Singh, R. Combinatorial effect of curcumin with docetaxel modu-lates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docet-axel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liu, H.; Wen, Y.; Huang, H.; Hao, J.; Lv, Y.; Qin, R.; Yang, X. Aspernolide A Inhibits the Proliferation of Human Laryngeal Carcinoma Cells through the Mitochondrial Apoptotic and STAT3 Signaling Pathways. Molecules 2019, 24, 1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gezici, S.; Şekeroğlu, N. Current Perspectives in the Application of Medicinal Plants Against Cancer: Novel Therapeutic Agents. Anticancer Agents Med. Chem. 2019, 19, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Huang, J.; Wang, B.; Gong, Y.; Fang, Y.; Liu, Y.; Wang, S.; Guo, Y.; Wang, H.; et al. Anti-tumor effects and mechanisms of Astragalus membranaceus (AM) and its specific immunopotentiation: Status and prospect. J. Ethnopharmacol. 2020, 258, 112797. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Hu, L.; Ying, J.; Zhang, M.; Qiu, X.; Lu, Y. Antitumor Potential of Marine Natural Products: A Mechanistic Investigation. Anti-Cancer Agents Med. Chem. 2018, 18, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nessa, M.U.; Beale, P.; Chan, C.; Yu, J.Q.; Huq, F. Studies on combination of platinum drugs cisplatin and oxaliplatin with phytochemicals anethole and curcumin in ovarian tumour models. Anticancer. Res. 2012, 32, 4843–4850. [Google Scholar]
- Yang, L.; Zhang, Z.H.; Jia, X.B. Effect of ginseng rare ginsenoside components combined with paclitaxel on A549 lung cancer. Zhongguo Zhong Yao Za Zhi 2018, 43, 1446–1452. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, T.; Zhao, P.; Choi, H.-Y.; Hao, J.; Huang, H.; Wu, C.; Yang, X.; Pang, K. New flavonoids from the roots of Sophora davidii (Franch.) Skeels and their glucose transporter 4 translocation activities. Bioorgan. Chem. 2021, 106, 104500. [Google Scholar] [CrossRef]
- Garg, A.D.; Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense respons-es. Immunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Childs, E.E.; Kuharsky, D.K.; Yin, X.-M. Activation of Pro-death Bcl-2 Family Proteins and Mitochondria Apoptosis Pathway in Tumor Necrosis Factor-α-induced Liver Injury. J. Biol. Chem. 2001, 276, 27432–27440. [Google Scholar] [CrossRef] [Green Version]
- Renvoizé, C.; Biola-Vidamment, A.; Pallardy, M.; Bréard, J. Apoptosis: Identification of dying cells. Cell Biol. Toxicol. 1998, 14, 111–120. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, 20180992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majtnerová, P.; Roušar, T. An overview of apoptosis assays detecting DNA fragmentation. Mol. Biol. Rep. 2018, 45, 1469–1478. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Kim, C.; Kim, B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A re-view. Nutrients 2018, 10, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopeina, G.S.; Prokhorova, E.A.; Lavrik, I.N.; Zhivotovsky, B. Alterations in the nucleocytoplasmic transport in apoptosis: Caspases lead the way. Cell Prolif. 2018, 51, e12467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortezaee, K.; Salehi, E.; Mahyari, H.M.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.V.; Hermel, E.; Castro-Obregon, S.; del Rio, G.; Ellerby, L.M.; Ellerby, H.M.; Bredesen, D.E. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J. Biol Chem 2001, 276, 33869–33874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Tan, Y.; Dourdin, N.; Wu, C.; De Veyra, T.; Elce, J.S.; Greer, P.A. Ubiquitous Calpains Promote Caspase-12 and JNK Activation during Endoplasmic Reticulum Stress-induced Apoptosis. J. Biol. Chem. 2006, 281, 16016–16024. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zhang, M.; Yin, H. Signaling Pathways Involved in Endoplasmic Reticulum Stress-Induced Neuronal Apoptosis. Int. J. Neurosci. 2012, 123, 155–162. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibi-tion of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towers, C.G.; Thorburn, A. Therapeutic Targeting of Autophagy. EBioMedicine 2016, 14, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Kaminskyy, V.; Zhivotovsky, B. Proteases in autophagy. Biochim. et Biophys. Acta (BBA)Proteins Proteom. 2012, 1824, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, H.; Choi, H.-Y.; Ma, Y.; Zhou, T.; Peng, Y.; Pang, K.; Shu, G.; Yang, X. Anti-esophageal Cancer Effect of Corilagin Extracted from Phmllanthi Fructus via the Mitochondrial and Endoplasmic Reticulum Stress Pathways. J. Ethnopharmacol. 2021, 269, 113700. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, P.; Huang, H.; Ma, Y.; Wu, C.; Yang, X.; Choi, H.-Y. Davidone C Induces the Death of Hepatocellular Carcinoma Cells by Promoting Apoptosis and Autophagy. Molecules 2021, 26, 5219. https://doi.org/10.3390/molecules26175219
Song P, Huang H, Ma Y, Wu C, Yang X, Choi H-Y. Davidone C Induces the Death of Hepatocellular Carcinoma Cells by Promoting Apoptosis and Autophagy. Molecules. 2021; 26(17):5219. https://doi.org/10.3390/molecules26175219
Chicago/Turabian StyleSong, Ping, Huiqi Huang, Yuanren Ma, Chaoqun Wu, Xinzhou Yang, and Ho-Young Choi. 2021. "Davidone C Induces the Death of Hepatocellular Carcinoma Cells by Promoting Apoptosis and Autophagy" Molecules 26, no. 17: 5219. https://doi.org/10.3390/molecules26175219
APA StyleSong, P., Huang, H., Ma, Y., Wu, C., Yang, X., & Choi, H.-Y. (2021). Davidone C Induces the Death of Hepatocellular Carcinoma Cells by Promoting Apoptosis and Autophagy. Molecules, 26(17), 5219. https://doi.org/10.3390/molecules26175219





