Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Derivatization
3.2. Saponification
3.3. Instrumental Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mozaffarian, D.; Rimm, E.B. Fish intake, contaminants, and human health evaluating the risks and the benefits. JAMA 2006, 296, 1885–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omega 3 Supplements Market Size, Share & Trends Analysis Report By Source (Fish Oil, Krill Oil), By Form (Soft Gels, Capsules), By Functionality, By End-User, By Distribution Channel, By Region, And Segment Forecasts, 2020–2027. Available online: https://www.grandviewresearch.com/industry-analysis/omega-3-supplement-market (accessed on 10 June 2021).
- Salute: Gli Integratori Alimentari Utilizzati da 32 Milioni di Italiani. Available online: https://www.censis.it/welfare-e-salute/salute-gli-integratori-alimentari-utilizzati-da-32-milioni-di-italiani (accessed on 10 June 2021).
- Gli Integratori di Omega 3 Servono Davvero? Secondo i Primi Dati di Uno Studio Decennale Sembrerebbe di No. Available online: https://ilfattoalimentare.it/integratori-omega-3-servono.html (accessed on 10 June 2021).
- Hamilton, K.; Brooks, P.; Holmes, M.; Cunningham, J.; Russell, F.D. Evaluation of the Composition of Omega-3 Fatty Acids in dietary oil supplements. Nutr. Diet. 2010, 67, 182–189. [Google Scholar] [CrossRef]
- Galuch, M.B.; Carbonera, F.; Magon, T.F.S.; Da Silveira, R.; Dos Santos, P.D.S.; Pizzo, J.S.; Santos, O.O.; Visentainer, J.V. Quality assessment of omega-3 supplements available in the Brazilian market. J. Braz. Chem. Soc. 2018, 29, 631–638. [Google Scholar] [CrossRef]
- Damerau, A.; Ahonen, E.; Kortesniemi, M.; Puganen, A.; Tarvainen, M.; Linderborg, K.M. Evaluation of the composition and oxidative status of omega-3 fatty acid supplements on the Finnish market using NMR and SPME-GC-MS in comparison with conventional methods. Food Chem. 2020, 330, 127194. [Google Scholar] [CrossRef] [PubMed]
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 Long-Chain Polyunsaturated Fatty Acid Content and Oxidation State of Fish Oil Supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolanowski, W. Omega-3 LC PUFA contents and oxidative stability of encapsulated fish oil dietary supplements. Int. J. Food Prop. 2010, 13, 498–511. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, R.; Kalwa, K.; Wilczynski, K.; Kobus, Z. The fatty acids composition of selected fish oils used as dietary supplements. Pol. J. Nat. Sci. 2019, 34, 115–126. [Google Scholar]
- Chee, K.M.; Gong, J.X.; Good Rees, D.M.; Meydanl, M.; Ausman, L.; Johnson, J.; Siguel, E.N.; Schaefer, E.J. Fatty acid content of marine oil capsules. Lipids 1990, 25, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.C.S.; Budge, S.M.; Jovica, F. Quality analysis of commercial fish oil preparations. J. Sci. Food Agric. 2013, 93, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, A.C.; Cladis, D.P.; Santerre, C.R. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 2015, 95, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Srigley, C.T.; Rader, J.I. Content and composition of fatty acids in marine oil omega-3 supplements. J. Agric. Food Chem. 2014, 62, 7268–7278. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Sherratt, S.C.R. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem. Biophys. Res. Commun. 2017, 483, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Olli, J.J.; Breivik, H.; Thorstad, O. Removal of persistent organic pollutants in fish oils using short-path distillation with a working fluid. Chemosphere 2013, 92, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Mendez, J.R.; Hoyos-Concha, J.L. Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Cienc. Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Fatty acids in encapsulated fish oils, Method 991.39. In Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2007. [Google Scholar]
- Nevigato, T.; Masci, M.; Orban, E.; Di Lena, G.; Casini, I.; Caproni, R. Analysis of fatty acids in 12 mediterranean fish species: Advantages and limitations of a new GC-FID/GC-MS based technique. Lipids 2012, 47, 741–753. [Google Scholar] [CrossRef] [PubMed]
- USDA, US Department of Agriculture, National Institute of Health, Methods and Application of Food Composition Laboratory. Dietary Supplement Ingredient Database Release 4.0 (DSID4). Omega-3 Fatty Acid Dietary Supplement Study. Research Summary. 2015 (2017, Minor Revisions). Available online: https://dietarysupplementdatabase.usda.nih.gov/Omega3.php (accessed on 15 June 2021).
- Kinsella, J.E.; Shimp, J.L.; Mai, J.; Weihrauch, J. Fatty acid content and composition of freshwater finfish. J. Am. Oil Chem. Soc. 1977, 54, 424–429. [Google Scholar] [CrossRef] [PubMed]
- European Union Regulation. Commission regulation (EEC) No 2568/91. In Analysis by Gas Chromatography of Methyl Esters of Fatty Acids: Use of Correction Factors; Official Journal of the European Communities: Luxembourg, 1991; No L 248/41-42. [Google Scholar]

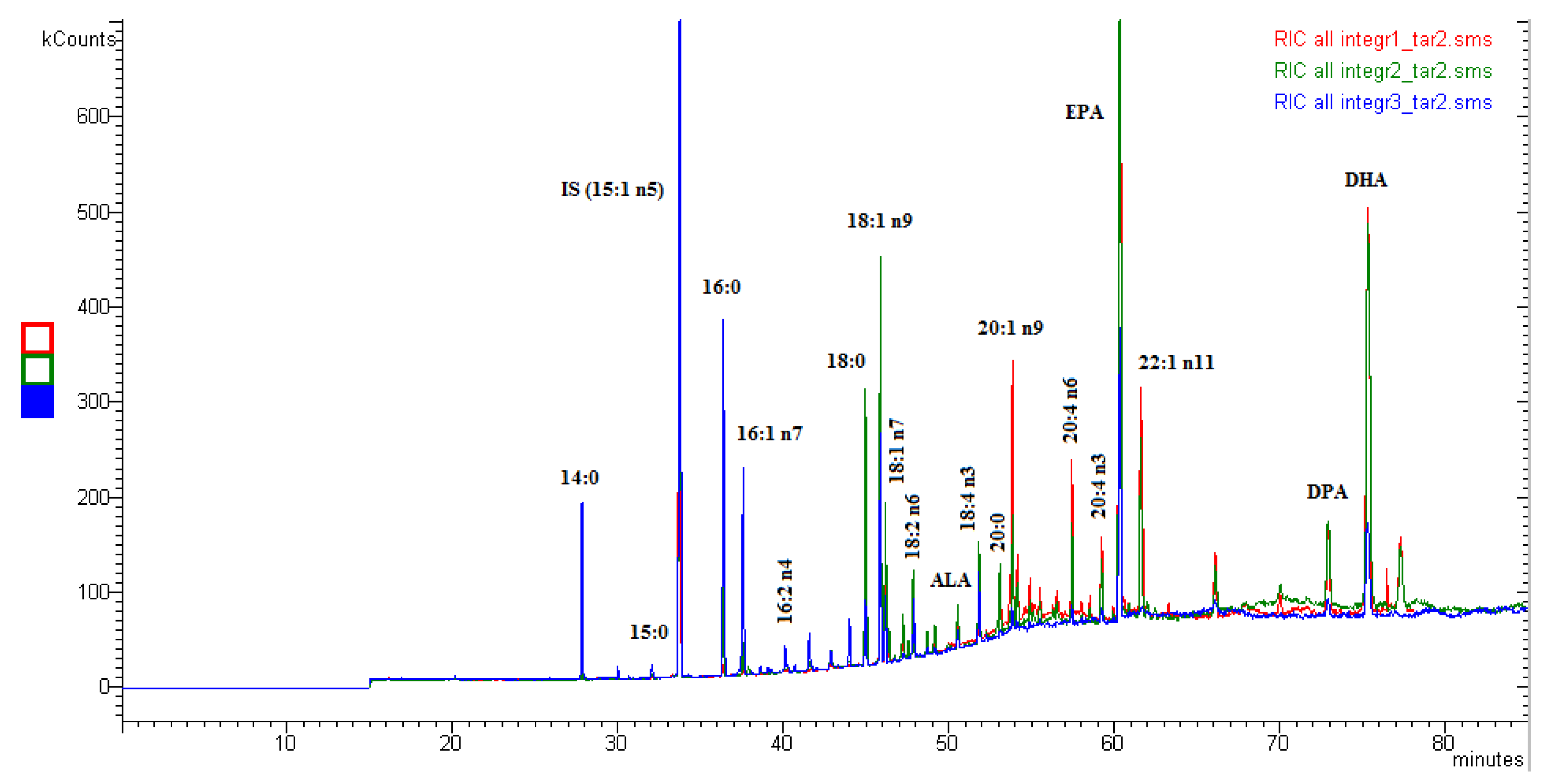
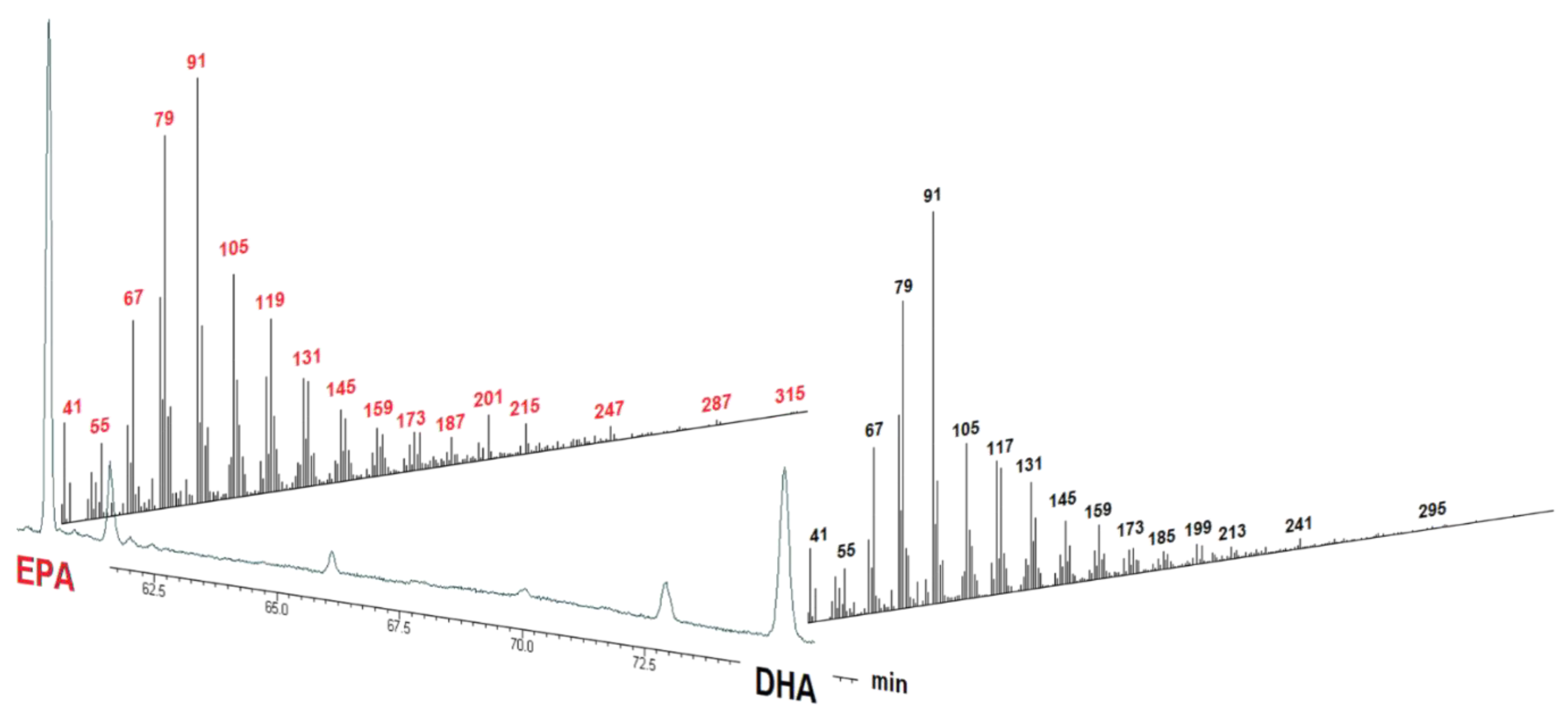
| Fatty Acids | Supplement No. 1 | Supplement No. 2 | Supplement No. 3 |
|---|---|---|---|
| C12:0 (lauric) | n.d. | n.d. | 0.14 |
| C13:0 (tridecanoic) | n.d. | n.d. | 0.03 |
| C14:0 (myristic) | 0.05 | 0.13 | 6.29 |
| C14:1 n-5 (myristoleic) | n.d. | n.d. | 0.05 |
| C15:0 (pentadecanoic) | 0.01 | 0.06 | 0.54 |
| C16:0 (palmitic) | 0.16 | 1.32 | 13.80 |
| C16:1 n-7 (palmitoleic) | 0.11 | 0.44 | 7.71 |
| C16:2 n-4 (palmitolinoleic) | n.d. | 0.08 | 1.21 |
| C17:0 (margaric) | 0.06 | 0.10 | 0.35 |
| C18:0 (stearic) | 2.94 | 4.27 | 2.77 |
| C18:1 n-9 (oleic) | 2.87 | 6.17 | 9.55 |
| C18:1 n-7 (vaccenic) | 1.29 | 2.48 | 2.72 |
| C18:2 n-6 (linoleic) | 0.52 | 1.33 | 0.99 |
| C19:0 (nonadecanoic) | 0.39 | 0.52 | 0.37 |
| C18:3 n-3 (α-linolenic or ALA) | 0.34 | 0.70 | 0.72 |
| C18:4 n-3 (Stearidonic) | 0.47 | 1.54 | 2.75 |
| C20:0 (arachidic) | 0.93 | 1.02 | 0.38 |
| C20:1 n-11 (gadoleic) | 0.73 | 0.26 | n.d. |
| C20:1 n-9 (gondoic) | 3.58 | 1.61 | 0.93 |
| C20:2 n-6 (dihomolinoleic) | 0.39 | 0.13 | n.d. |
| C20:4 n-6 (arachidonic) | 2.56 | 1.59 | 0.78 |
| C20:4 n-3 (omega-3 arachidonic) | 1.58 | 1.32 | 0.69 |
| C20:5 n-3 (eicosapentaenoic or EPA) | 32.41 | 25.76 | 16.68 |
| C22:1 n-11 (cetoleic) | 2.60 | 1.42 | n.d. |
| C22:1 n-9 (erucic) | 0.18 | 0.44 | n.d. |
| C22:5 n-3 (docosapentaenoic or DPA) | 2.71 | 2.91 | 1.37 |
| C22:6 n-3 (docosahexaenoic or DHA) | 19.68 | 20.06 | 12.89 |
| EPA + DHA | 52.09 | 45.82 | 29.57 |
| Σn-3 | 57.19 | 52.29 | 35.10 |
| Σn-6 | 3.47 | 3.05 | 1.77 |
| Σn-6/Σn-3 (dimensionless value) | 0.06 | 0.06 | 0.05 |
| Σ saturated fatty acids (ΣSFA) | 4.54 | 7.42 | 24.67 |
| Σ monounsaturated fatty acids (ΣMUFA) | 11.36 | 12.82 | 20.96 |
| Σ polyunsaturated fatty acids (ΣPUFA) | 60.66 | 55.42 | 38.08 |
| Other fatty acids | 13.58 | 12.07 | 8.72 |
| Sum (total FAs) | 90.14 | 87.73 | 92.43 |
| Supplement | EPA | DHA | ||||
|---|---|---|---|---|---|---|
| Label | Measured | % | Label | Measured | % | |
| Supplement no. 1 | 40 | 32.41 | 81 | 20 | 19.68 | 98 |
| Supplement no. 2 | 33 | 25.76 | 78 | 22 | 20.06 | 91 |
| Supplement no. 3 | 15 | 16.68 | 111 | 10 | 12.89 | 129 |
| Fatty Acid | Units | Test Material | Measured Values a | ||
|---|---|---|---|---|---|
| Assigned Value | Satisfactory Range | Mean | RPD (%) | ||
| 18:3 ω-3 | % | 0.95 | 0.86–1.05 | 0.95 | 6.32 |
| 20:5 ω-3 | % | 12.93 | 11.64–14.23 | 11.10 | 2.97 |
| 22:5 ω-3 | % | 1.88 | 1.69–2.07 | 1.74 | 3.45 |
| 22:6 ω-3 | % | 10.66 | 9.59–11.73 | 11.99 | 7.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nevigato, T.; Masci, M.; Caproni, R. Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules 2021, 26, 5015. https://doi.org/10.3390/molecules26165015
Nevigato T, Masci M, Caproni R. Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules. 2021; 26(16):5015. https://doi.org/10.3390/molecules26165015
Chicago/Turabian StyleNevigato, Teresina, Maurizio Masci, and Roberto Caproni. 2021. "Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study" Molecules 26, no. 16: 5015. https://doi.org/10.3390/molecules26165015
APA StyleNevigato, T., Masci, M., & Caproni, R. (2021). Quality of Fish-Oil-Based Dietary Supplements Available on the Italian Market: A Preliminary Study. Molecules, 26(16), 5015. https://doi.org/10.3390/molecules26165015