Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin
Abstract
:1. Introduction
2. Results
2.1. Quantitative Determination of the Podophyllotoxin Content in the Juniper Extracts
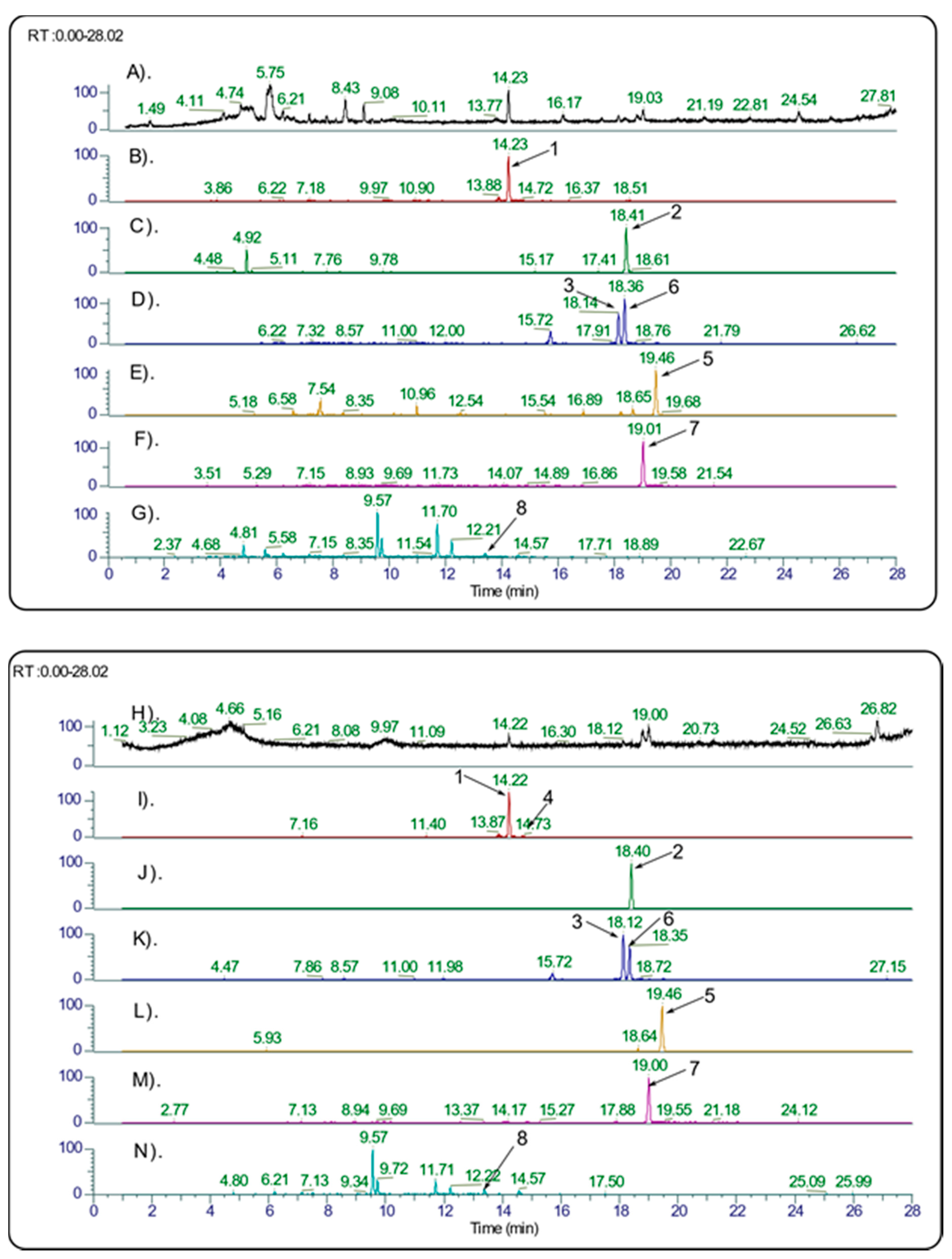
2.2. Identification of PPT Derivatives and Other Lignans in the Cytotoxic Juniper Extracts
2.3. Identification of Juniperus Species with Efficient Antiproliferative Activity of Their Extracts
3. Discussion
3.1. Quantification of PPT and Identification of Other Lignans in the Juniper Leaf Extracts
3.2. Selection of Juniper Species with High Antiproliferative Activity, Based on the PPT Content of Their Extracts
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Extraction Procedure
4.4. Cell Culture and MTT-Assays
4.5. Data Processing and Statistics
4.6. LC-ESI-MS/MS Analyses
4.7. UHPLC-HRMS Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Adams, R.P. Junipers of the World: The Genus Juniperus, 4th ed.; Trafford Publishing Co.: Bloomington, IN, USA, 2014; Available online: https://www.amazon.com/Junipers-World-Genus-Juniperus-4th/dp/1490723250 (accessed on 1 July 2021)ISBN 978-1-4907-2325-9.
- Krüssmann, G. Manual of Cultivated Conifers; Timber Press: Portland, OR, USA, 1991; Available online: https://www.amazon.com/Manual-Cultivated-Conifers-Gerd-Krüssmann/dp/1604691115 (accessed on 1 July 2021)ISBN 978-1604691115.
- Bhardwaj, K.; Silva, A.; Atanassova, M.; Sharma, R.; Nepovimova, E.; Musilek, K.; Sharma, R.; Alghuthaymi, M.; Dhanjal, D.; Nicoletti, M.; et al. Conifers Phytochemicals: A Valuable Forest with Therapeutic Potential. Molecules 2021, 26, 3005. [Google Scholar] [CrossRef]
- Dawson, W.R.; Ebbell, B. The Papyrus Ebers; The Greatest Egyptian Medical Document. J. Egypt. Archaeol. 1938, 24, 250. [Google Scholar] [CrossRef]
- Hartwell, J.L. Plants used against cancer. A survey. Lloydia 1971, 34, 386–425. Available online: https://www.ncbi.nlm.nih.gov/pubmed/5173435 (accessed on 1 July 2021). [PubMed]
- Lewis, W.H.; Elvin-Lewis, M.P.F. Medical Botany: Plants Affecting Human Health; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Graham, J.; Quinn, M.; Fabricant, D.; Farnsworth, N. Plants used against cancer—An extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Lechner-Knecht, S. Sacred healing plants in Nepal. Dtsch. Apoth. Ztg. 1982, 122, 2122–2129. [Google Scholar]
- Gordien, A.Y.; Gray, A.I.; Franzblau, S.G.; Seidel, V. Antimycobacterial terpenoids from Juniperus communis L. (Cuppressaceae). J. Ethnopharmacol. 2009, 126, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.D.; O’Neill, T.; Picot, N.; Johnson, J.A.; Robichaud, G.A.; Webster, D.; Gray, C.A. Anti-mycobacterial natural products from the Canadian medicinal plant Juniperus communis. J. Ethnopharmacol. 2012, 143, 695–700. [Google Scholar] [CrossRef]
- Orchard, A.; Van Vuuren, S. Commercial Essential Oils as Potential Antimicrobials to Treat Skin Diseases. Evidence-Based Complement. Altern. Med. 2017, 2017, 4517971. [Google Scholar] [CrossRef] [Green Version]
- Gardner, D.R.; Panter, K.E.; James, L.F.; Stegelmeier, B.L. Abortifacient effects of lodgepole pine (Pinus contorta) and common juniper (Juniperus communis) on cattle. Vet. Hum. Toxicol. 1998, 40, 260–263. [Google Scholar]
- Johnson, W. Final Report on the Safety Assessment of Juniperus communis Extract, Juniperus oxycedrus Extract, Juniperus oxycedrus Tar, Juniperus phoenicea Extract, and Juniperus virginiana Extract. Int. J. Toxicol. 2001, 20, 41–56. [Google Scholar] [CrossRef]
- Borsche, W.; Niemann, J. Über Podophyllin. Eur. J. Org. Chem. 1932, 494, 126–142. [Google Scholar] [CrossRef]
- Lipke, M.M. An Armamentarium of Wart Treatments. Clin. Med. Res. 2006, 4, 273–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canel, C.; Moraes, R.M.; Dayan, F.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef]
- Guerram, M.; Jiang, Z.-Z.; Zhang, L.-Y. Podophyllotoxin, a medicinal agent of plant origin: Past, present and future. Chin. J. Nat. Med. 2012, 10, 161–169. [Google Scholar] [CrossRef]
- Stähelin, H.F.; von Wartburg, A. The chemical and biological route from podophyllotoxin glucoside to etoposide: Memorial award lecture. Cancer Res. 1991, 51, 5–15. Available online: https://www.ncbi.nlm.nih.gov/pubmed/1988106 (accessed on 1 July 2021).
- Shah, Z.; Gohar, U.; Jamshed, I.; Mushtaq, A.; Mukhtar, H.; Zia-Ui-Haq, M.; Toma, S.; Manea, R.; Moga, M.; Popovici, B. Podophyllotoxin: History, Recent Advances and Future Prospects. Biomolecules 2021, 11, 603. [Google Scholar] [CrossRef]
- Leander, K.; Rosen, B. Medicinal Uses for Podophyllotoxin. U.S. Patent 4,788,216, 29 November 1988. Available online: https://patents.google.com/patent/US4788216A/en (accessed on 1 July 2021).
- Fay, D.A.; Ziegler, H.W. Botanical Source Differentiation of Podophyllum Resin by HPLC. J. Liq. Chromatogr. 1985, 8, 1501–1505. [Google Scholar] [CrossRef]
- Pandey, H.; Nandi, S.K.; Kumar, A.; Palni, U.T.; Palni, L.M.S. Podophyllotoxin content in Podophyllum hexandrum Royle plants of known age of seed origin and grown at a lower altitude. Acta Physiol. Plant. 2007, 29, 121–126. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, T.; Sharma, N.; Kumar, V.; Sood, H.; Chauhan, R.S. Expression analysis of biosynthetic pathway genes vis-à-vis podophyllotoxin content in Podophyllum hexandrum Royle. Protoplasma 2015, 252, 1253–1262. [Google Scholar] [CrossRef]
- Cushman, K.E.; Maqbool, M.; Lata, H.; Bedir, E.; Khan, I.A.; Moraes, R.M. Podophyllotoxin Content and Yield of American Mayapple Leaves in Sun and Shade. HortScience 2005, 40, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Zheljazkov, V.D.; Jones, A.M.; Avula, B.; Maddox, V.; Rowe, D.E. Lignan and Nutrient Concentrations in American Mayapple (Podophyllum peltatum L.) in the Eastern United States. HortScience 2009, 44, 349–353. [Google Scholar] [CrossRef]
- Sultan, P.; Shawl, A.; Ramteke, P.; Kour, A.; Qazi, P. Assessment of diversity in Podophyllum hexandrum by genetic and phytochemical markers. Sci. Hortic. 2008, 115, 398–408. [Google Scholar] [CrossRef]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar]
- Seca, A.M.L.; Silva, A.M.S. Recent Progress in Medicinal Plants, Vol. 16. In The Chemical Composition of the Juniperus Genus (1970–2004); Govil, J.N., Singh, V.K., Eds.; Studium Press LLC: Houston, TX, USA, 2006; pp. 401–522. Available online: https://www.researchgate.net/publication/283432291_The_chemical_composition_of_the_Juniperus_Genus_1970-2004 (accessed on 1 July 2021).
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-Dziki, U. LC-ESI-MS/MS-MRM Profiling of Polyphenols and Antioxidant Activity Evaluation of Junipers of Different Origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupré, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagège, D.; Lamblin, F.; Lainé, E.; et al. Podophyllotoxin and Deoxypodophyllotoxin in Juniperus bermudiana and 12 Other Juniperus Species: Optimization of Extraction, Method Validation, and Quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef]
- Bedir, E.; Khan, I.; Moraes, R.M. Bioprospecting for podophyllotoxin. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002; pp. 545–549. Available online: https://www.hort.purdue.edu/newcrop/ncnu02/pdf/moraes-545.pdf (accessed on 1 July 2021)ISBN 0-970756-5-5.
- Cushman, K.E.; Maqbool, M.; Gerard, P.D.; Bedir, E.; Lata, H.; Moraes, R.M. Variation of Podophyllotoxin in Leaves of Eastern Red Cedar (Juniperus virginiana). Planta Med. 2003, 69, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Gawde, A.; Cantrell, C.L.; Zheljazkov, V.D. Dual extraction of essential oil and podophyllotoxin from Juniperus virginiana. Ind. Crop. Prod. 2009, 30, 276–280. [Google Scholar] [CrossRef]
- Gawde, A.; Zheljazkov, V.D.; Maddox, V.; Cantrell, C.L. Bioprospection of Eastern red cedar from nine physiographic regions in Mississippi. Ind. Crop. Prod. 2009, 30, 59–64. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. Chemometric Evaluation of the Anti-cancer Pro-drug Podophyllotoxin and Potential Therapeutic Analogues in Juniperus and Podophyllum Species. Phytochem. Anal. 2010, 22, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Donega, M.A.; Astatkie, T.; Heidel, B. Podophyllotoxin Concentration in Junipers in the Big Horn Mountains in Wyoming. HortScience 2012, 47, 1696–1697. [Google Scholar] [CrossRef]
- Benzina, S.; Harquail, J.; Jean, S.; Beauregard, A.-P.; Colquhoun, C.D.; Carroll, M.; Bos, A.; Gray, C.A.; Robichaud, G.A. Deoxypodophyllotoxin Isolated from Juniperus communis Induces Apoptosis in Breast Cancer Cells. Anti-Cancer Agents Med. Chem. 2014, 15, 79–88. [Google Scholar] [CrossRef]
- Feliciano, A.S.; del Corral, J.M.M.; Gordaliza, M.; Castro, A. Lignans from Juniperus sabina. Phytochemistry 1990, 29, 1335–1338. [Google Scholar] [CrossRef]
- Hartwell, J.L.; Johnson, J.M.; Fitzgerald, D.B.; Belkin, M. Podophyllotoxin from Juniperus Species; Savinin. J. Am. Chem. Soc. 1953, 75, 235–236. [Google Scholar] [CrossRef]
- Ivanova, D.I.; Tashev, A.N.; Nedialkov, P.T.; Ilieva, Y.E.; Atanassova, T.N.; Olech, M.; Nowak, R.; Angelov, G.; Tsvetanova, F.V.; Iliev, I.A.; et al. Antioxidant and antiproliferative activity of Juniperus L. species of Bulgarian and foreign origin and their anticancer metabolite identification. Bulg. Chem. Commun. 2018, 50C, 144–150. Available online: http://www.bcc.bas.bg/BCC_Volumes/Volume_50_Special_C_2018/pdf/BCC-50-C-2018-144-150-Ivanova-28.pdf (accessed on 1 July 2021).
- Zheljazkov, V.; Cantrell, C.; Semerdjieva, I.; Radoukova, T.; Stoyanova, A.; Maneva, V.; Kačániová, M.; Astatkie, T.; Borisova, D.; Dincheva, I.; et al. Essential Oil Composition and Bioactivity of Two Juniper Species from Bulgaria and Slovakia. Molecules 2021, 26, 3659. [Google Scholar] [CrossRef] [PubMed]
- Och, M.; Och, A.; Ciesla, L.; Kubrak, T.; Pecio, Ł.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2014, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.S.; Phuong, N.T.; Park, K.R.; Kim, Y.H.; Kim, K.H.; Kang, J.S. Distribution of (−)-yatein in Cupressaceae family analysed by high performance liquid chromatography. Arch. Pharmacal Res. 2004, 27, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinié, R.; Corbin, C.; Montguillon, J.; Banuls, L.M.Y.; Renouard, S.; Lamblin, F.; Dupré, P.; et al. Investigation of the Lignan Content in Extracts from Linum, Callitris and Juniperus Species in Relation to Their In Vitro Antiproliferative Activities. Planta Medica 2016, 83, 574–581. [Google Scholar] [CrossRef] [Green Version]
- Cairnes, D.A.; Ekundayo, O.; Kingston, D.G.I. Plant Anticancer Agents. X. Lignans From Juniperus phoenicea. J. Nat. Prod. 1980, 43, 495–497. [Google Scholar] [CrossRef]
- Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.; Mojica, M.Y.A.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.I.; Álvarez, L. Cytotoxic Podophyllotoxin Type-Lignans from the Steam Bark of Bursera fagaroides var. fagaroides. Molecules 2012, 17, 9506–9519. [Google Scholar] [CrossRef] [Green Version]
- Donoso-Fierro, C.; Tiezzi, A.; Ovidi, E.; Ceccarelli, D.; Triggiani, D.; Mastrogiovanni, F.; Taddei, A.R.; Pérez, C.; Becerra, J.; Silva, M.; et al. Antiproliferative activity of yatein isolated from Austrocedrus chilensis against murine myeloma cells: Cytological studies and chemical investigations. Pharm. Biol. 2014, 53, 378–385. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lin, S.-Z.; Chang, J.-Y.; Cheng, Y.-L.; Tsai, N.-M.; Chen, S.-P.; Chang, W.-L.; Harn, H.-J. In vitro and in vivo studies of a novel potential anticancer agent of isochaihulactone on human lung cancer A549 cells. Biochem. Pharmacol. 2006, 72, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Natural lignans from Arctium lappa modulate P-glycoprotein efflux function in multidrug resistant cancer cells. Phytomedicine 2015, 22, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of arctigenin and matairesinol against the T-cell lymphoma cell line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Hemmati, S.; Fuss, E.; Alfermann, A.W. A combined HPLC-UV and HPLC-MS method for the identification of lignans and its application to the lignans of Linum usitatissimum L. and L. bienne Mill. Phytochem. Anal. 2006, 17, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hendrawati, O.; Woerdenbag, H.; Michiels, P.J.; Aantjes, H.G.; Van Dam, A.; Kayser, O. Identification of lignans and related compounds in Anthriscus sylvestris by LC–ESI-MS/MS and LC-SPE–NMR. Phytochemistry 2011, 72, 2172–2179. [Google Scholar] [CrossRef]
- Lanotte, M.; Martin-Thouvenin, V.; Najman, S.; Balerini, P.; Valensi, F.; Berger, R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991, 77, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.-K.; Tsui, S.-K.; Kwan, S.-Y.; Su, X.-L.; Lin, R.-C. Identification and characterization of Podophyllum emodi by API-LC/MS/MS. J. Mass Spectrom. 2000, 35, 1246–1251. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshöft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef]
- Koulman, A.; Bos, R.; Medarde, M.; Pras, N.; Quax, W.J. A Fast and Simple GC MS Method for Lignan Profiling in Anthriscus sylvestris and Biosynthetically Related Plant Species. Planta Medica 2001, 67, 858–862. [Google Scholar] [CrossRef]
- Khaled, M.; Jiang, Z.-Z.; Zhang, L.-Y. Deoxypodophyllotoxin: A promising therapeutic agent from herbal medicine. J. Ethnopharmacol. 2013, 149, 24–34. [Google Scholar] [CrossRef]
- Adams, R.P.; Johnson, S.T.; Anderson, J.; Rushforth, K.; Farhat, P.; Valentin, N.; Siljak-Yakovlev, S. The origin of Juniperus x pfitzeriana, an allotetraploid hybrid of J. chinensis x J. sabina. Phytologia 2019, 101, 164–174. Available online: https://www.juniperus.org/uploads/2/2/6/3/22639912/416._-_phyto101_2_164-174adamsetal_xfitzeriana_w_2_seagreen_6-11-19.pdf (accessed on 1 July 2021).
- Adams, R.P.; Boratynski, A.; Mataraci, T.; Tashev, A.N.; Schwarzbach, A.E. Discovery of Juniperus sabina var. balkanensis R. P. Adams and A. N. Tashev in western Turkey (Anatolia). Phytologia 2017, 99, 22–31. Available online: https://www.juniperus.org/uploads/2/2/6/3/22639912/375-_2017_phyto_99_1_22-31adams_boratynski_taskev_dist_v_balkanensis.pdf (accessed on 1 July 2021).
- Adams, R.P.; Farhat, P.; Shuka, L.; Siljak-Yakovlev, S. Discovery of Juniperus sabina var. balkanensis R. P. Adams and A. N. Tashev in Albania and relictual polymorphisms found in nrDNA. Phytologia 2018, 100, 187–194. Available online: https://www.juniperus.org/uploads/2/2/6/3/22639912/402._-_2019_phyto100_3_187-194adams_et_al_discovery_balkanensisalkbaniatypo4-27-19.pdf (accessed on 1 July 2021).
- Adams, R.P.; Schwarzbach, A.E.; Tashev, A.N. Chloroplast capture by a new variety, Juniperus sabina var. balkanensis R. P. Adams and A. N. Tashev, from the Balkan peninsula: A putative stabilized relictual hybrid between J. sabina and ancestral J. thurifera. Phytologia 2016, 98, 100–111. Available online: https://www.juniperus.org/uploads/2/2/6/3/22639912/361_-_2016_phyto_98_2_100-111adams_tashev_schwarzback_balcanensis_new_sp__rev_2-18-16.pdf (accessed on 1 July 2021).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Konstantinov, S.M.; Eibl, H.; Berger, M.R. BCR-ABL influences the antileukaemic efficacy of alkylphosphocholines. Br. J. Haematol. 1999, 107, 365–374. [Google Scholar] [CrossRef] [PubMed]
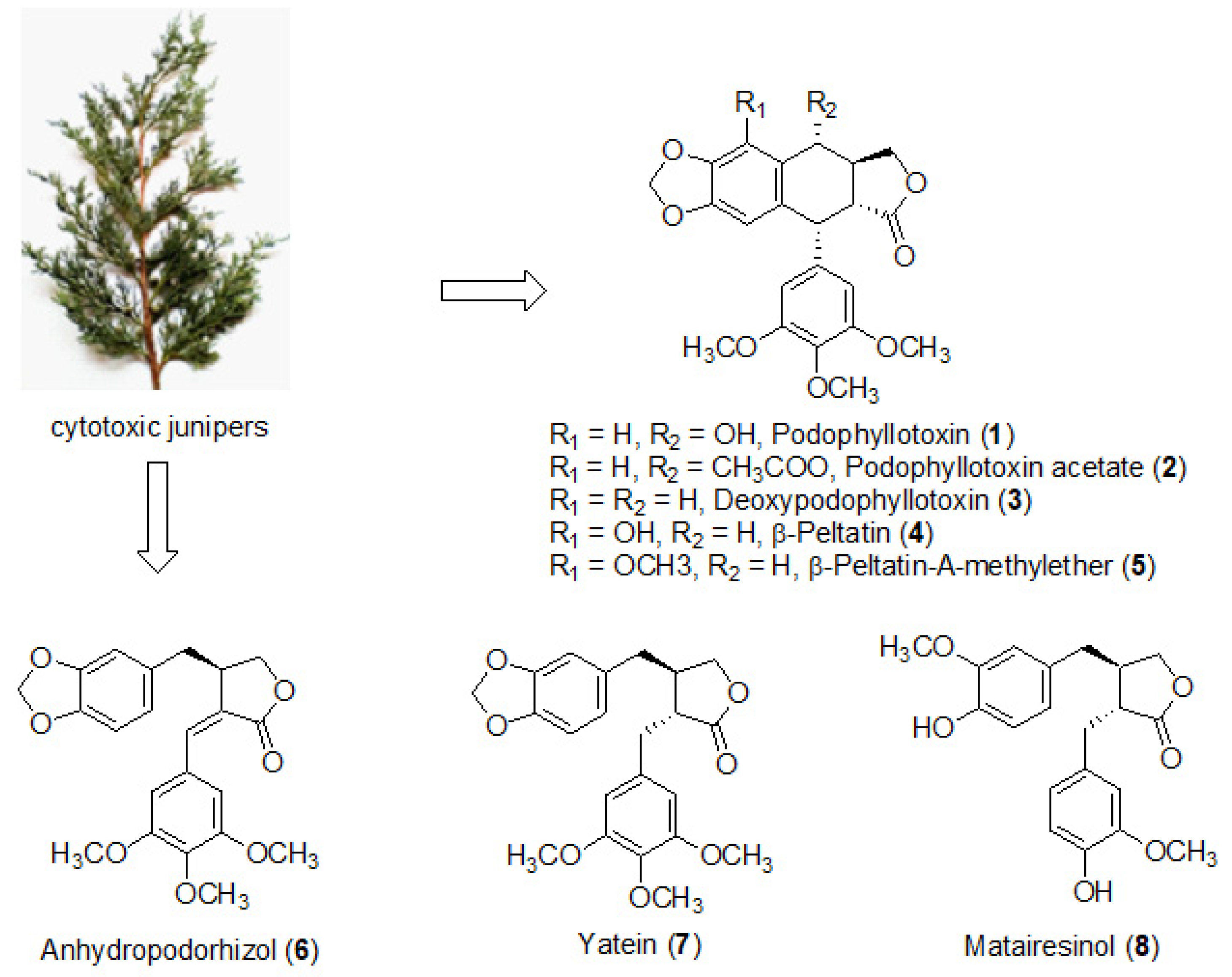
| Extract № | Specimen Number | Juniper Species | F/M | Organ Used | Original Source Accession Summary | %PPT ± SD in the Dry Extract | NB-4 IC50 ± SD [μg/mL] | Q% |
|---|---|---|---|---|---|---|---|---|
| 1 | AA 1746–81/A | J. virginiana L. | F | leaves | wild, USA, Bald Head Cliff, Maine, Ogunquit, 1981 | 0.91 ± 0.01 | 0.21 ± 0.01 | 12 |
| 2 | AA 1746–81/A | J. virginiana L. | F | galbuli | wild, USA, Bald Head Cliff, Maine, Ogunquit, 1981 | 0.23 ± 0.01 | 0.54 ± 0.03 | 6 |
| 3 | AA 4714/A | J. virginiana ‘Cinerascens’ | F | leaves | Berlin, Späth Arb., 1901 | 1.03 ± 0.03 | 0.37 ± 0.03 | 12 |
| 4 | SOM 174406 | J. virginiana L. | ME | leaves | cultivar, UFA, Sofia, BG | 0.37 ± 0.01 | 0.48 ± 0.10 | 15 |
| 5 | AA 14882/A | J. virginiana ‘Glauca’ | F | leaves | Rochester Park, New York, 1903 | 0.74 ± 0.01 | 0.29 ± 0.02 | 8 |
| 6 | AA 14882/A | J. virginiana ‘Glauca’ | F | galbuli | Rochester Park, New York, 1903 | 0.04 ± 0.01 | 0.26 ± 0.01 | 9 |
| 7 | AA 1136–61/A | J. virginiana ‘Grey Owl’ | F | leaves | Dominion Arb., Ottawa, Canada, 1961 | 0.39 ± 0.01 | 0.72 ± 0.10 | 11 |
| 8 | AA 1136–61/A | J. virginiana ‘Grey Owl’ | F | galbuli | Dominion Arb., Ottawa, Canada, 1961 | 0.09 ± 0.01 | 0.49 ± 0.20 | 7 |
| 9 | AA 211–57/A | J. scopulorum ‘Moon light’ | M | leaves | USA, D. Hill Nurs., Dundee, Illinois, 1957 | 0.85 ± 0.02 | 0.20 ± 0.04 | 18 |
| 10 | AA 14868/E | J. sabina L. | F | leaves | wild, 1904, Uzbekistan | 0.50 ± 0.01 | 0.34 ± 0.01 | 13 |
| 11 | SOM 177009 | J. sabina var. balkanensis Adams & Tashev | M | leaves | wild, from eastern Rhodopes, BG | 0.20 ± 0.01 | 0.29 ± 0.03 | 16 |
| 12 | AA 1164–56/A | J. horizontalis Moench | F | leaves | D. Hill Nurs., USA, Dundee, Illinois, 1956 | 0.38 ± 0.01 | 0.23 ± 0.03 | 12 |
| 13 | AA 1164–56/A | J. horizontalis Moench | F | galbuli | D. Hill Nurs., USA, Dundee, Illinois, 1956 | 0.03 ± 0.01 | 2.43 ± 0.22 | 7 |
| 14 | AA 74–42/C | J. × media ‘Pfitzeriana Argentea’ | M | leaves | Morris Arb, Pennsylvania, USA, 1942 | 0.42 ± 0.01 | 0.51 ± 0.07 | 11 |
| 15 | AA 183–62/A | J. × media ‘Óld Gold’ | M | leaves | Grootendorst Nurs., Holland, 1962 | 0.47 ± 0.01 | 0.42 ± 0.04 | 10 |
| 16 | AA 639–48/A | J. × media ‘Richeson’ | M | leaves | Armstrong Nurs., Ontario, CA, 1948 | 0.22 ± 0.01 | 0.43 ± 0.03 | 13 |
| 17 | AA 1–51/A | J. chinensis ‘Pfitzer Mattews Blue’ | M | leaves | Interstate Nursery, Iowa, USA, 1951 | 1.30 ± 0.05 | 0.24 ± 0.01 | 13 |
| 18 | AA 219–61/A | J. chinensis ‘Plumosa Aurea’ | M | leaves | Pennsylvania, USA, 1961 | 0.22 ± 0.02 | 0.45 ± 0.02 | 10 |
| 19 | AA 265–33/A | J. chinensis L. | M | leaves | California, USA, 1933 | BQL | 0.5 ± 0.3 | 14 |
| 20 | AA 14809/A | J. chinensis L. | F | leaves | Royal Botanic Gardens, UK, Kew, 1908 | BQL | 1.0 ± 0.1 | 15 |
| 21 | AA 14809/A | J. chinensis L. | F | galbuli | Royal Botanic Gardens, UK, Kew, 1908 | BQL | 0.8 ± 0.1 | 7 |
| 22 | SOM 174400 | J. communis L. | F | leaves | wild from Rhodopes, BG | BQL | 1.0 ± 0.4 | 15 |
| 23 | SOM 174400 | J. communis L. | F | galbuli | wild from Rhodopes, BG | BQL | 3.6 ± 1.7 | 13 |
| 24 | AA 49–66/A | J. communis ‘Laxa’ | M | leaves | U. S. Natl. Arb., 1966, Washington | BQL | 0.8 ± 0.2 | 16 |
| 25 | AA 4176–1/A | J. communis ‘Oblonga Pendula’ | M | leaves | USA, Biltmore Estate, North Carolina, 1907 | BQL | 4.7 ± 0.6 | 17 |
| 26 | AA 280–98/A | J. formosana Hayata | F | leaves | wild, from Taiwan, 1998 | BQL | 1.7 ± 0.3 | 17 |
| 27 | SOM 174401 | J. sibirica Burgsd. | F | leaves | wild, from Vitosha, BG | ND | 3 ± 1 | 15 |
| 28 | SOM 174401 | J. sibirica Burgsd. | F | galbuli | wild, from Vitosha, BG | BQL | 15 ± 3 | 9 |
| 29 | SOM 174402 | J. pigmaea K. Koch | F | leaves | wild from Rhodopes, BG | BQL | 5 ± 1 | 15 |
| 30 | SOM 174402 | J. pigmaea K. Koch | F | galbuli | wild from Rhodopes, BG | BQL | 29 ± 7 | 11 |
| 31 | AA 20–89/A | J. squamata ‘Meyeri’ | F | leaves | Hicks Nurs., Westbury, NY, 1989 | BQL | 6.3 ± 0.5 | 10 |
| 32 | AA 642–88/B | J. pinchotii Sudw. | M | leaves | wild, USA, Oklahoma, Kiowa reserv., 1988 | BQL | 17 ± 2 | 19 |
| 33 | SOM 174403 | J. deltoides R. P. Adams | F | leaves | wild from Rhodopes, BG | BQL | 66 ± 8 | 16 |
| 34 | SOM 174403 | J. deltoides R. P. Adams | F | galbuli | wild from Rhodopes, BG | ND | 70 ± 5 | 17 |
| 35 | AA 276–86/A | J. ashei J. Buchholz | M | leaves | wild, USA, Oklahoma, Murray, 1986 | BQL | 130 ± 18 | 28 |
| 36 | SOM 174404 | J. excelsa M. Bieb. | F | leaves | wild, Struma riverside, BG | ND | 137 ± 12 | 19 |
| 37 | SOM 174404 | J. excelsa M. Bieb. | F | galbuli | wild, Struma riverside, BG | ND | 188 ± 55 | 16 |
| - | control | Podophyllotoxin | - | standard compound | - | 0.005 ± 0.001 | - |
| Compound | RT [min] | Molecular Weight | [M + H]+ [m/z] | Fragment Ions [m/z] | Collision Energy [eV] | DP [V] | EP [V] | CEP [V] | CXP [V] |
|---|---|---|---|---|---|---|---|---|---|
| PPT | 3.60 | 414 | 415 | 397 | 18 | 30 | 10 | 23.6 | 2 |
| 247 | 20 | 30 | 10 | 23.6 | 2 | ||||
| 229 | 20 | 30 | 10 | 23.6 | 2 |
| Compound | LOD [ng mL−1] | LOQ [ng mL−1] | r2 | Equation | Linearity Range [ng mL−1] |
|---|---|---|---|---|---|
| PPT | 5 | 12.5 | 0.9992 | y = 87.3x − 144 | 12.5 to 500 |
| Compound | RT min | Molecular Formula | [M + H]+ | HRMS/MS Fragments ** | Extract № | Ref | ||
|---|---|---|---|---|---|---|---|---|
| Calcd. * | Found | Δppm * | ||||||
| Podophyllotoxin | 14.23 | C22H22O8 | 415.1387 | 415.1385 | −0.61 | 397.1277 (100), 313.1066 (10), 247.0598 (85), 229.0493 (20), 185.0596 (5) | 1, 3–5, 7, 9–12, 14–18 *** | [51,52] |
| Podophyllotoxin acetate | 18.41 | C24H24O9 | 457.1496 | 457.1493 | 0.57 | 397.1287 (80), 355.1169 (70), 313.1070 (100), 229.0492 (50), 185.0596 (25) | 7, 9, 10, 14–16, 18 | [46] |
| Deoxypodophyllotoxin | 18.14 | C22H22O7 | 399.1438 | 399.1437 | −0.23 | 231.0649 (100), 187.0752 (10) | 1, 3–5, 7, 9–12, 14–20, 22, 24–27, 29 | [51,52] |
| β-Peltatin | 14.72 | C22H22O8 | 415.1387 | 415.1390 | 0.54 | 247.0599 (100), 203.0701 (5), 189.0545 (2) | 1, 4, 5, 14–20, | [51,52] |
| β-Peltatin-A-methyl-ether | 19.47 | C23H24O8 | 429.1544 | 429.1546 | 0.57 | 261.0755 (100), 217.0860 (10) | 1, 3, 4, 7, 9, 10, 12, 14–19, 22, 24, 25 | [51,52] |
| Anhydropodorhizol | 18.35 | C22H22O7 | 399.1438 | 399.1440 | 0.38 | 381.1327 (30), 363.1227 (10), 231.0649 (90), 203.0701 (40), 187.0752 (20), 181.0857 (10), 135.0441 (15) | 1, 3–5, 7, 9–12, 14–19 | [52] |
| Yatein | 19.02 | C22H24O7 | 401.1595 | 401.1598 | 0.74 | 383.1484 (100), 365.1381 (5), 223.0963 (5), 181.0858 (30), 161.0596 (10), 135.0441 (5) | 1, 3, 5, 7, 9–12, 14–20, 26 | [51,52] |
| Matairesinol | 13.40 | C20H22O6 | 359.1489 | 359.1490 | 0.15 | 341.1379 (60), 323.1274 (25), 223.0964 (10), 163.0752 (10), 137.0596 (100) | 1, 3–5, 7, 9, 10, 20, 24, 25, 29 | [51] |
| Extract № | Juniperus Representatives | K-562 | BV-173 | T-24 | HT-29 |
|---|---|---|---|---|---|
| 4 | J. virginiana | 0.4 ± 0.1 | 0.14 ± 0.06 | 1.3 ± 0.5 | 5 ± 1 |
| 7 | J. virginiana ‘Grey Owl’ | 0.4 ± 0.3 | 0.11 ± 0.05 | 4.0 ± 3.5 | 8 ± 5 |
| 9 | J. scopulorum ‘Moon light’ | 0.3 ± 0.1 | 0.22 ± 0.03 | 0.8 ± 0.2 | 2 ± 1 |
| 10 | J. sabina | 0.4 ± 0.1 | 0.24 ± 0.03 | 0.8 ± 0.3 | 2 ± 1 |
| 11 | J. sabina var. balkanensis | 0.2 ± 0.1 | 0.16 ± 0.03 | 0.7 ± 0.3 | 2 ± 1 |
| 22 | J. communis | 1.6 ± 1.2 | 1.6 ± 0.2 | 19 ± 12 | 17 ± 7 |
| control | Podophyllotoxin | 0.006 ± 0.003 | 0.007 ± 0.002 | 0.007 ± 0.002 | 0.007 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, D.I.; Nedialkov, P.T.; Tashev, A.N.; Olech, M.; Nowak, R.; Ilieva, Y.E.; Kokanova-Nedialkova, Z.K.; Atanasova, T.N.; Angelov, G.; Najdenski, H.M. Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules 2021, 26, 5179. https://doi.org/10.3390/molecules26175179
Ivanova DI, Nedialkov PT, Tashev AN, Olech M, Nowak R, Ilieva YE, Kokanova-Nedialkova ZK, Atanasova TN, Angelov G, Najdenski HM. Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules. 2021; 26(17):5179. https://doi.org/10.3390/molecules26175179
Chicago/Turabian StyleIvanova, Diana I., Paraskev T. Nedialkov, Alexander N. Tashev, Marta Olech, Renata Nowak, Yana E. Ilieva, Zlatina K. Kokanova-Nedialkova, Teodora N. Atanasova, George Angelov, and Hristo M. Najdenski. 2021. "Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin" Molecules 26, no. 17: 5179. https://doi.org/10.3390/molecules26175179
APA StyleIvanova, D. I., Nedialkov, P. T., Tashev, A. N., Olech, M., Nowak, R., Ilieva, Y. E., Kokanova-Nedialkova, Z. K., Atanasova, T. N., Angelov, G., & Najdenski, H. M. (2021). Junipers of Various Origins as Potential Sources of the Anticancer Drug Precursor Podophyllotoxin. Molecules, 26(17), 5179. https://doi.org/10.3390/molecules26175179










