A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis
Abstract
:1. Introduction
2. General Aspects of the Chromatographic Analysis of Aroma
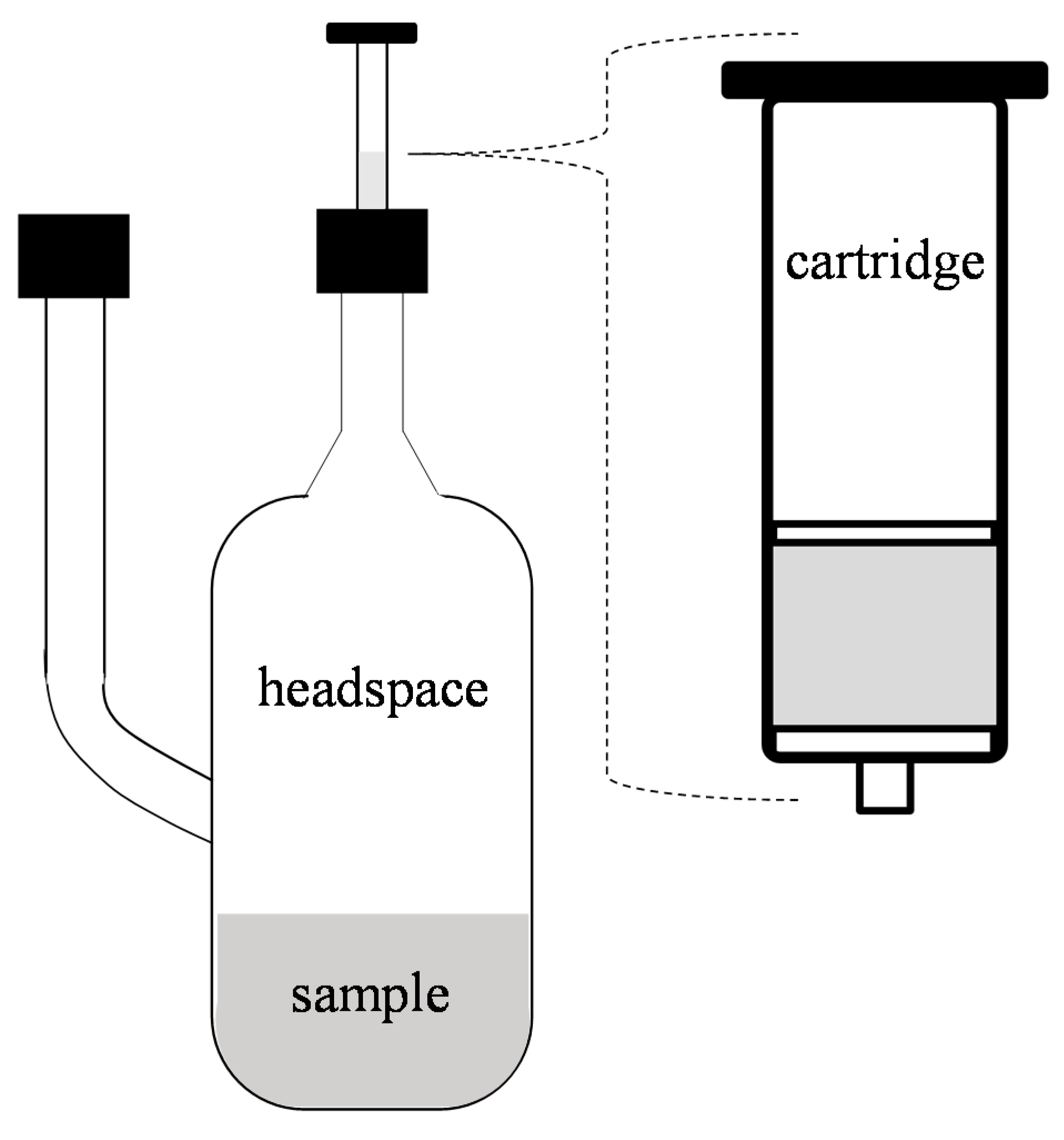
2.1. Extract Preparation
2.2. Separation and Identification of Compounds Responsible for Food Aroma Using Gas Chromatography (GC)
3. Techniques to Evaluate the Odoriferous Importance of Chemical Compounds
| Fruit | Volatile Extraction Technique | Chromatographic Detection | Classification Technique for Odoriferous Compounds | Reference |
|---|---|---|---|---|
| Yellow tamarillo | SAFE distillation | GC-MS-O | AEDA | García et al. [51] |
| Hardy kiwi | SAFE distillation | GC-MS-O | AEDA | Lindhorst and Steinhaus [52] |
| Cherimoya | SPME | GC-MS/GC-FID-O | AEDA | Pino and Roncal [53] |
| Sour guava | SAFE distillation | GC-FID-O | AEDA | Cuadrado-Silva et al. [39] |
| Ningxia goji berries | SPME | GC-MS-O | AEDA | Lu et al. [41] |
| Terebinth | SAFE distillation | GC-MS-O | AEDA | Amanpour et al. [38] |
| Lucuma | SAFE distillation | GC-MS-O | AEDA | Inga et al. [54] |
| “Honeycrisp” apple | Liquid extraction | GC-MS-O | AEDA | Yan et al. [42] |
| Cascara | SAFE distillation | GC-MS/GC-FID-O | AEDA | Pua et al. [55] |
| Mango | SAFE distillation | GC-MS | OAV | Bonneau et al. [45] |
| Orange | HS-SPME | GC-MS | OAV | Rodríguez et al. [56] |
| Mulberry | SPME | GC-MS/GC-FID-O | OAV | Zhu et al. [44] |
| Peach | SPME | GC-MS/GC-FID-O | OAV | Zhuand and Xiao [57] |
| Gabiroba | HS-SPME | GC-MS-O | OSME | Ferreira et al. [50] |
| Passion fruit | HS | GC-MS-O | OSME | Janzantti and Monteiro [16] |
| Mango | SPE | GC-MS-O | OSME | Liu et al. [50] |
| Bayberry | HS-SPME | GC-MS-O | - | Cheng et al. [58] |
| Tomato | SPME-PFPD | GC-MS-O | - | Du et al. [59] |
| Black velvet tamarind | SAFE distillation | GC-MS-O | OAV | Lasekan and See [60] |
| Bayberry | SPME | GC-MS-O | - | Cheng et al. [61] |
| Strawberry | HS-SPME | GC-MS | - | Li et al. [62] |
| Murici, bacuri, and sapodilla | HSSE | GC-MS | Uekane et al. [63] | |
| Quince | SPME | GC-MS-O | Choi et al. [64] | |
| Omija | HSSE | GC-MS | Kim et al. [65] | |
| Watermelon | SPME | GC-MS-O | Mendoza-Enano et al. [66] | |
| Pistachio | HS-SPME | GC-MS | Şahanand Bozkurt [67] | |
| Blueberries | Liquid extraction | GC-QTOF-MS | Yuan et al. [68] |
4. Studies of Aroma Composition of Fruits
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carini, F.; Coughtrey, P.; Kinnersley, R. Radionuclide transfer to fruits: A critical review. Introduction. J. Environ. Radioact. 2001, 52, 123–129. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.; De Castro, I.R.R.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica 2010, 26, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Abrafrutas. National Producers Want More Space in a Global Market That Already Moves More than $130 Billion per Year. Asia, Europe and the United States Are on the Radar of Brazilians. Available online: https://abrafrutas.org/2018 (accessed on 27 February 2020).
- Aung, M.M.; Chang, Y.S. Traceability in a food supply chain: Safety and quality perspectives. Food Control. 2014, 39, 172–184. [Google Scholar] [CrossRef]
- Zellner, B.d.A.; Dugo, P.; Dugo, G.; Mondello, L. Gas chromatography–olfactometry in food flavour analysis. J. Chro-Matography A 2008, 1186, 123–143. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Jeleń, H.H.; Majcher, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Dutcosky, S. Análise Sensorial de Alimentos [Food Sensory Analysis]; Champagnat PUCPress: Curitiba, Brazil, 2019; 540p. [Google Scholar]
- Franco, M. Aroma e Sabor de Alimentos: Temas Atuais [Food Aroma and Flavor: Current Issues]; Varela: São Paulo, Brazil, 2004. [Google Scholar]
- Van Ruth, S. Methods for gas chromatography-olfactometry: A review. Biomol. Eng. 2001, 17, 121–128. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A Review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Grosch, W. Determination of potent odourants in foods by Aroma Extract Dilution Analysis (AEDA) and calculation of Odour Activity Values (OAVs). Flavour Fragr. J. 1994, 9, 147–158. [Google Scholar] [CrossRef]
- Biasioli, F.; Gasperi, F.; Yeretzian, C.; Märk, T.D. PTR-MS monitoring of VOCs and BVOCs in food science and technology. Trac Trends Anal. Chem. 2011, 30, 968–977. [Google Scholar] [CrossRef]
- Ridgway, K.; Lalljie, S.P.; Smith, R. Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef]
- Zapata, J.; López, R.; Ferreira, V. Estrategias Automáticas Para el anÁlisis de Compuestos voláTiles: SPME e ITEX; Publicia: Chișinău, Germany, 2013; 316p. [Google Scholar]
- Janzantti, N.; Monteiro, M. HS-GC-MS-O analysis and sensory acceptance of passion fruit during maturation. J. Food Sci. Technol. 2017, 54, 2594–2601. [Google Scholar] [CrossRef] [Green Version]
- Thomazini, M.; Franco, M. Metodologia para análise dos constituintes voláteis do sabor. Bol. Soc. Bras. Cienc. Tecnol. Aliment. 2000, 34, 52–59. [Google Scholar]
- Ibarz, M.; Ferreira, V.; Hernández-Orte, P.; Loscos, N.; Cacho, J. Optimization and evaluation of a procedure for the gas chroma-tographic-mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J. Chromatogr. A 2006, 1116, 217–229. [Google Scholar] [CrossRef]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M.L.; Ferreira, V.; Orte, M.P.H. Glycosidically Bound Aroma Compounds and Impact Odorants of Four Strawberry Varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Identification of novel antioxidant peptides generated in Spanish dry-cured ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.; Sala, L.; Ores, J.D.C.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A Review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [Green Version]
- Culleré, L.; Ferreira, V.; Venturini, M.E.; Marco, P.; Blanco, D. Chemical and sensory effects of the freezing process on the aroma profile of black truffles (Tuber melanosporum). Food Chem. 2013, 136, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Garcia, C.; Stevenson, R.; Atkinson, R.; Winz, R.; Quek, S.-Y. Changes in the bound aroma profiles of ‘Hayward’ and ‘Hort16A’ kiwifruit (Actinidia spp.) during ripening and GC-olfactometry analysis. Food Chem. 2013, 137, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Quek, S.-Y.; Stevenson, R.J.; Winz, R.A. Characterisation of bound volatile compounds of a low flavour kiwifruit species: Actinidia eriantha. Food Chem. 2012, 134, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Meret, M.; Brat, P.; Mertz, C.; Lebrun, M.; Günata, Z. Contribution to aroma potential of Andean blackberry (Rubus glaucus Benth.). Food Res. Int. 2011, 44, 54–60. [Google Scholar] [CrossRef]
- Jalili, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2019, 152, 104319. [Google Scholar] [CrossRef]
- San-Juan, F.; Petka, J.; Cacho, J.; Ferreira, V.; Escudero, A. Producing headspace extracts for the gas chromatography-olfactometric evaluation of wine aroma. Food Chem. 2010, 123, 188–195. [Google Scholar] [CrossRef]
- Van Ruth, S.; O’Connor, C. Evaluation of three gas chromatography-olfactometry methods: Comparison of odour intensi-ty-concentration relationships of eight volatile compounds with sensory headspace data. Food Chem. 2001, 74, 341–347. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.; Pet’Ka, J.; Aznar, M.; Cacho, J. Quantitative gas chromatography-olfactometry. Analytical characteristics of a panel of judges using a simple quantitative scale as gas chromatography detector. J. Chromatogr. A 2003, 1002, 169–178. [Google Scholar] [CrossRef]
- Inczedy, J.; Lengyel, T.; Ure, A.; Gelencsér, A.; Hulanicki, A. Compendium of Analytical Nomenclature; Blackwell Science: Hoboken, NJ, USA, 1998. [Google Scholar]
- De-la-Fuente-Blanco, A.; Ferreira, V. Gas Chromatography Olfactometry (GC-O) for the (Semi) Quantitative Screening of Wine Aroma. Foods 2020, 9, 1892. [Google Scholar] [CrossRef] [PubMed]
- Delahunty, C.; Eyres, G.; Dufour, J.P. Gas Chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Barbará, J.A.; Nicolli, K.; Souza-Silva, E.A.; Biasoto, A.; Welke, J.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2019, 308, 125552. [Google Scholar] [CrossRef] [PubMed]
- Van Ruth, S. Evaluation of two gas chromatography-olfactometry methods: The detection frequency and perceived intensity method. J. Chromatogr. A 2004, 1054, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, F.; Grosch, W. Identification of the most intense volatile flavour compounds formed during autoxidation of linoleic acid. Eur. Food Res. Technol. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Wardencki, W.; Chmiel, T.; Dymerski, T. Gas Chromatography-Olfactometry (GC-O), electronic noses (e-noses) and electronic tongues (e-tongues) for in vivo food flavour measurement. Instrum. Assess. Food Sens. Qual. 2013, 195–229. [Google Scholar] [CrossRef]
- Amanpour, A.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of key aroma compounds in fresh and roasted terebinth fruits using aroma extract dilution analysis and GC-MS-Olfactometry. Microchem. J. 2018, 145, 96–104. [Google Scholar] [CrossRef]
- Cuadrado-Silva, C.T.; Pozo-Bayón, M.; Osorio, C. Identification of aroma compounds and precursors of sour guava (Psidium friedrichsthalianum Nied.) following a sensomics approach. Eur. Food Res. Technol. 2016, 243, 1–10. [Google Scholar] [CrossRef]
- Sonmezdag, A.S. Characterization of aroma and aroma-active composition of Gaziantep cheese by solvent-assisted flavor evaporation (SAFE) and aroma extract dilution analysis (AEDA). J. Food Process. Preserv. 2018, 43, e13840. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Quan, J.; An, W.; Zhao, J.; Xi, W. Identification of characteristic aroma volatiles of Ningxia goji berries (Lycium barbarum L.) and their developmental changes. Int. J. Food Prop. 2017, 20, S214–S227. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography-olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC-MS) and flame photometric detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Bonneau, A.; Boulanger, R.; Lebrun, M.; Maraval, I.; Gunata, Z. Aroma compounds in fresh and dried mango fruit (Mangifera indica L. cv. K ent): Impact of drying on volatile composition. Int. J. Food Sci. Technol. 2016, 51, 789–800. [Google Scholar] [CrossRef]
- Miranda-Lopez, R.; Libbey, L.M.; Watson, B.T.; McDaniel, M.R. Odor Analysis of pinot noir wines from grapes of different maturities by a Gas Chromatography-olfactometry technique (Osme). J. Food Sci. 1992, 57, 985–993. [Google Scholar] [CrossRef]
- Costa, A.; Garruti, D.; Madruga, M. The power of odour volatiles from unifloral melipona honey evaluated by gas chroma-tography-olfactometry Osme techniques. J. Sci. Food Agric. 2019, 99, 4493–4497. [Google Scholar] [CrossRef]
- Le Guen, S.; Prost, C.; Demaimay, M. Critical comparison of three olfactometric methods for the identification of the most potent odorants in cooked mussels (Mytilus edulis). J. Agric. Food Chem. 2000, 48, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic characterization of mangoes (Mangifera indica L.) using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, D.d.F.; Garruti, D.d.S.; Barin, J.; Cichoski, A.; Wagner, R. Characterization of odor-active compounds in gabiroba fruits (Campomanesia xanthocarpa O. Berg). J. Food Qual. 2016, 39, 90–97. [Google Scholar] [CrossRef]
- García, J.M.; Prieto, L.J.; Guevara, A.; Malagon, D.; Osorio, C. Chemical studies of yellow tamarillo (Solanum betaceum Cav.) fruit flavor by using a molecular sensory approach. Molecules 2016, 21, 1729. [Google Scholar] [CrossRef]
- Lindhorst, A.C.; Steinhaus, M. Aroma-active compounds in the fruit of the hardy kiwi (Actinidia arguta) cultivars Ananasnaya, Bojnice, and Dumbarton Oaks: Differences to common kiwifruit (Actinidia deliciosa ‘Hayward’). Eur. Food Res. Technol. 2015, 242, 967–975. [Google Scholar] [CrossRef]
- Pino, J.A.; Roncal, E. Characterisation of odour-active compounds in cherimoya (Annona cherimola Mill.) fruit. Flavour Fragr. J. 2015, 31, 143–148. [Google Scholar] [CrossRef]
- Inga, M.; García, J.M.; Galvez, A.C.A.; Campos, D.; Osorio, C. Chemical characterization of odour-active volatile compounds during lucuma (Pouteria lucuma) fruit ripening. CyTA J. Food 2019, 17, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Pua, A.; Choo, W.X.D.; Goh, R.M.V.; Liu, S.Q.; Cornuz, M.; Ee, K.-H.; Sun, J.; Lassabliere, B.; Yu, B. A systematic study of key odourants, non-volatile compounds, and antioxidant capacity of cascara (dried Coffea arabica pulp). LWT 2020, 138, 110630. [Google Scholar] [CrossRef]
- Rodríguez, A.; Peris, J.E.; Redondo, A.; Shimada, T.; Costell, E.; Carbonell, I.; Rojas, C.; Peña, L. Impact of d-limonene synthase up- or down-regulation on sweet orange fruit and juice odor perception. Food Chem. 2017, 217, 139–150. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography-olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Chen, S.; Wu, D.; Liu, D.; Ye, X. Characterization of aroma-active volatiles in three Chinese bayberry (Myrica rubra) cultivars using GC-MS-olfactometry and an electronic nose combined with principal component analysis. Food Res. Int. 2015, 72, 8–15. [Google Scholar] [CrossRef]
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, O.; See, N.S. Key volatile aroma compounds of three black velvet tamarind (Dialium) fruit species. Food Chem. 2015, 168, 561–565. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Chen, S.; Xia, Q.; Liu, D.; Ye, X. Sensory evaluation, physicochemical properties and aroma-active profiles in a diverse collection of Chinese bayberry (Myrica rubra) cultivars. Food Chem. 2016, 212, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.; JI, M.-L.; Chao, Y.; Ling, L.; Gao, D.-S.; Fu, X.L. Effects of molybdenum on nutrition, quality, and flavour compounds of strawberry (Fragaria × ananassa Duch. cv. Akihime) fruit. J. Integr. Agric. 2017, 16, 1502–1512. [Google Scholar] [CrossRef]
- Uekane, T.M.; Nicolotti, L.; Griglione, A.; Bizzo, H.R.; Rubiolo, P.; Bicchi, C.; Rocha-Leão, M.H.M.; Rezende, C.M. Studies on the volatile fraction composition of three native Amazonian-Brazilian fruits: Murici (Byrsonima crassifolia L., Malpighiaceae), bacuri (Platonia insignis M., Clusiaceae), and sapodilla (Manilkara sapota L., Sapotaceae). Food Chem. 2017, 219, 13–22. [Google Scholar] [CrossRef]
- Choi, J.Y.; Lee, S.M.; Lee, H.J.; Kim, Y.-S. Characterization of aroma-active compounds in Chinese quince (Pseudocydonia sinensis Schneid) by aroma dilution analyses. Food Res. Int. 2017, 105, 828–835. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, Y.-Y.; Lee, K.-G.; Jang, H.W. Instrumental volatile flavor analysis of omija (Schisandra chinesis Baillon) using headspace stir-bar sorptive extraction-gas chromatography-mass spectrometry and its relationship to human sensory perceptions. Food Res. Int. 2018, 120, 650–655. [Google Scholar] [CrossRef]
- Mendoza-Enano, M.; Stanley, R.; Frank, D. Linking consumer sensory acceptability to volatile composition for improved shelf-life: A case study of fresh-cut watermelon (Citrullus lanatus). Postharvest Biol. Technol. 2019, 154, 137–147. [Google Scholar] [CrossRef]
- Şahan, A.; Bozkurt, H. Effects of harvesting time and irrigation on aroma active compounds and quality parameters of pistachio. Sci. Hortic. 2020, 261, 108905. [Google Scholar] [CrossRef]
- Yuan, F.; Yan, J.; Yan, X.; Liu, H.; Pan, S. Comparative transcriptome analysis of genes involved in volatile compound synthesis in blueberries (Vaccinium virgatum) during postharvest storage. Postharvest Biol. Technol. 2020, 170, 111327. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Macoris, M.S.; Garruti, D.S.; Monteiro, M. Influence of the cultivation system in the aroma of the volatile compounds and total antioxidant activity of passion fruit. LWT 2012, 46, 511–518. [Google Scholar] [CrossRef]
- Tucker, G.; Seymour, G.; Taylor, J.; Tucker, G. Biochemistry of Fruit Ripening; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Drawert, F.; Heimann, W.; Emberger, R.; Tressl, R. Über die Biogenese von Aromastoffen bei Pflanzen und Früchten, II. Enzy-matische Bildung von Hexen-(2)-al-(1), Hexanal und deren Vorstufen. Justus Liebigs Ann. Chem. 1966, 694, 200–208. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant. J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, R.; Chassagne, D.; Crouzet, J. Free and bound flavour components of amazonian fruits. 1: Bacuri. Flavour Fragr. J. 1999, 14, 303–311. [Google Scholar] [CrossRef]
- Culleré, L.; Ferreira, V.; Chevret, B.; Venturini, M.E.; Gimeno, A.C.S.; Blanco, D. Characterisation of aroma active compounds in black truffles (Tuber melanosporum) and summer truffles (Tuber aestivum) by gas chromatography-olfactometry. Food Chem. 2010, 122, 300–306. [Google Scholar] [CrossRef]
- Yamashita, I.; Iino, K.; Nemoto, Y.; Yoshikawa, S. Studies on flavor development in strawberries. 4. Biosynthesis of volatile alcohol and esters from aldehyde during ripening. J. Agric. Food Chem. 1977, 25, 1165–1168. [Google Scholar] [CrossRef]
- Hatanaka, A.; Sekiya, J.; Kajiwara, T. Distribution of an enzyme system producing cis-3-hexenal and n-hexanal from linolenic and linoleic acids in some plants. Phytochemistry 1978, 17, 869–872. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grimm, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Chyau, C.C.; Chen, S.Y.; Wu, C.M. Differences of volatile and nonvolatile constituents between mature and ripe guava (Psidium guajava Linn.) fruits. J. Agric. Food Chem. 1992, 40, 846–849. [Google Scholar] [CrossRef]
- Egea, M.B.; Pereira-Netto, A.B.; Cacho, J.; Ferreira, V.; Lopez, R. Comparative analysis of aroma compounds and sensorial features of strawberry and lemon guavas (Psidium cattleianum Sabine). Food Chem. 2014, 164, 272–277. [Google Scholar] [CrossRef] [PubMed]

| Chemical Compounds | Fruits | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayberry 1 | Bayberry 2 | Cherimoya 3 | Cascara 4 | Gabiroba 5 | Hardy Kiwi 6 | “Honeycrisp” Apple 7 | Lucuma 8 | Mango 9 | Mulberry 10 | Ningxia Goji Berries 11 | Passion 12 | Peach 13 | Quince 14 | Sour Guava 15 | Tamarind 16 | Terebinth 17 | Tomato 18 | Watermelon 19 | |
| (Z)-3-hexenal | X | ||||||||||||||||||
| (E)-2-hexenal | X | X | X | X | X | X | X | X | |||||||||||
| (E)-2-Nonenal | X | X | |||||||||||||||||
| (E)-2-Octenal | X | X | |||||||||||||||||
| (E,E)-2,4-Hexadienal | X | ||||||||||||||||||
| (Z)-3-hexen-1-ol | X | ||||||||||||||||||
| (Z)-3-hexenal | X | X | X | ||||||||||||||||
| (Z)-6-nonenal | X | ||||||||||||||||||
| (Z)-β-Ocimene | X | X | |||||||||||||||||
| 1,3-octanediol | X | ||||||||||||||||||
| 1,8-cineol | X | ||||||||||||||||||
| 1-hexanol | X | X | X | X | |||||||||||||||
| 1-Octen-3-one | X | X | |||||||||||||||||
| 2,3-butanediol | |||||||||||||||||||
| 2,3-butanedione | X | ||||||||||||||||||
| 2-methyl-1,3-dithiolane | X | ||||||||||||||||||
| 3-mercaptohexanol | X | ||||||||||||||||||
| 3-methylbutyl 3-methylbutanoate | X | ||||||||||||||||||
| 3-methylbutyl butanoate | X | ||||||||||||||||||
| 3-octanone | X | ||||||||||||||||||
| 4-Hydroxy-2,5-dimethyl-3(2H)-furanone | X | ||||||||||||||||||
| 4-Oxoisophorone | X | ||||||||||||||||||
| 6-Methyl-5-hepten-2-one | X | X | |||||||||||||||||
| benzaldehyde | X | ||||||||||||||||||
| butyl butanoate | X | ||||||||||||||||||
| cinnamyl acetate | X | ||||||||||||||||||
| cis-4,5-epoxy-(2E)-undec-2-enal | X | ||||||||||||||||||
| cis-linalool oxide | X | ||||||||||||||||||
| Citral | X | ||||||||||||||||||
| diethyl carbonate | X | ||||||||||||||||||
| dimethyl disulphide | X | ||||||||||||||||||
| ethanol | X | ||||||||||||||||||
| ethyl (E)-2-butenoate | X | ||||||||||||||||||
| ethyl 2-methylbutenoate | X | ||||||||||||||||||
| ethyl 2-methylpropanoate | X | ||||||||||||||||||
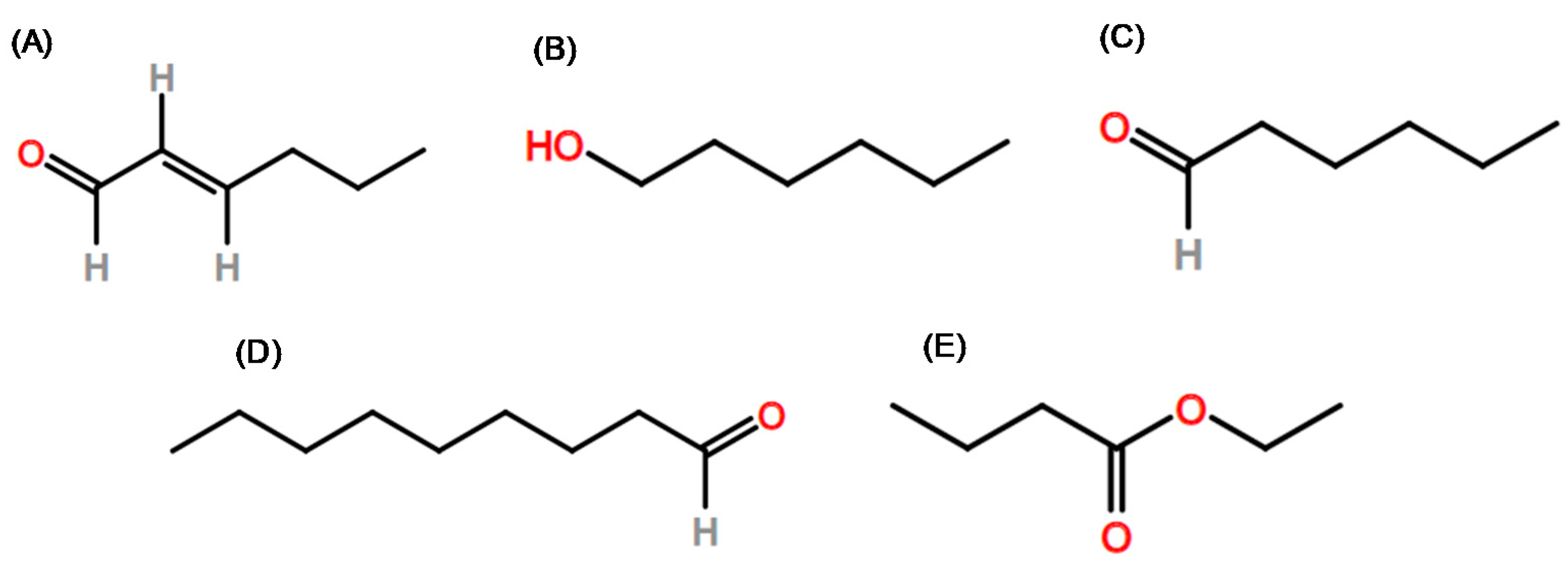
| ethyl butanoate | X | X | X | X | |||||||||||||||
| ethyl cis-3-hexenoate | X | ||||||||||||||||||
| ethyl hexanoate | X | X | X | ||||||||||||||||
| geranyl acetone | X | ||||||||||||||||||
| Guaiacol | X | ||||||||||||||||||
| hexanal | X | X | X | X | X | X | X | X | |||||||||||
| hexyl 2-methylbutyrate | X | ||||||||||||||||||
| isoamylol | X | ||||||||||||||||||
| isocaryophyllene | X | ||||||||||||||||||
| Linalool | X | ||||||||||||||||||
| methional | X | X | |||||||||||||||||
| methyl 2-methylbutanoate | X | ||||||||||||||||||
| nonanal | X | X | X | X | X | ||||||||||||||
| Ocimene | X | ||||||||||||||||||
| p-cymene | X | ||||||||||||||||||
| propyl acetate | X | ||||||||||||||||||
| Sotolone | X | ||||||||||||||||||
| terpinen-4-ol | |||||||||||||||||||
| terpinolene | X | ||||||||||||||||||
| trans-4,5-epoxy-(2E)-dec-2-enal | X | ||||||||||||||||||
| α-farnesene | X | ||||||||||||||||||
| α-Pinene | X | X | |||||||||||||||||
| β-Damascenone | X | ||||||||||||||||||
| β-Ionone | X | ||||||||||||||||||
| β-Myrcene | X | ||||||||||||||||||
| γ-decalactone | X | ||||||||||||||||||
| γ-terpinene | X | ||||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egea, M.B.; Bertolo, M.R.V.; Oliveira Filho, J.G.d.; Lemes, A.C. A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules 2021, 26, 5181. https://doi.org/10.3390/molecules26175181
Egea MB, Bertolo MRV, Oliveira Filho JGd, Lemes AC. A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules. 2021; 26(17):5181. https://doi.org/10.3390/molecules26175181
Chicago/Turabian StyleEgea, Mariana Buranelo, Mirella Romanelli Vicente Bertolo, Josemar Gonçalves de Oliveira Filho, and Ailton Cesar Lemes. 2021. "A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis" Molecules 26, no. 17: 5181. https://doi.org/10.3390/molecules26175181
APA StyleEgea, M. B., Bertolo, M. R. V., Oliveira Filho, J. G. d., & Lemes, A. C. (2021). A Narrative Review of the Current Knowledge on Fruit Active Aroma Using Gas Chromatography-Olfactometry (GC-O) Analysis. Molecules, 26(17), 5181. https://doi.org/10.3390/molecules26175181










