Recent Advances in In Silico Target Fishing
Abstract
1. Introduction
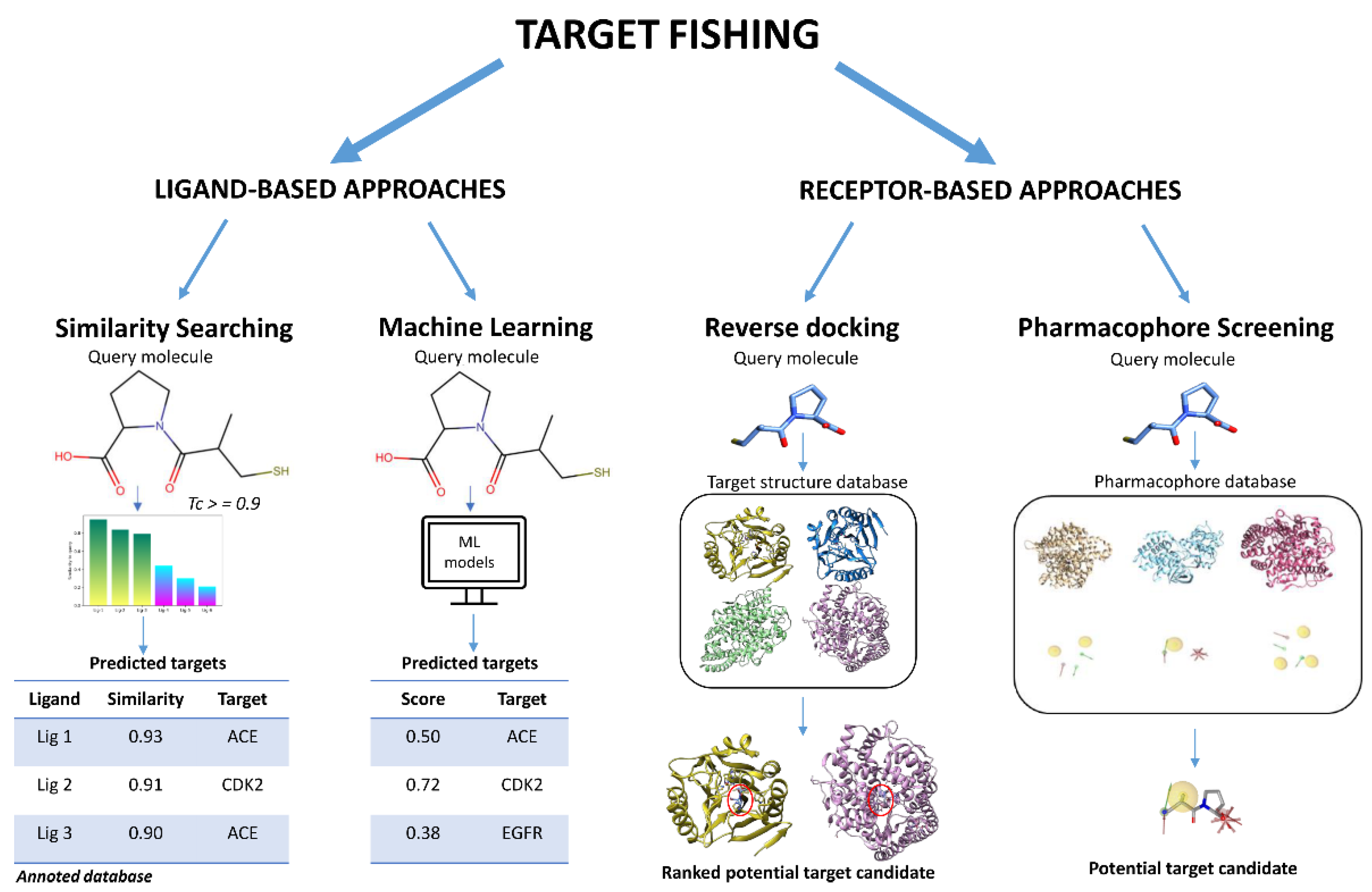
2. Ligand-Based Approaches
2.1. 2D-Structure Similarity Searching
2.2. Mixed 2D/3D-Structure Similarity Searching
2.3. Machine Learning
3. Receptor-Based Approaches
3.1. Reverse Docking
3.2. Pharmacophore-Based Target Fishing Approach
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boran, A.D.W.; Iyengar, R. Systems approaches to polypharmacology and drug discovery. Curr. Opin. Drug Discov. Dev. 2010, 13, 297–309. [Google Scholar]
- Xie, L.; Xie, L.; Bourne, P.E. Structure-based systems biology for analyzing off-target binding. Curr. Opin. Struct. Biol. 2011, 21, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.; Pries, V.; Hedberg, C.; Waldmann, H. Target identification for small bioactive molecules: Finding the needle in the haystack. Angew. Chemie Int. Ed. 2013, 52, 2744–2792. [Google Scholar] [CrossRef]
- Lounkine, E.; Keiser, M.J.; Whitebread, S.; Mikhailov, D.; Hamon, J.; Jenkins, J.L.; Lavan, P.; Weber, E.; Doak, A.K.; Côté, S.; et al. Large-scale prediction and testing of drug activity on side-effect targets. Nature 2012, 486, 361–367. [Google Scholar] [CrossRef]
- Anighoro, A.; Bajorath, J.; Rastelli, G. Polypharmacology: Challenges and opportunities in drug discovery. J. Med. Chem. 2014, 57, 7874–7887. [Google Scholar] [CrossRef]
- Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L. Review. One drug for multiple targets: A computational perspective. J. Mex. Chem. Soc. 2016, 60, 168–181. [Google Scholar]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Maggiora, G.; Vogt, M.; Stumpfe, D.; Bajorath, J. Molecular Similarity in Medicinal Chemistry. J. Med. Chem. 2014, 57, 3186–3204. [Google Scholar] [CrossRef]
- Papadatos, G.; Gaulton, A.; Hersey, A.; Overington, J.P. Activity, assay and target data curation and quality in the ChEMBL database. J. Comput. Aided. Mol. Des. 2015, 29, 885–896. [Google Scholar] [CrossRef]
- Wang, Y.; Bryant, S.H.; Cheng, T.; Wang, J.; Gindulyte, A.; Shoemaker, B.A.; Thiessen, P.A.; He, S.; Zhang, J. PubChem BioAssay: 2017 update. Nucleic Acids Res. 2017, 45, D955–D963. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Lomelino, C.L.; Andring, J.T.; McKenna, R. Crystallography and Its Impact on Carbonic Anhydrase Research. Int. J. Med. Chem. 2018, 2018, 9419521. [Google Scholar] [CrossRef]
- Li, Y.H.; Yu, C.Y.; Li, X.X.; Zhang, P.; Tang, J.; Yang, Q.; Fu, T.; Zhang, X.; Cui, X.; Tu, G.; et al. Therapeutic target database update 2018: Enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018, 46, D1121–D1127. [Google Scholar] [CrossRef]
- Nettles, J.H.; Jenkins, J.L.; Bender, A.; Deng, Z.; Davies, J.W.; Glick, M. Bridging Chemical and Biological Space: “Target Fishing” Using 2D and 3D Molecular Descriptors. J. Med. Chem. 2006, 49, 6802–6810. [Google Scholar] [CrossRef]
- Willett, P.; Barnard, J.M.; Downs, G.M. Chemical Similarity Searching. J. Chem. Inf. Comput. Sci. 1998, 38, 983–996. [Google Scholar] [CrossRef]
- Koshland, D.E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc. Natl. Acad. Sci. USA 1958, 44, 98–104. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Senese, S.; Damoiseaux, R.; Torres, J.Z. 3D Chemical Similarity Networks for Structure-Based Target Prediction and Scaffold Hopping. ACS Chem. Biol. 2016, 11, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.S.; Morris, G.M.; Finn, P.W.; Sharma, R.; Moretti, L.; Cooper, R.I.; Richards, W.G. ElectroShape: Fast molecular similarity calculations incorporating shape, chirality and electrostatics. J. Comput. Aided. Mol. Des. 2010, 24, 789–801. [Google Scholar] [CrossRef]
- ElGamacy, M.; Van Meervelt, L. A fast topological analysis algorithm for large-scale similarity evaluations of ligands and binding pockets. J. Cheminform. 2015, 7, 42. [Google Scholar] [CrossRef]
- Peón, A.; Li, H.; Ghislat, G.; Leung, K.-S.; Wong, M.-H.; Lu, G.; Ballester, P.J. MolTarPred: A web tool for comprehensive target prediction with reliability estimation. Chem. Biol. Drug Des. 2019, 94, 1390–1401. [Google Scholar] [CrossRef]
- Rogers, D.; Hahn, M. Extended-Connectivity Fingerprints. J. Chem. Inf. Model. 2010, 50, 742–754. [Google Scholar] [CrossRef]
- Peón, A.; Naulaerts, S.; Ballester, P.J. Predicting the Reliability of Drug-target Interaction Predictions with Maximum Coverage of Target Space. Sci. Rep. 2017, 7, 3820. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, C.; Wipf, P.; Liu, H.; Su, W.; Xie, X.-Q. TargetHunter: An in silico target identification tool for predicting therapeutic potential of small organic molecules based on chemogenomic database. AAPS J. 2013, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Peng, J.; Xu, Y.; Wang, Y.; Zhou, N.; Xing, J.; Luo, X.; Jiang, H.; Zheng, M. TarPred: A web application for predicting therapeutic and side effect targets of chemical compounds. Bioinformatics 2015, 31, 2049–2051. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Xu, Y.; Li, S.; Wang, Y.; Peng, J.; Luo, C.; Luo, X.; Zheng, M.; Chen, K.; Jiang, H. In Silico target fishing: Addressing a “Big Data” problem by ligand-based similarity rankings with data fusion. J. Cheminform. 2014, 6, 33. [Google Scholar] [CrossRef]
- Alberga, D.; Trisciuzzi, D.; Montaruli, M.; Leonetti, F.; Mangiatordi, G.F.; Nicolotti, O. A New Approach for Drug Target and Bioactivity Prediction: The Multifingerprint Similarity Search Algorithm (MuSSeL). J. Chem. Inf. Model. 2019, 59, 586–596. [Google Scholar] [CrossRef]
- Landrum G RDKit: Open-Source Cheminformatics. Available online: https://www.rdkit.org (accessed on 1 June 2021).
- O’Boyle, N.M.; Morley, C.; Hutchison, G.R. Pybel: A Python wrapper for the OpenBabel cheminformatics toolkit. Chem. Cent. J. 2008, 2, 5. [Google Scholar] [CrossRef]
- Steinbeck, C.; Han, Y.; Kuhn, S.; Horlacher, O.; Luttmann, E.; Willighagen, E. The Chemistry Development Kit (CDK): An Open-Source Java Library for Chemo- and Bioinformatics. J. Chem. Inf. Comput. Sci. 2003, 43, 493–500. [Google Scholar] [CrossRef]
- Wang, X.; Pan, C.; Gong, J.; Liu, X.; Li, H. Enhancing the Enrichment of Pharmacophore-Based Target Prediction for the Polypharmacological Profiles of Drugs. J. Chem. Inf. Model. 2016, 56, 1175–1183. [Google Scholar] [CrossRef]
- Awale, M.; Reymond, J.-L. The polypharmacology browser: A web-based multi-fingerprint target prediction tool using ChEMBL bioactivity data. J. Cheminform. 2017, 9, 11. [Google Scholar] [CrossRef]
- Venkatraman, V.; Pérez-Nueno, V.I.; Mavridis, L.; Ritchie, D.W. Comprehensive Comparison of Ligand-Based Virtual Screening Tools Against the DUD Data set Reveals Limitations of Current 3D Methods. J. Chem. Inf. Model. 2010, 50, 2079–2093. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Michielin, O.; Zoete, V. Shaping the interaction landscape of bioactive molecules. Bioinformatics 2013, 29, 3073–3079. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Armstrong, M.S.; Finn, P.W.; Morris, G.M.; Richards, W.G. Improving the accuracy of ultrafast ligand-based screening: Incorporating lipophilicity into ElectroShape as an extra dimension. J. Comput. Aided. Mol. Des. 2011, 25, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef]
- Lagunin, A.; Stepanchikova, A.; Filimonov, D.; Poroikov, V. PASS: Prediction of activity spectra for biologically active substances. Bioinformatics 2000, 16, 747–748. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Rensi, S.E.; Torng, W.; Altman, R.B. Machine learning in chemoinformatics and drug discovery. Drug Discov. Today 2018, 23, 1538–1546. [Google Scholar] [CrossRef]
- Gertrudes, J.C.; Maltarollo, V.G.; Silva, R.A.; Oliveira, P.R.; Silva, K.M.H. and A.B.F. da Machine Learning Techniques and Drug Design. Curr. Med. Chem. 2012, 19, 4289–4297. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Shukla, T.; Huang, X.; Ussery, D.W.; Wang, S. Machine Learning Methods in Drug Discovery. Molecules 2020, 25, 5277. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Webb, G.I.; Keogh, E.; Miikkulainen, R. Naïve Bayes. Encycl. Mach. Learn. 2010, 15, 713–714. [Google Scholar]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Jenkins, J.L.; Bender, A.; Davies, J.W. In silico target fishing: Predicting biological targets from chemical structure. Drug Discov. Today Technol. 2006, 3, 413–421. [Google Scholar] [CrossRef]
- Tsoumakas, G.; Katakis, I. Multi-Label Classification: An Overview. Int. J. Data Warehous. Min. 2007, 3, 1–13. [Google Scholar] [CrossRef]
- Speck-Planche, A.; Cordeiro, M.N.D.S. Multi-Target QSAR Approaches for Modeling Protein Inhibitors. Simultaneous Prediction of Activities against Biomacromolecules Present in Gram-Negative Bacteria. Curr. Top. Med. Chem. 2015, 15, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Zhou, Y.; Li, J.; Li, W.; Liu, G.; Tang, Y. Prediction of chemical-protein interactions: Multitarget-QSAR versus computational chemogenomic methods. Mol. Biosyst. 2012, 8, 2373–2384. [Google Scholar] [CrossRef] [PubMed]
- Nidhi; Glick, M.; Davies, J.W.; Jenkins, J.L. Prediction of biological targets for compounds using multiple-category Bayesian models trained on chemogenomics databases. J. Chem. Inf. Model. 2006, 46, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Caruana, R. Multitask Learning. Mach. Learn. 1997, 28, 41–75. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Liaw, A.; Sheridan, R.P.; Svetnik, V. Demystifying Multitask Deep Neural Networks for Quantitative Structure–Activity Relationships. J. Chem. Inf. Model. 2017, 57, 2490–2504. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, R.; Bajorath, J. Prediction of Compound Profiling Matrices, Part II: Relative Performance of Multitask Deep Learning and Random Forest Classification on the Basis of Varying Amounts of Training Data. ACS Omega 2018, 3, 12033–12040. [Google Scholar] [CrossRef]
- de la Vega de León, A.; Chen, B.; Gillet, V.J. Effect of missing data on multitask prediction methods. J. Cheminform. 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Tropsha, A. Best Practices for QSAR Model Development, Validation, and Exploitation. Mol. Inform. 2010, 29, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, M.; Kim, D. Utilizing random Forest QSAR models with optimized parameters for target identification and its application to target-fishing server. BMC Bioinform. 2017, 18, 567. [Google Scholar] [CrossRef]
- van Westen, G.J.P.; Wegner, J.K.; IJzerman, A.P.; van Vlijmen, H.W.T.; Bender, A. Proteochemometric modeling as a tool to design selective compounds and for extrapolating to novel targets. Med. Chem. Commun. 2011, 2, 16–30. [Google Scholar] [CrossRef]
- Geppert, H.; Humrich, J.; Stumpfe, D.; Gärtner, T.; Bajorath, J. Ligand Prediction from Protein Sequence and Small Molecule Information Using Support Vector Machines and Fingerprint Descriptors. J. Chem. Inf. Model. 2009, 49, 767–779. [Google Scholar] [CrossRef]
- Ning, X.; Rangwala, H.; Karypis, G. Multi-Assay-Based Structure−Activity Relationship Models: Improving Structure−Activity Relationship Models by Incorporating Activity Information from Related Targets. J. Chem. Inf. Model. 2009, 49, 2444–2456. [Google Scholar] [CrossRef]
- Lapinsh, M.; Prusis, P.; Petrovska, R.; Uhlén, S.; Mutule, I.; Veiksina, S.; Wikberg, J.E.S. Proteochemometric modeling reveals the interaction site for Trp9 modified alpha-MSH peptides in melanocortin receptors. Proteins 2007, 67, 653–660. [Google Scholar] [CrossRef]
- Wen, M.; Zhang, Z.; Niu, S.; Sha, H.; Yang, R.; Yun, Y.; Lu, H. Deep-Learning-Based Drug–Target Interaction Prediction. J. Proteome Res. 2017, 16, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Paricharak, S.; Cortés-Ciriano, I.; IJzerman, A.P.; Malliavin, T.E.; Bender, A. Proteochemometric modelling coupled to in silico target prediction: An integrated approach for the simultaneous prediction of polypharmacology and binding affinity/potency of small molecules. J. Cheminform. 2015, 7, 15. [Google Scholar] [CrossRef]
- Gamo, F.-J.; Sanz, L.M.; Vidal, J.; de Cozar, C.; Alvarez, E.; Lavandera, J.-L.; Vanderwall, D.E.; Green, D.V.S.; Kumar, V.; Hasan, S.; et al. Thousands of chemical starting points for antimalarial lead identification. Nature 2010, 465, 305–310. [Google Scholar] [CrossRef]
- Grenet, I.; Merlo, K.; Comet, J.-P.; Tertiaux, R.; Rouquié, D.; Dayan, F. Stacked Generalization with Applicability Domain Outperforms Simple QSAR on in Vitro Toxicological Data. J. Chem. Inf. Model. 2019, 59, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Miao, W.; Cui, J.; Fang, C.; Su, S.; Li, H.; Hu, L.; Lu, Y.; Chen, G. Efficient Corrections for DFT Noncovalent Interactions Based on Ensemble Learning Models. J. Chem. Inf. Model. 2019, 59, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Cockroft, N.T.; Cheng, X.; Fuchs, J.R. STarFish: A Stacked Ensemble Target Fishing Approach and its Application to Natural Products. J. Chem. Inf. Model. 2019, 59, 4906–4920. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, K.T.; Bietz, S.; Briem, H.; Henzler, A.M.; Urbaczek, S.; Rarey, M. Facing the challenges of structure-based target prediction by inverse virtual screening. J. Chem. Inf. Model. 2014, 54, 1676–1686. [Google Scholar] [CrossRef]
- Lee, A.; Lee, K.; Kim, D. Using reverse docking for target identification and its applications for drug discovery. Expert Opin. Drug Discov. 2016, 11, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, I.D.; Blaney, J.M.; Oatley, S.J.; Langridge, R.; Ferrin, T.E. A geometric approach to macromolecule-ligand interactions. J. Mol. Biol. 1982, 161, 269–288. [Google Scholar] [CrossRef]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tufféry, P. fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38, W582. [Google Scholar] [CrossRef]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef]
- Liu, H.; Lin, F.; Yang, J.-L.; Wang, H.-R.; Liu, X.-L. Applying Side-chain Flexibility in Motifs for Protein Docking. Genom. Insights 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Luger, D.; Poli, G.; Wieder, M.; Stadler, M.; Ke, S.; Ernst, M.; Hohaus, A.; Linder, T.; Seidel, T.; Langer, T.; et al. Identification of the putative binding pocket of valerenic acid on GABAA receptors using docking studies and site-directed mutagenesis. Br. J. Pharmacol. 2015, 172, 5403–5413. [Google Scholar] [CrossRef]
- Wang, J.C.; Chu, P.Y.; Chen, C.M.; Lin, J.H. idTarget: A web server for identifying protein targets of small chemical molecules with robust scoring functions and a divide-and-conquer docking approach. Nucleic Acids Res. 2012, 40, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Z.; Kang, L.; Zhang, H.; Yang, K.; Yul, K.; Luo, X.; Zhu, W.; Chen, K.; Shen, J.; et al. TarFisDock: A web server for identifying drug targets with docking approach. Nucleic Acids Res. 2006, 34, 219–224. [Google Scholar] [CrossRef]
- Luo, H.; Chen, J.; Shi, L.; Mikailov, M.; Zhu, H.; Wang, K.; He, L.; Yang, L. DRAR-CPI: A server for identifying drug repositioning potential and adverse drug reactions via the chemical-protein interactome. Nucleic Acids Res. 2011, 39, 492–498. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, P.; Cao, X.H.; Du, D.; Ye, H.; Huang, H.; Li, C.; Qin, S.; Wan, C.; Shi, L.; et al. DPDR-CPI, a server that predicts Drug Positioning and Drug Repositioning via Chemical-Protein Interactome. Sci. Rep. 2016, 6, 35996. [Google Scholar] [CrossRef]
- Warren, G.L.; Andrews, C.W.; Capelli, A.M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A critical assessment of docking programs and scoring functions. J. Med. Chem. 2006, 49, 5912–5931. [Google Scholar] [CrossRef]
- Lapillo, M.; Tuccinardi, T.; Martinelli, A.; Macchia, M.; Giordano, A.; Poli, G. Extensive Reliability Evaluation of Docking-Based Target-Fishing Strategies. Int. J. Mol. Sci. 2019, 20, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, X.; He, W.; Fan, Y.; Chen, Y.; Chen, X. The interprotein scoring noises in glide docking scores. Proteins Struct. Funct. Bioinform. 2012, 80, 169–183. [Google Scholar] [CrossRef]
- Liu, J.; Su, M.; Liu, Z.; Li, J.; Li, Y.; Wang, R. Enhance the performance of current scoring functions with the aid of 3D protein-ligand interaction fingerprints. BMC Bioinform. 2017, 18, 343. [Google Scholar] [CrossRef]
- Sato, T.; Honma, T.; Yokoyama, S. Combining machine learning and pharmacophore-based interaction fingerprint for in silico screening. J. Chem. Inf. Model. 2010, 50, 170–185. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Koch, O. The Development of Target-Specific Machine Learning Models as Scoring Functions for Docking-Based Target Prediction. J. Chem. Inf. Model. 2019, 59, 1238–1252. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Allen, W.J.; Balius, T.E.; Mukherjee, S.; Brozell, S.R.; Moustakas, D.T.; Lang, P.T.; Case, D.A.; Kuntz, I.D.; Rizzo, R.C. DOCK 6: Impact of new features and current docking performance. J. Comput. Chem. 2015, 36, 1132–1156. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Z.; Zhi, D.G. Ligand—Protein inverse docking and its potential use in the computer search of protein targets of a small molecule. Proteins Struct. Funct. Genet. 2001, 43, 217–226. [Google Scholar] [CrossRef]
- Zhang, Z.; Miao, L.; Lv, C.; Sun, H.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells. Cell Death Dis. 2013, 4, e001343. [Google Scholar] [CrossRef]
- Ye, L.; He, Y.; Ye, H.; Liu, X.P.; Yang, L.L.; Cao, Z.W.; Tang, K.L. Pathway-pathway network-based study of the therapeutic mechanisms by which salvianolic acid B regulates cardiovascular diseases. Chin. Sci. Bull. 2012, 57, 1672–1679. [Google Scholar] [CrossRef][Green Version]
- Cui, Z.; Sheng, Z.; Yan, X.; Cao, Z.; Tang, K. In silico insight into potential anti-alzheimer’s disease mechanisms of icariin. Int. J. Mol. Sci. 2016, 17, 113. [Google Scholar] [CrossRef]
- Schomburg, K.T.; Rarey, M. Benchmark data sets for structure-based computational target prediction. J. Chem. Inf. Model. 2014, 54, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Hu, M.; Liu, K.; Peng, J. Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction. Toxicol. Vitr. 2010, 24, 1482–1490. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, P.; Li, F.; Tao, L.; Ding, H.; Rui, Y.; Cao, Z.; Zhang, W. Therapeutic Effects of Astragaloside IV on Myocardial Injuries: Multi-Target Identification and Network Analysis. PLoS ONE 2012, 7, e44938. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Meng, P.; Zhang, B.; Kang, H.; Wen, H.; Schluesener, H.; Cao, Z.; Zhang, Z. Computer-aided identification of protein targets of four polyphenols in Alzheimer’s disease (AD) and validation in a mouse AD model. J. Biomed. Res. 2019, 33, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, H.; Zhang, H.; Liu, X.; Kang, L.; Luo, X.; Zhu, W.; Chen, K.; Wang, X.; Jiang, H. PDTD: A web-accessible protein database for drug target identification. BMC Bioinform. 2008, 9, 104. [Google Scholar] [CrossRef]
- Iyer, P.; Bolla, J.; Kumar, V.; Gill, M.S.; Sobhia, M.E. In silico identification of targets for a novel scaffold, 2-thiazolylimino-5-benzylidin-thiazolidin-4-one. Mol. Divers. 2015, 19, 855–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yin, J.; Hu, W.; Zhang, H. Glycogen Phosphorylase: A Drug Target of Amino Alcohols in Echinococcus granulosus, Predicted by a Computer-Aided Method. Front. Microbiol. 2020, 11, 557039. [Google Scholar] [CrossRef]
- Wang, F.; Wu, F.X.; Li, C.Z.; Jia, C.Y.; Su, S.W.; Hao, G.F.; Yang, G.F. ACID: A free tool for drug repurposing using consensus inverse docking strategy. J. Cheminform. 2019, 11, 73. [Google Scholar] [CrossRef]
- Tuccinardi, T.; Poli, G.; Romboli, V.; Giordano, A.; Martinelli, A. Extensive consensus docking evaluation for ligand pose prediction and virtual screening studies. J. Chem. Inf. Model. 2014, 54, 2980–2986. [Google Scholar] [CrossRef]
- Poli, G.; Martinelli, A.; Tuccinardi, T. Reliability analysis and optimization of the consensus docking approach for the development of virtual screening studies. J. Enzym. Inhib. Med. Chem. 2016, 31, 167–173. [Google Scholar] [CrossRef]
- Antonia, S.; Bekas, N.; Katsikoudi, A.; Tzakos, A.G.; Horacio, P. DIA-DB: A Web-Accessible Database for the Prediction of Diabetes Drugs. In Bioinformatics and Biomedical Engineering; IWBBIO 2015; Lecture Notes in Computer Science; Ortuño, F., Rojas, I., Eds.; Springer: Cham, Switzerland, 2015; Volume 9044. [Google Scholar] [CrossRef]
- Horacio, P.; Apostolides, Z. Exploring African Medicinal Plants for Potential Virtual Screening Web Server. Molecules 2019, 24, 2002. [Google Scholar] [CrossRef]
- Pasznik, P.; Rutkowska, E.; Niewieczerzal, S.; Cielecka-piontek, J. Potential off-target effects of beta-blockers on gut hormone receptors: In silico study including GUT-DOCK—A web service for small-molecule docking. PLoS ONE 2019, 14, e0210705. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, R.; Renard, B.Y. Docking small peptides remains a great challenge: An assessment using AutoDock Vina. Brief. Bioinform. 2015, 16, 1045–1056. [Google Scholar] [CrossRef]
- Wolber, G.; Seidel, T.; Bendix, F.; Langer, T. Molecule-pharmacophore superpositioning and pattern matching in computational drug design. Drug Discov. Today 2008, 13, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Al-Asri, J.; Fazekas, E.; Lehoczki, G.; Perdih, A.; Görick, C.; Melzig, M.F.; Gyémánt, G.; Wolber, G.; Mortier, J. From carbohydrates to drug-like fragments: Rational development of novel α-amylase inhibitors. Bioorg. Med. Chem. 2015, 23, 6725–6732. [Google Scholar] [CrossRef] [PubMed]
- Mortier, J.; Prévost, J.R.C.; Sydow, D.; Teuchert, S.; Omieczynski, C.; Bermudez, M.; Frédérick, R.; Wolber, G. Arginase Structure and Inhibition: Catalytic Site Plasticity Reveals New Modulation Possibilities. Sci. Rep. 2017, 7, 13616. [Google Scholar] [CrossRef] [PubMed]
- Rognan, D. Structure-based approaches to target fishing and ligand profiling. Mol. Inform. 2010, 29, 176–187. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.J.; Muskal, S.M. Pharmacophore Fingerprinting. 2. Application to Primary Library Design. J. Chem. Inf. Comput. Sci. 2000, 40, 117–125. [Google Scholar] [CrossRef]
- Steindl, T.M.; Schuster, D.; Laggner, C.; Langer, T. Parallel screening: A novel concept in pharmacophore modeling and virtual screening. J. Chem. Inf. Model. 2006, 46, 2146–2157. [Google Scholar] [CrossRef]
- Kaserer, T.; Beck, K.R.; Akram, M.; Odermatt, A.; Schuster, D.; Willett, P. Pharmacophore models and pharmacophore-based virtual screening: Concepts and applications exemplified on hydroxysteroid dehydrogenases. Molecules 2015, 20, 22799–22832. [Google Scholar] [CrossRef]
- Sanders, M.P.A.; Verhoeven, S.; De Graaf, C.; Roumen, L.; Vroling, B.; Nabuurs, S.B.; De Vlieg, J.; Klomp, J.P.G. Snooker: A structure-based pharmacophore generation tool applied to class A GPCRs. J. Chem. Inf. Model. 2011, 51, 2277–2292. [Google Scholar] [CrossRef]
- Meagher, K.L.; Carlson, H.A. Incorporating protein flexibility in structure-based drug discovery: Using HIV-1 protease as a test case. J. Am. Chem. Soc. 2004, 126, 13276–13281. [Google Scholar] [CrossRef]
- Mortier, J.; Dhakal, P.; Volkamer, A. Truly target-focused pharmacophore modeling: A novel tool for mapping intermolecular surfaces. Molecules 2018, 23, 1959. [Google Scholar] [CrossRef]
- Pérez-Sánchez, H.; Den Haan, H.; Pérez-Garrido, A.; Penã-Garciá, J.; Chakraborty, S.; Erdogan Orhan, I.; Senol Deniz, F.S.; Villalgordo, J.M. Combined Structure and Ligand-Based Design of Selective Acetylcholinesterase Inhibitors. J. Chem. Inf. Model. 2021, 61, 467–480. [Google Scholar] [CrossRef]
- Ab Ghani, N.S.; Ramlan, E.I.; Firdaus-Raih, M. Drug ReposER: A web server for predicting similar amino acid arrangements to known drug binding interfaces for potential drug repositioning. Nucleic Acids Res. 2019, 47, W350–W356. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Tinivella, A.; Gagliardelli, L.; Beneventano, D.; Rastelli, G. LigAdvisor: A versatile and user-friendly web-platform for drug design. Nucleic Acids Res. 2021, 49, W326–W335. [Google Scholar] [CrossRef]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef]
- Ballard, C.; Aarsland, D.; Cummings, J.; O’Brien, J.; Mills, R.; Molinuevo, J.L.; Fladby, T.; Williams, G.; Doherty, P.; Corbett, A.; et al. Drug repositioning and repurposing for Alzheimer disease. Nat. Rev. Neurol. 2020, 16, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, 5–7. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ling, Q.Z.; Chen, S.J. Identification of a Potential Target of Capsaicin by Computational Target Fishing. Evid. Based Complement. Altern. Med. 2015, 2015, 983951. [Google Scholar] [CrossRef]
- Chen, S.J.; Cui, M.C. Systematic understanding of the mechanism of salvianolic acid A via computational target fishing. Molecules 2017, 22, 644. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Azfaralariff, A.; Law, D.; Najm, A.A.; Sanusi, S.A.; Lim, S.J.; Cheah, Y.H.; Fazry, S. Comprehensive computational target fishing approach to identify Xanthorrhizol putative targets. Sci. Rep. 2021, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chaput, L.; Villoutreix, B.O. Virtual screening web servers: Designing chemical probes and drug candidates in the cyberspace. Brief. Bioinform. 2021, 22, 1790–1818. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.P.; Campana, P.R.V.; de Oliveira Scoaris, D.; de Almeida, V.L.; Lopes, J.C.D.; Shaw, J.M.H.; Silva, C.G. Combined in vitro studies and in silico target fishing for the evaluation of the biological activities of diphylleia cymosa and podophyllum hexandrum. Molecules 2018, 23, 3303. [Google Scholar] [CrossRef] [PubMed]
| Web Tool | Description | URL |
|---|---|---|
| SwissTargetPrediction | A combination of 2D and 3D similarity with known ligands | http://www.swisstargetprediction.ch |
| CSNAP3D | 3D chemical similarity using a network algorithms score | http://services.mbi.ucla.edu/CSNAP |
| MolTarPred | 2D Similarity search based on ECFP4 fingerprints | http://moltarpred.marseille.inserm.fr |
| TargetHunter | 2D Similarity search based on ECFP6 fingerprints | http://www.cbligand.org/TargetHunter |
| TarPred | Molecular similarity searching with KNN-based fusion score | http://www.dddc.ac.cn/tarpred |
| STarFish | A stacking approach combining multiple multi-target QSAR models | https://github.com/ntcockroft/STarFish |
| MolTarPred | 2D Similarity search based on ECFP4 fingerprints | http://moltarpred.marseille.inserm.fr |
| Web Tool | Description | URL |
|---|---|---|
| INVDOCK | Reverse docking approach using an in-house database | http://bidd.group/group/softwares/invdock.htm |
| idTarget | Reverse docking approach based on Divide-and-conquer method and using all protein structures in the PDB | http://idtarget.rcas.sinica.edu.tw |
| TarFisDock | Reverse docking using the Potential Drug Target Database (PDTD) | http://www.dddc.ac.cn/tarfisdock |
| DPDR-CPI | Reverse docking using proteins from PDB and PDBBind, combined with ML models for target predictions | http://cpi.bio-x.cn/dpdr |
| ACID | Reverse docking with an automated consensus inverse docking protocol | http://chemyang.ccnu.edu.cn/ccb/server/ACID |
| DIA-DB | Identification of potential antidiabetic drugs based on similarity search and reverse docking | http://bio-hpc.eu/software/dia-db |
| GUT-DOCK | Identification of small-molecule interactions with gut hormone GPCRs based on reverse docking | https://gut-dock.miningmembrane.com |
| Drug ReposER | Pharmacophore approach based on sub-structural similarity to the binding interfaces of known drug binding sites. | http://mfrlab.org/drugreposer/ |
| LigAdvisor | 2D ligand-receptor interactions based on similarity estimations | https://ligadvisor.unimore.it |
| PLIP | Binding site alignment or similarity among molecular structures and residue sequences | https://projects.biotec.tu-dresden.de/plip-web |
| PharmMapper | Target identification based on pharmacophore mapping procedure | http://59.78.96.61/pharmmapper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galati, S.; Di Stefano, M.; Martinelli, E.; Poli, G.; Tuccinardi, T. Recent Advances in In Silico Target Fishing. Molecules 2021, 26, 5124. https://doi.org/10.3390/molecules26175124
Galati S, Di Stefano M, Martinelli E, Poli G, Tuccinardi T. Recent Advances in In Silico Target Fishing. Molecules. 2021; 26(17):5124. https://doi.org/10.3390/molecules26175124
Chicago/Turabian StyleGalati, Salvatore, Miriana Di Stefano, Elisa Martinelli, Giulio Poli, and Tiziano Tuccinardi. 2021. "Recent Advances in In Silico Target Fishing" Molecules 26, no. 17: 5124. https://doi.org/10.3390/molecules26175124
APA StyleGalati, S., Di Stefano, M., Martinelli, E., Poli, G., & Tuccinardi, T. (2021). Recent Advances in In Silico Target Fishing. Molecules, 26(17), 5124. https://doi.org/10.3390/molecules26175124








