Involvement of the γ Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines
Abstract
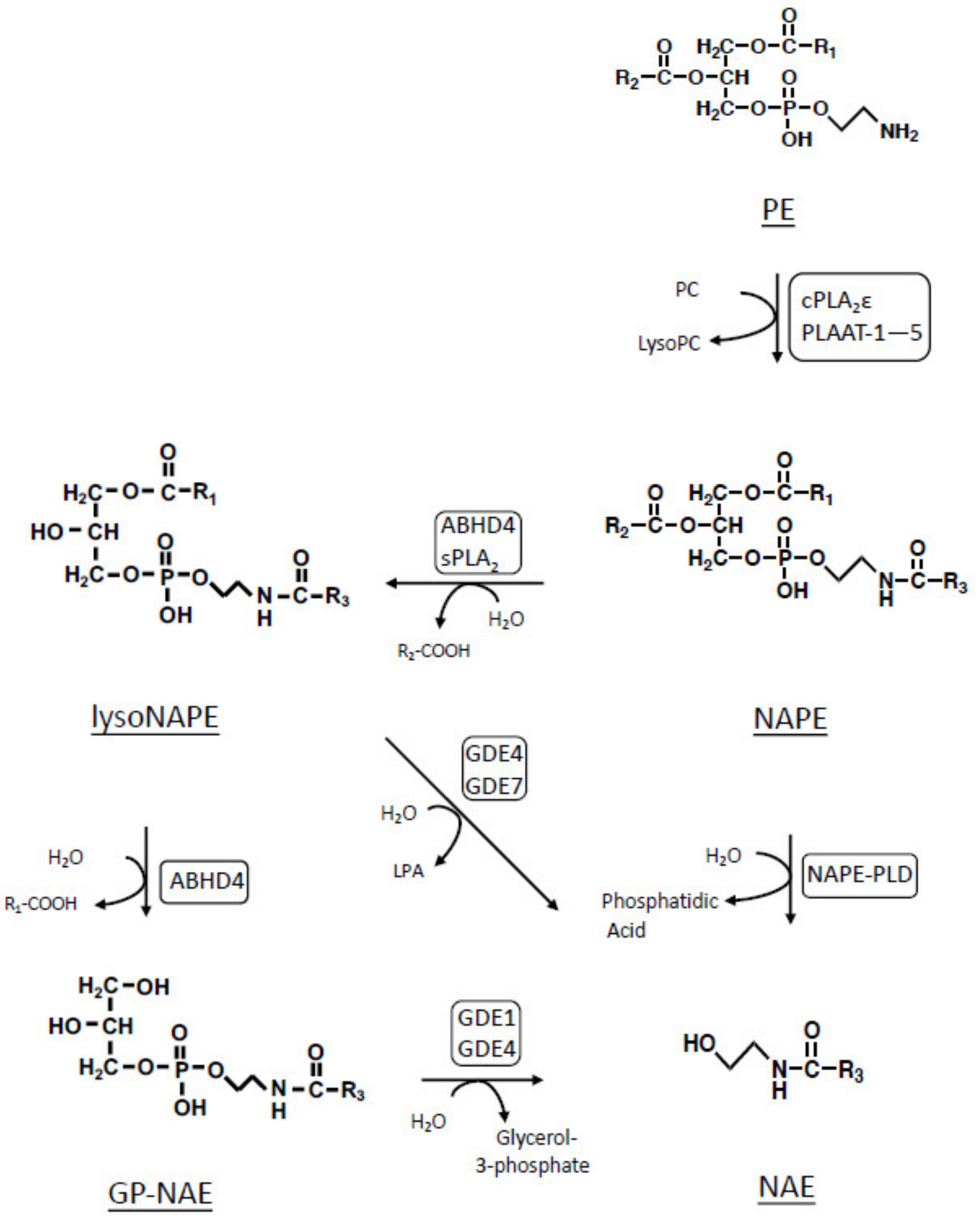
:1. Introduction
2. Results and Discussion
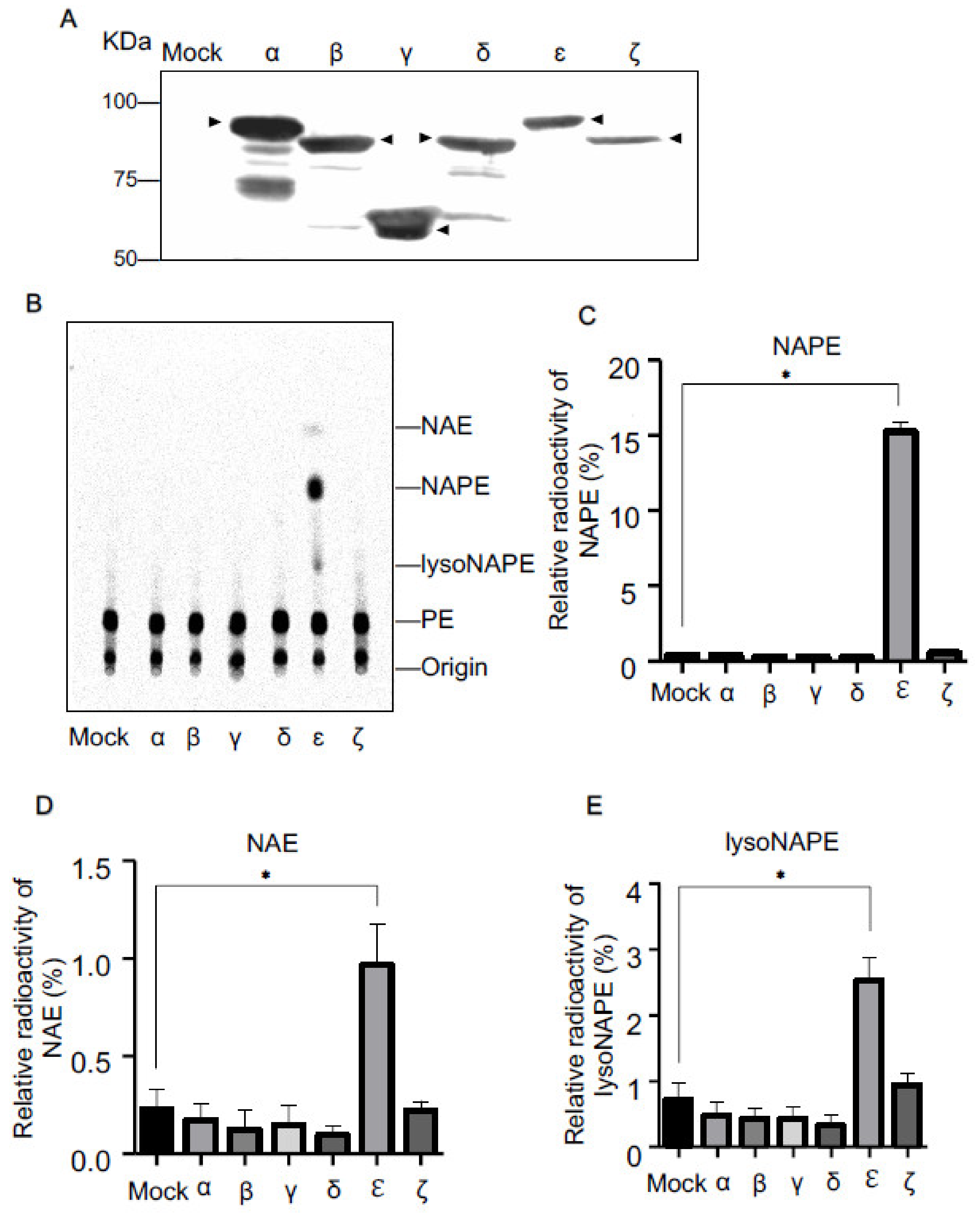
2.1. N-Acyltransferase Activity of cPLA2 Isoforms in Living Cells
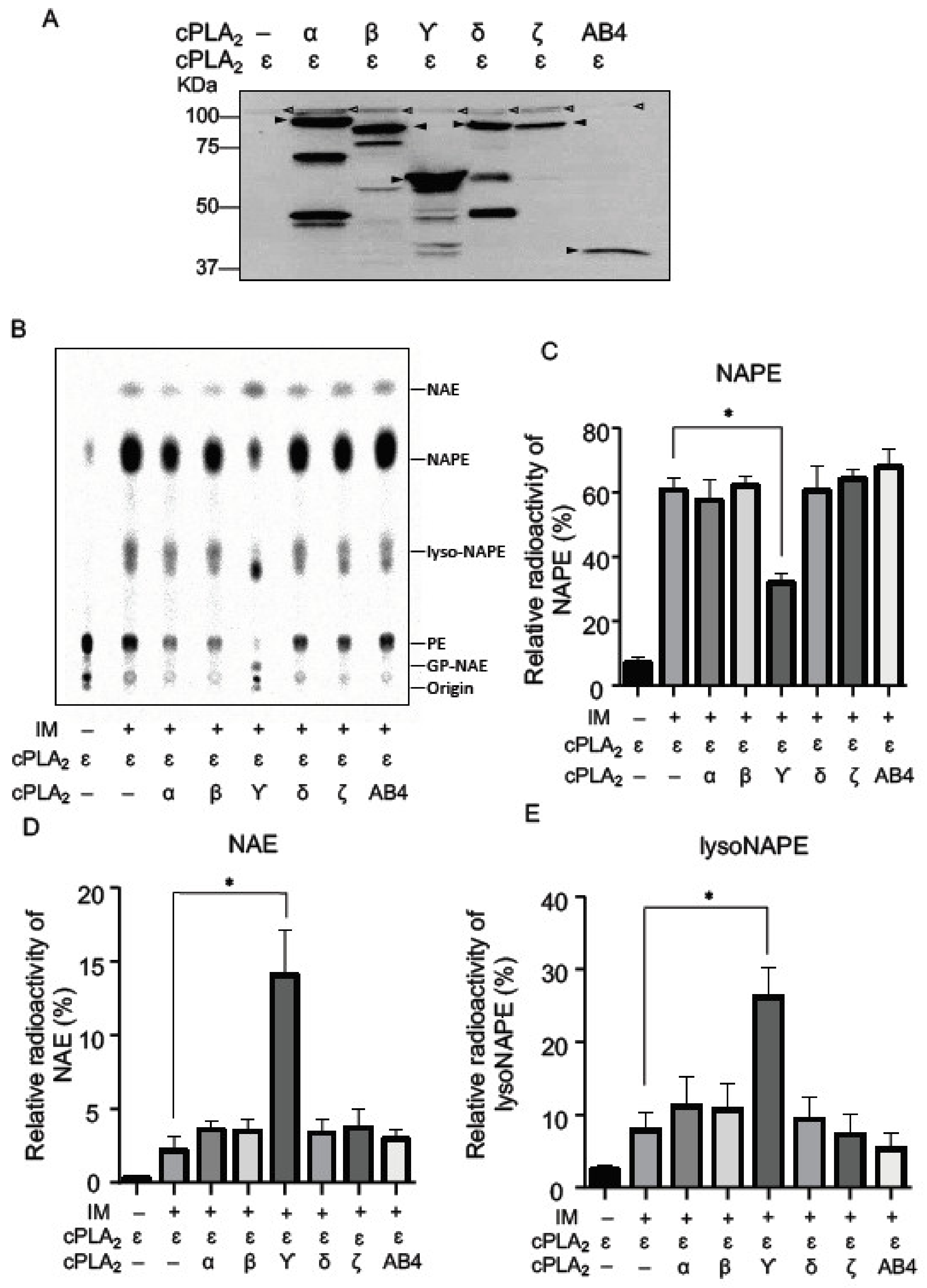
2.2. NAPE-PLA1/A2 Activity of cPLA2 Isoforms in Living Cells
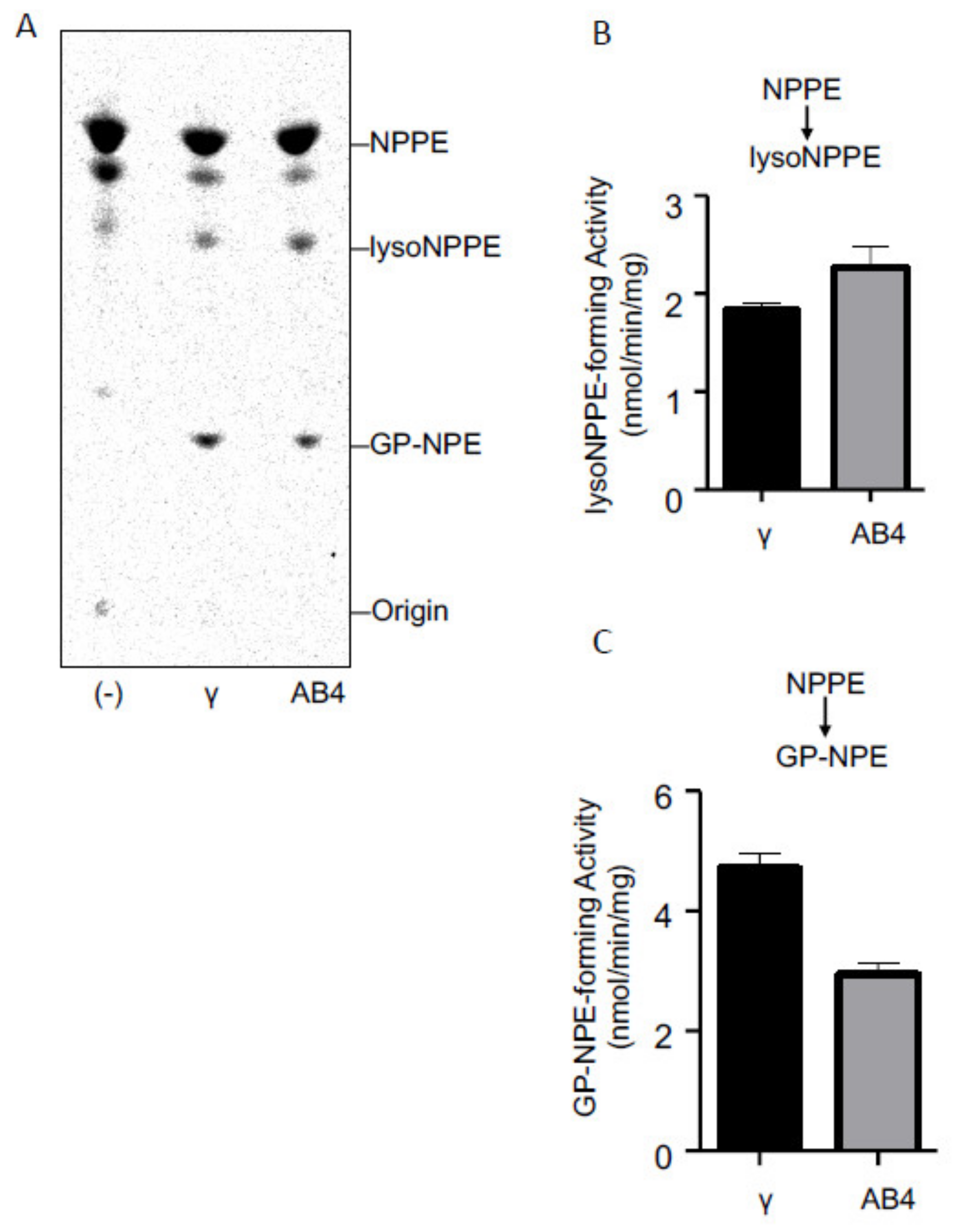
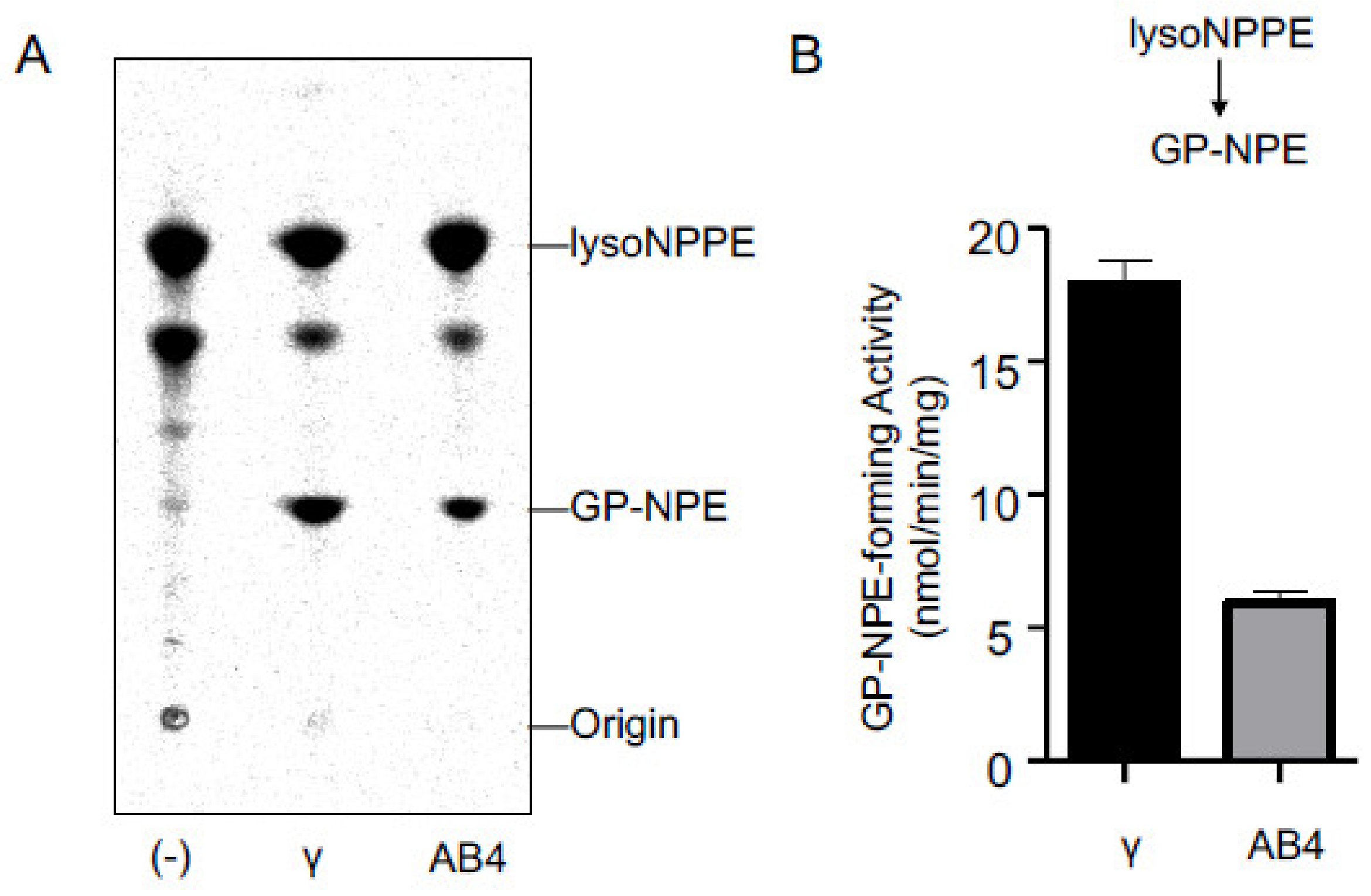
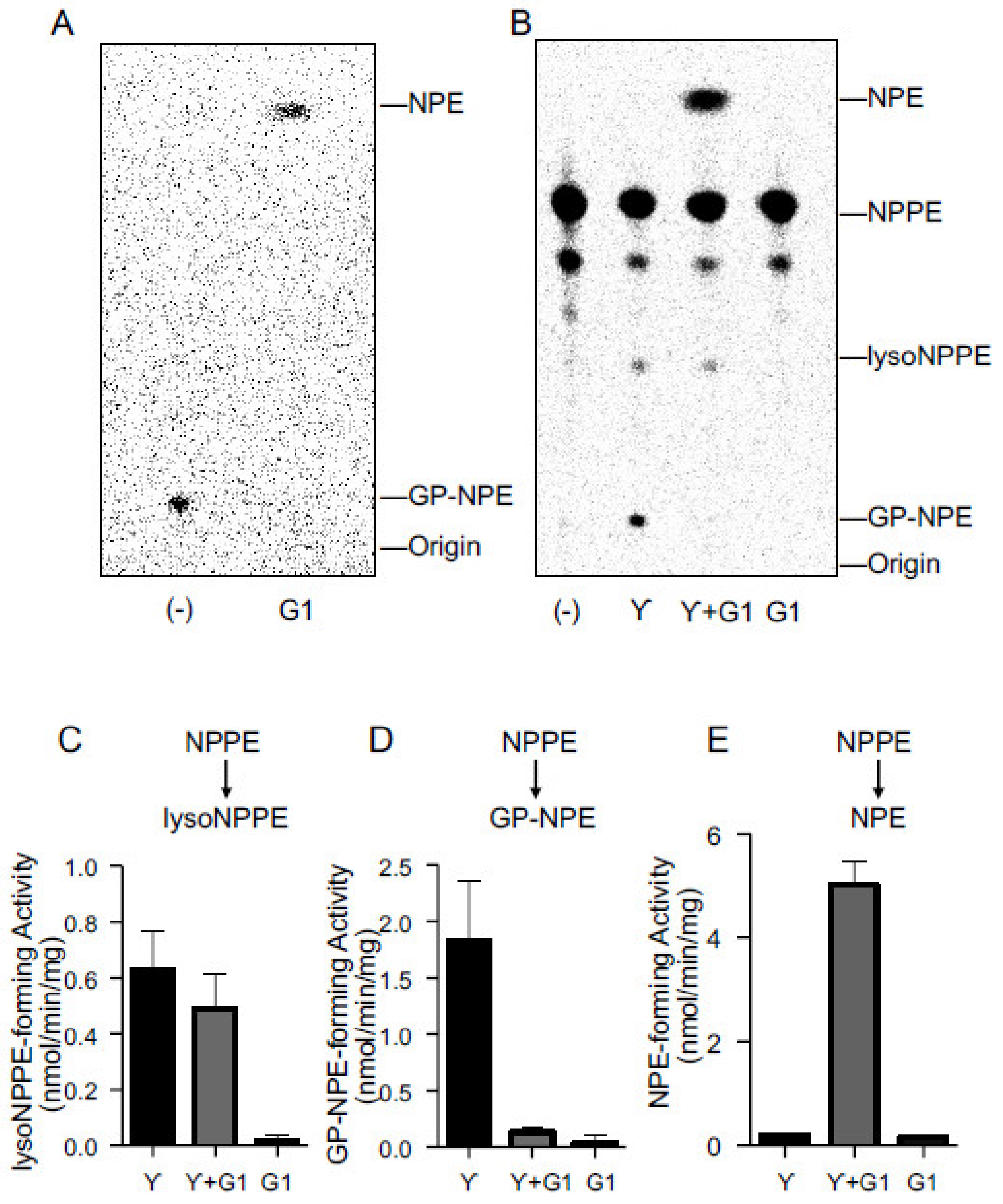
2.3. Activities of Purified cPLA2γ and ABHD4
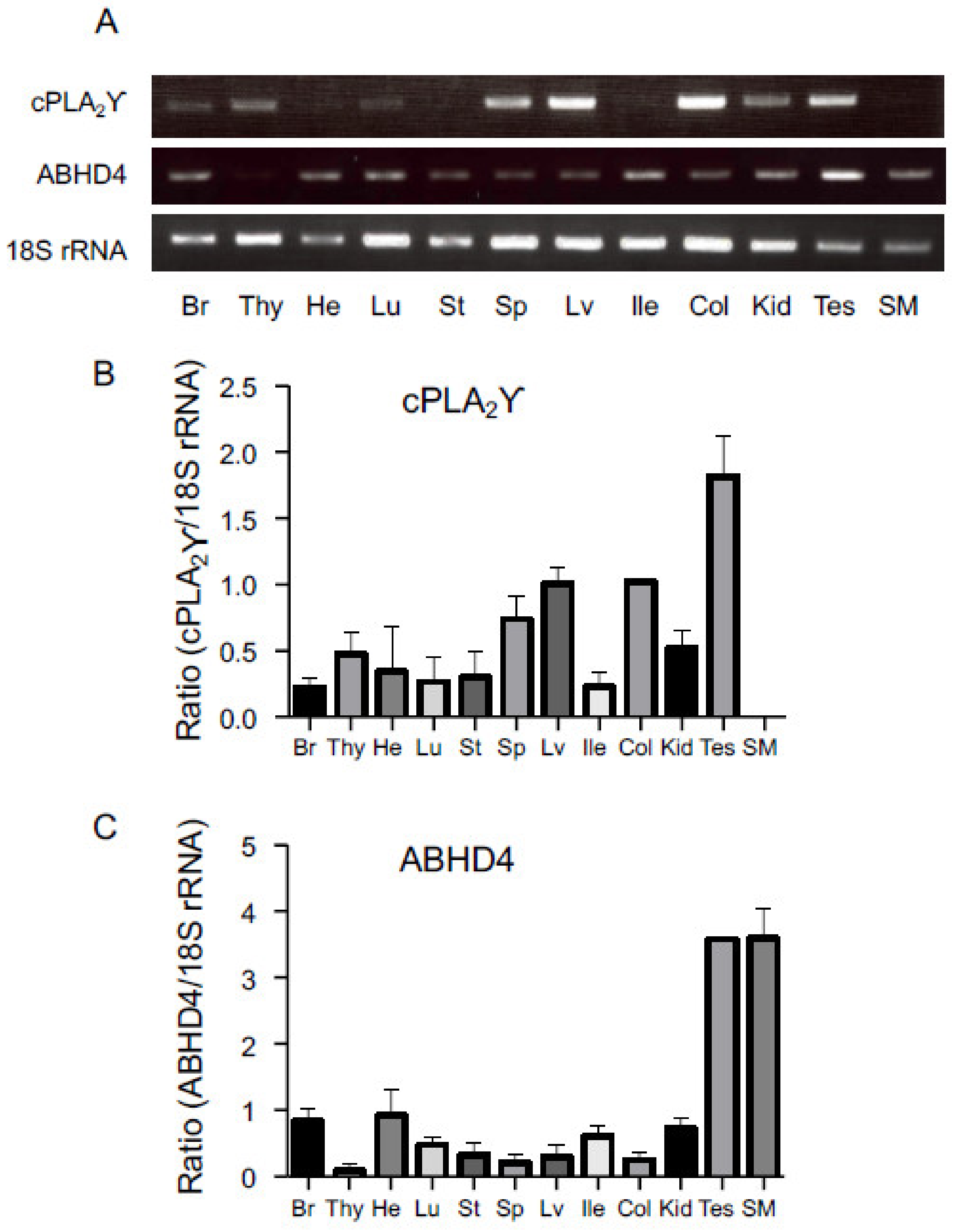
2.4. Tissue Distributions of cPLA2γ and ABHD4
3. Materials and Methods
3.1. Materials
3.2. Construction of Expression Vectors
3.3. Metabolic Labeling
3.4. Expression and Purification of Recombinant Proteins
3.5. Enzyme Assay
3.6. Statistical Analysis
3.7. Western Blotting
3.8. Reverse Transcription-PCR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid, H.H.O.; Schmid, P.C.; Natarajan, V. N-acylated glycerophospholipids and their derivatives. Prog. Lipid Res. 1990, 29, 1–43. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Lo Verme, J.; Fu, J.; Astarita, G.; la Rana, G.; Russo, R.; Calignano, A.; Piomelli, D. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 2005, 67, 15–19. [Google Scholar] [CrossRef]
- Rodríguez de Fonseca, F.; Navarro, M.; Gómez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodríguez, E.; Giuffrida, A.; loVerme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212. [Google Scholar] [CrossRef] [Green Version]
- Ueda, N.; Tsuboi, K.; Uyama, T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA). Prog. Lipid Res. 2010, 49, 299–315. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta 2017, 1862, 1546–1561. [Google Scholar] [CrossRef]
- Jin, X.H.; Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J. Biol. Chem. 2007, 282, 3614–3623. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Parsons, W.H.; Kamat, S.S.; Cravatt, B.F. A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat. Chem. Biol. 2016, 12, 669–671. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J. Biol. Chem. 2004, 279, 5298–5305. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, V.; Schmid, P.C.; Reddy, P.V.; Schmid, H.H.O. Catabolism of N-acylethanolamine phospholipids by dog brain preparations. J. Neurochem. 1984, 42, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Okamoto, Y.; Ikematsu, N.; Inoue, M.; Shimizu, Y.; Uyama, T.; Wang, J.; Deutsch, D.G.; Burns, M.P.; Ulloa, N.M.; et al. Enzymatic formation of N-acylethanolamines from N-acylethanolamine plasmalogen through N-acylphosphatidylethanolamine-hydrolyzing phospholipase D-dependent and -independent pathways. Biochim. Biophys. Acta 2011, 1811, 565–577. [Google Scholar] [CrossRef]
- Sun, Y.X.; Tsuboi, K.; Okamoto, Y.; Tonai, T.; Murakami, M.; Kudo, I.; Ueda, N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem. J. 2004, 380, 749–756. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 2006, 281, 26465–26472. [Google Scholar] [CrossRef] [Green Version]
- Simon, G.M.; Cravatt, B.F. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J. Biol. Chem. 2008, 283, 9341–9349. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.; Saghatelian, A.; Simon, G.M.; Cravatt, B.F. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry 2006, 45, 4720–4726. [Google Scholar] [CrossRef] [Green Version]
- Inoue, M.; Tsuboi, K.; Okamoto, Y.; Hidaka, M.; Uyama, T.; Tsutsumi, T.; Tanaka, T.; Ueda, N.; Tokumura, A. Peripheral tissue levels and molecular species compositions of N-acyl-phosphatidylethanolamine and its metabolites in mice lacking N-acyl-phosphatidylethanolamine-specific phospholipase D. J. Biochem. 2017, 162, 449–458. [Google Scholar] [CrossRef]
- Ghosh, M.; Tucker, D.E.; Burchett, S.A.; Leslie, C.C. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 2006, 45, 487–510. [Google Scholar] [CrossRef]
- Binte Mustafiz, S.S.; Uyama, T.; Morito, K.; Takahashi, N.; Kawai, K.; Hussain, Z.; Tsuboi, K.; Araki, N.; Yamamoto, K.; Tanaka, T.; et al. Intracellular Ca2+-dependent formation of N-acyl-phosphatidylethanolamines by human cytosolic phospholipase A2ε. Biochim. Biophys. Acta 2019, 1864, 158515. [Google Scholar] [CrossRef]
- Lee, H.C.; Simon, G.M.; Cravatt, B.F. ABHD4 regulates multiple classes of N-acyl phospholipids in the mammalian central nervous system. Biochemistry 2015, 54, 2539–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Underwood, K.W.; Song, C.; Kriz, R.W.; Chang, X.J.; Knopf, J.L.; Lin, L.L. A novel calcium-independent phospholipase A2, cPLA2-gamma, that is prenylated and contains homology to cPLA2. J. Biol. Chem. 1998, 273, 21926–21932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, A.; Tanaka, K.; Kamata, R.; Kumazawa, T.; Suzuki, N.; Koga, H.; Waku, K.; Sugiura, T. Subcellular localization and lysophospholipase/transacylation activities of human group IVC phospholipase A2 (cPLA2gamma). Biochim. Biophys. Acta 2009, 1791, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Okamoto, Y.; Rahman, I.A.; Uyama, T.; Inoue, T.; Tokumura, A.; Ueda, N. Glycerophosphodiesterase GDE4 as a novel lysophospholipase D: A possible involvement in bioactive N-acylethanolamine biosynthesis. Biochim. Biophys. Acta 2015, 1851, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Uyama, T.; Kawai, K.; Binte Mustafiz, S.S.; Tsuboi, K.; Araki, N.; Ueda, N. Phosphatidylserine-stimulated production of N-acyl-phosphatidylethanolamines by Ca2+-dependent N-acyltransferase. Biochim. Biophys. Acta 2018, 1863, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| cDNA (Accession Number) | Direction | Sequence (Restriction Sites and a Tag Sequence) | Tissue |
|---|---|---|---|
| cPLA2α (NM_008869) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataagtctttcatagatccttatcagcac-3′ (Spe I site, in-frame FLAG sequence) | Thymus |
| reverse | 5′-cgcgcggccgcttacacagtgggtttacttagaaa-3′ (Not I site) | ||
| cPLA2β (NM_145378) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataaggctctgcaaacctgcccagtctac-3′ (Spe I site, in-frame FLAG sequence) | Brain |
| reverse | 5′-cgcgcggccgctcactccggcctaaactgtttgcg-3′ (Not I site) | ||
| cPLA2γ (NM_001004762) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataaggaactaagctctggggtctgccct-3′ (Spe I site, in-frame FLAG sequence) | Brain |
| reverse | 5′-cgcgcggccgcttaatccttagatatgttgtggga-3′ (Not I site) | ||
| cPLA2δ (NM_001024137) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataagtggagtggagatagaagagtaggc-3′ (Spe I site, in-frame FLAG sequence) | Testis |
| reverse | 5′-cgcgcggccgctcacgtcttcactcccaatggcct-3′ (Not I site) | ||
| cPLA2ζ (NM_001024145) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataagccctggactctccagccaaagtgg-3′ (Spe I site, in-frame FLAG sequence) | Large intestine |
| reverse | 5′-cgcgcggccgctcagcccccaacccttcccccagc-3′ (Not I site) | ||
| ABHD4 (NM_134076) | forward | 5′-cgcactagtggaaaatggattacaaggatgacgacgataaggctgatgatctggagcagcagcctcag-3′ (Spe I site, in-frame FLAG sequence) | Brain |
| reverse | 5′-cgcgcggccgctcagtcaactgagttgcagatctcttc-3′ (Not I site) |
| Gene | Direction | Sequence |
|---|---|---|
| cPLA2γ | forward | 5′-tgaggtgagcgaggatcagctgaag-3′ |
| reverse | 5′-atgagtcagatagttttactgtccc-3′ | |
| ABHD4 | forward | 5′-ggcacagtttgggaggattcctggc-3′ |
| reverse | 5′-gaggtgcacggatctcactagggtc-3′ | |
| 18S rRNA | forward | 5′-gtaacccgttgaaccccatt-3′ |
| reverse | 5′-ccatccaatcggtagtagcg-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Uyama, T.; Rahman, S.M.K.; Sikder, M.M.; Hussain, Z.; Tsuboi, K.; Miyake, M.; Ueda, N. Involvement of the γ Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines. Molecules 2021, 26, 5213. https://doi.org/10.3390/molecules26175213
Guo Y, Uyama T, Rahman SMK, Sikder MM, Hussain Z, Tsuboi K, Miyake M, Ueda N. Involvement of the γ Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines. Molecules. 2021; 26(17):5213. https://doi.org/10.3390/molecules26175213
Chicago/Turabian StyleGuo, Yiman, Toru Uyama, S. M. Khaledur Rahman, Mohammad Mamun Sikder, Zahir Hussain, Kazuhito Tsuboi, Minoru Miyake, and Natsuo Ueda. 2021. "Involvement of the γ Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines" Molecules 26, no. 17: 5213. https://doi.org/10.3390/molecules26175213
APA StyleGuo, Y., Uyama, T., Rahman, S. M. K., Sikder, M. M., Hussain, Z., Tsuboi, K., Miyake, M., & Ueda, N. (2021). Involvement of the γ Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines. Molecules, 26(17), 5213. https://doi.org/10.3390/molecules26175213







