Abstract
Paeonol is a naturally existing bioactive compound found in the root bark of Paeonia suffruticosa and it is traditionally used in Chinese medicine for the prevention and management of cardiovascular diseases. To date, a great deal of studies has been reported on the pharmacological effects of paeonol and its mechanisms of action in various diseases and conditions. In this review, the underlying mechanism of action of paeonol in cardiovascular disease has been elucidated. Recent studies have revealed that paeonol treatment improved endothelium injury, demoted inflammation, ameliorated oxidative stress, suppressed vascular smooth muscle cell proliferation, and repressed platelet activation. Paeonol has been reported to effectively protect the cardiovascular system either employed alone or in combination with other traditional medicines, thus, signifying it could be a hypothetically alternative or complementary atherosclerosis treatment. This review summarizes the biological and pharmacological activities of paeonol in the treatment of cardiovascular diseases and its associated underlying mechanisms for a better insight for future clinical practices.
1. Introduction
Cardiovascular disease (CVD) is one of the major causes of death in the world [1]. The World Health Organization estimates that CVD is responsible for the deaths of approximately 30,000 people each day and accounted for 15.2 million deaths worldwide in 2016 [2,3]. The United Nations in 2011, recognized non-communicable diseases, including CVD as a major concern for global health and dramatically planned to reduce the effect of this disease in all regions [4]. The global awareness was expanded with increased efforts to reduce CVD and other non-communicable diseases [5,6].
The mechanism of CVD from the molecular perspective has been studied extensively for the past decades. Oxidative stress and inflammation were found to be predominantly associated with the pathogenesis of CVD. The inflammation could have been triggered by oxidative stress which results from the excessive generation of reactive oxygen species/reactive nitrogen species (ROS/RNS). This contributes to low-density lipoprotein (LDL) oxidation, endothelial dysfunction or apoptosis, atherosclerotic plaque formation, plaque rupture, vascular remodeling, and atherothrombosis [7,8,9].
Natural products offer unique structural and chemical diversity that serves as a source of novel drug and therapeutic agents [10]. Paeonol is a phenolic compound mainly isolated from the root bark and/or cortex of genus Paeonia, predominantly found in Paeonia suffruticosa Andrew (Cortex. Moutan) compared to Paeonia ostia T. Hong and J. X. Zhang, Paeonia clusii subsp. clusii F.C. Stearn, Paeonia mascula subsp. hellenica Tzanoud, Paeonia parnassica Tzanoud, Paeonia lactiflora Pall, and Paeonia broteri Boiss and Reut [11,12]. A number of studies revealed that paeonol possesses many physiological activities, including vascular dilation [13], inhibit platelet aggregation, [14] formation of free radicals [15], and prevent cardiovascular diseases [16,17]. In recent years, a great number of studies on the pharmacological effects of paeonol and its mechanism of action have been reported. This article reviews the mechanism of action of paeonol in cardiovascular diseases particularly looking into the anti-oxidant, anti-inflammatory, anti-apoptotic, and its regulation of vascular tone.
2. Pharmacological Features of Paeonol
2.1. Bioactive Compounds in Cortex Moutan
Several phytochemical studies performed on Cortex Moutan had identified and isolated approximately 80 bioactive compounds. The chemical structure of paeonol is as shown in Figure 1. Based on the structural formula, these bioactive compounds are divided into monoterpenoid glycosides, flavonoids, tannins, phenols, and paeonols. Paeonol (2′-hydroxy-4′-methoxyacetophenone) appeared to be the main representative bioactive compound in paeonol [18,19] and till today, twelve derivatives of paeonol have been synthesized (Figure 1). Among bioactive compounds derived from Cortex Moutan, paeonol has been extensively investigated for anti-cardiovascular activity and its mechanisms of action in CVD [20,21,22,23,24,25,26,27,28,29,30].
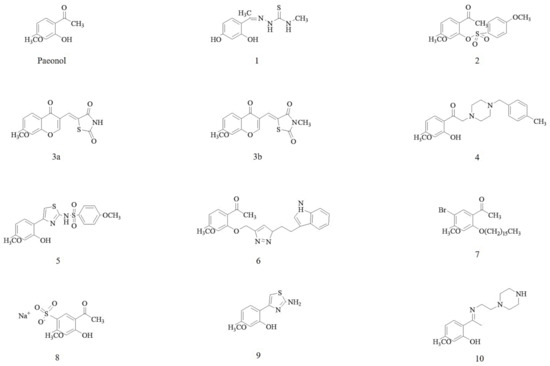
Figure 1.
Chemical structures of paeonol and its derivatives (1–10). The derivatives are formed with thiosemicarbazone-like analogs (1), phenylsulfonyl-like analogs (2), chromonylthiazolidines-like analogs (3a and 3b), donepezil-like analogs (4), aminothiazole-like analogs (5), tryptamine hybrid analogs (6), alkyl ether analogs (7), paeononlsilatie sodium (8), aminothiazole-like analogs (9), and piperazin-like analogs (10).
2.2. Pharmacokinetics and Drug Delivery of Paeonol
In the aspect of absorption, paeonol has been studied in different parameters, including, pH, drug concentration, osmotic pressure, and perfusion. Based on the results, it was noted that paeonol is absorbed by the intestinal tract and rapidly enters portal circulation upon oral administration [11,18]. Due to having a short half-life and maximum time, it is delivered rapidly in the bloodstreams to several target organs, including heart, liver, kidney, and brain without long-term accumulation and excreted from the body in a short time, conferring a great safety [31]. It has been reported that paeonol absorption is the first-order metabolism as it does not depend on its concentration. It can be absorbed easily in acidic conditions, particularly hypertonic solutions. Its absorption rate is constant similarly to its perfusion rate [32]. Chen et al. reported that experimental rats could absorb paeonol rapidly and efficiently via the intranasal route of administration [11,12]. Besides, the metabolism of paeonol has generated six metabolites as found in the urine samples of 19 Chinese volunteers who received 80 mg paeonol tablets for two days consecutively, three times per day. It has also shown that paeonol is metabolized by demethylation and hydroxylation in phase 1 metabolism followed by sulfuric acid and glucuronic acid conjugation in phase 2 metabolism [11,12].
Intriguingly, Li et al. revealed that the retention time and levels of paeonol in the heart and brain could be significantly increased via co-administration with other phytochemicals, as evidenced with danshensu in CVD [33,34]. Nonetheless, the therapeutic efficacy of paeonol was shown to be limited by the first-pass effect and poor bioavailability due to low aqueous solubility [18]. To counteract these issues, several nanotechnology-assisted drug delivery systems, for example, microemulsion gel, transethosome, porous microsphere, liquid crystalline nanoparticles, and microsponge, were developed, particularly to treat cancer and skin diseases [35,36,37,38,39,40]. These delivery systems had greatly enhanced the therapeutic effects of paeonol due to their high encapsulation capacity and stability, together with better control of drug release and retention time in the targeting tissues. Collectively, only a few drug systems that scheme for paeonol have been designed to test in cardiovascular diseases. For example, Chen et al. designed nanoemulsions for paeonol that effectively increase its protective effect in the cardiovascular system by enhancing its oral transport and absorption, and bioavailability due to evading p-glycoprotein-mediated drug efflux [41]. Besides, the better delivery of paeonol has been observed with its loading into stent microparticles or poly (butyl-2-cyanoacrylate) nanocapsules, resulting in greater prevention of restenosis and stent thrombosis after percutaneous coronary intervention [35,42]. Given the benefits of using nanotechnology-assisted drug delivery of paeonol, various nanoformulations should be designed and tested, with the aim of improving its therapeutic efficacy and prevention against CVD in clinical settings.
3. Mechanism of Action of Paeonol in Cardiovascular Diseases
Numerous studies have shown that paeonol has several therapeutic effects against cardiovascular diseases and protects the cardiovascular system via several mechanisms.
3.1. Anti-Oxidant Mechanism
Exaggerated free radicals released by unopposed oxidative stress levels led to the increase in pathophysiology changes. Cardiovascular disease manifests major impact by this abnormality via mtDNA (mitochondrial DNA) damage, cellular apoptosis, and enzyme degradation [43]. MtDNA damage led to atherosclerosis via functional changes in the cell and disturbance of the mitochondrial respiratory chain [44]. Experimental and clinical studies have demonstrated endothelial dysfunction (ED) as an early predictor of cardiovascular pathology. ROS disturbs the function of endothelial nitric oxide synthase (eNOS) hence leading to impair nitric oxide (NO) bioavailability; a potent vasodilator, which contributes to essential hypertension [45]. Furthermore, ED manifested by lipid-rich atherosclerotic plaque rupture led to atherogenesis and myocardial infarction. This finding was proved by the development of oxidized low-density lipoproteins (ox-LDL) via ROS release. This bad lipid was shown to be oxidatively modified by vascular wall cellularity; endothelial cells, smooth muscle cells, and macrophages [46].
Oxidative stress occurs upon imbalance between the prooxidant-antioxidant condition in cells resulting in cellular apoptosis, hence promoting atherosclerosis [47]. An in vitro study using human umbilical vascular endothelial cells (HUVECs) showed 10 and 50 µM of paeonol significantly inhibited the overproduction of ROS when exposed by ox-LDL. A subsequent investigation by the same group revealed that the up-regulation of lectin-like low-density lipoprotein receptor-1 (LOX-1) protein expressions that were increased by ox-LDL were effectively suppressed by paeonol. This study further delineates the inhibition of ROS overproduction downregulated of p38 MAPK phosphorylation, NF-κB nuclear translocation, caspase-3 activation, and Bcl-2 production pathway, hence improved cell viability [48]. This result suggests that paeonol reduces ox-LDL in inducing apoptosis via ROS.
In atherosclerosis progression, vascular smooth muscle apoptosis or necrosis might result from cell senescence. The accumulation of its end products accumulates lipid peroxidation hence produce ROS [49]. Jamal et al. study highlighted the protective effect of paeonol in reducing the senescence effect in endothelial cells [50]. Pre-treatment with an optimum dose of 30 µM paeonol prior to H2O2 treatment normalized the pro-apoptotic p53 protein expression, as measured by fluorescent assay and western blotting. Downregulation of p53 protein suggests improvement of cellular senescence activity. Furthermore, paeonol increased Sirt1 expression, BrdU incorporation, and cell growth profile. Paeonol also reduced Ac-H3 K14 expression, Ac-H4 K16 expression, and SA-beta-galactosidase suggesting endothelial cells protection. This study suggests the relationship of paeonol in preventing aging-associated diseases, particularly, atherosclerosis and various cardiovascular disease [50].
Another study showed that paeonol was able to inhibit atherosclerotic plaque via downregulation of heme oxygenase-1 (HO-1) and upregulation of ATP-binding cassette transporter A1 (ABCA1) either via calpain activity reduction or wogonin increment in RAW264.7 macrophages and apolipoprotein E-deficient (ApoE−/−) mice. HO-1 mediates ABCA1, a cholesterol efflux regulatory protein to reduce the ox-LDL formation and inhibit foam cell formation. Treatment with paeonol, 5–50 µM decreased the expression of CD36 via inactivation of c-Jun-AP-1 pathway and increased the expression of ABCA1, hence reduced foam cells formation which further reduced intracellular lipid accumulation. Furthermore, the protein level of antioxidant enzymes, HO-1 in macrophages showed a dose-dependent increment in response to paeonol, thus demonstrating paeonol helps to regulate the atherosclerotic plaque formations via anti-oxidant properties [51].
3.2. Anti-Inflammatory Mechanism
Chronic inflammation leads to infiltration of inflammatory sensors such as tissue-resident macrophages, mast cells, and dendritic cells which induce the production of mediators including cytokines such as interleukins (ILs) and tumor necrosis factor-alpha (TNFα), chemokines, bioactive amines, and product of proteolytic cascades such as bradykinin. The overproduction of these mediators will eventually contribute to the progression of tissue damage [52]. The association between inflammation and cardiovascular disease, while seeming relatively recent, the root of this concept, stretches far back in time. Chronic inflammation is a common hallmark of cardiovascular diseases by accelerating atherosclerosis, destabilizing plaque, compromising endothelial functions, and causing arterial stiffness [53]. The inflammatory process leading to cardiovascular diseases includes an impaired function of endothelial cells, oxidative stress, accumulation of immune cells, production of TNFα, and several interleukins [54]. There have been studies relating inflammation as a risk factor in poor prognosis in ischemic damage as well as myocardial infarction [55]. Continuous generation of ROS by activated immune effector cells such as macrophages are also associated with chronic inflammatory conditions as the LDLs are oxidized by these ROS and then exert pro-atherogenic effects [56]. Activated endothelial cells then express adhesive molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular molecule 1 (ICAM-1), and selectins. These determine mononuclear cell recruitment into the vascular wall, together with the secretion of chemoattractant mediators such as complement factors, interleukin (IL)–8, and monocyte chemoattractant protein-1 (MCP-1). Monocytes differentiate into macrophages that become foam cells by ox-LDL absorption and then release a number of proinflammatory cytokines [57]. These inflammatory cascades impair the endothelial functions and the mechanical properties of the arteries. The decline in bioavailability of nitric oxide and the increase of the vasoactive peptides lead to arterial stiffening which in turn further causes endothelial dysfunction [53]. In the case of the inflammatory cascade in ischemic myocardial injury, cellular fragments will trigger the resident immune cells through the engagement of pattern recognition receptors (PRRs) for example membrane toll-like receptors, which activate inflammasomes in the heart, play a crucial role in cardiac remodeling. Excessive inflammation will induce cardiomyocyte apoptosis [55].
There have been reports documenting the anti-inflammatory properties of paeonol via various pathways and mechanisms. Pan et al. studied the effect of paeonol on TNF-α stimulated VCAM-1 expression of rat aortic endothelial cells (RAECs) [58]. In baseline conditions, VCAM-1 is not expressed but is rapidly induced in a pre-atherosclerotic state. Based on their investigation, it is shown that paeonol significantly inhibits TNF-α induced VCAM-1 expression as well as inhibits monocytic cell adhesion to TNF-α activated RAECs. Furthermore, their study also demonstrated the role of MAPK (ERK ½, JNK, and p38) in the process of VCAM-1 expression. MAPK was shown to block the activity of ERK ½, and p38. This evidence supports that paeonol reduces the monocytic cell adhesion to TNF-α induced RAECs by inhibiting the expression of VCAM-1 protein via the down-regulation of ERK ½ and p38. They found that paeonol was effectual in the prevention and treatment of atherosclerosis in the initial and progression stages through the inhibition of the inflammatory response [58].
There are also studies demonstrating paeonol exhibits anti-inflammatory effects through the suppression of TNFα, IL-1ß, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and prostaglandin E2 (PGE2) production in a rat model of carrageenan-evoked thermal hyperalgesia [59]. In models of hyperlipidemia, paeonol inhibited serum and aorta lipid peroxidation and decreased serum oxidative modification caused by ox-LDL in healthy people [18]. Kim et al. demonstrated via in vitro studies that paeonol significantly inhibited essential angiogenesis pathways such as proliferation and migration in fibroblast growth factor (FGF) stimulated HUVECs under pathological angiogenic conditions [59]. This finding indicates that paeonol is able to inhibit the early process of angiogenesis [59].
Another approach of paeonol as an anti-inflammatory is through the expression of miRNA-126 which has been reported specific to endothelial cells and also affects the pathophysiological processes of CVD such as atherosclerosis [60]. Yuan et al. conducted a study using ox-LDL stimulated vascular endothelial cells (VECs) isolated from rat thoracic aorta as an atherosclerosis model [60]. Ox-LDL significantly induced damage to VECs resulting in lower expression of miR-126, while treatment with paeonol promoted miR-126 expression to inhibit monocyte adhesion to ox-LDL-injured VECs via the downregulation of VCAM-1 expression. The same group demonstrated that paeonol blocks the activation of the PI3K/Akt/NF-κB signaling pathway which is a major pro-inflammatory signaling pathway in response to extracellular stress via promoting miR-126 expression. This finding suggests the possibility that miR-126 may serve as a therapeutic target along with paeonol as a treatment for atherosclerosis [60].
Another study by Liu et al. investigated the anti-inflammatory of paeonol via the expression of miRNA-21 [61]. Similar to the study by Yuan et al. ox-LDL stimulated VECs isolated from rat thoracic aortas were used as the study model [61]. The miR-21 may be critical in the progression of cardiovascular diseases as it is expressed abundantly in VECs, cardiomyocytes, and cardiac fibroblast. Based on the data obtained in this study, paeonol pre-treatment increased the survival rate of the ox-LDL stimulated VECs. The paeonol treatment also showed a significant decrease in ox-LDL-induced miR-21 expression as well as reversed the phosphatase and tensin homolog (PTEN) expression which is a downstream target gene for miR-21. Furthermore, paeonol decreased the ox-LDL induced TNF-α release. The transfection of miR-21 mimics or inhibitors combined with paeonol annulled or enhanced the TNF-α release respectively. Thus, there is a possibility the effect of paeonol on the release of TNF-α is miR-21 dependent [61].
Liu et al. carried out an in vivo and in vitro experiment to investigate the protective effect of paeonol on inflammatory response through the exosomal regulation of miR-223 [62]. High-fat diet ApoE−/− mice were used to replicate the atherosclerosis model and HUVECs were used for the in vitro testing. There were several major findings from this research which include paeonol restricted the development of atherosclerosis lesion and inflammation by augmenting the miR-223 expression and inhibiting the STAT3 pathway on the aorta. Besides that, they also proved that paeonol, both in vivo and in vitro, could decline the expression levels of IL-1ß and IL6, cytokines that accelerate atherosclerosis and decrease VCAM-1 and ICAM-1. In addition, paeonol also enhanced the expression of miR-223 for both exosomes and co-cultured HUVECs which consequently inhibits the STAT3 pathway. The miR-223 is another possible target to be considered as a therapeutic target with the conjunction of paeonol [62].
Furthermore, there was an in-vivo study conducted by Ma et al. that investigated the potential of paeonol as a cardio-protective agent on the no-reflow phenomenon which is described as inadequate perfusion of the myocardium [63]. This is commonly caused by swelling of the endothelial tissue, leucocytes accumulation, and vasoconstriction which all lead to acute inflammation. The pre-treatment with paeonol reduced the no-reflow area in concurrence with a significant decline in the release of serum creatinine kinase (CK) and lactate dehydrogenase (LDH) as well as reduced troponin T (TnT) and C-reactive protein (CRP) levels, suggesting that paeonol pre-treatment decreased myocardial ischemic damage, most likely through modulation of the microvasculature. These demonstrate that paeonol can be used as an alternative in the treatment of myocardial no-reflow and other cardiovascular diseases [63].
In a model of ApoE−/− mice, paeonol’s efficacy on cholesterol metabolism was investigated [64]. Paeonol significantly reduced the ox-LDL-induced cholesterol accumulation in macrophages due to cholesterol efflux. In addition, paeonol up-regulated the ATP-binding membrane cassette transport protein A1 (ABCA1) protein and mRNA but did not change the ABCG1 protein level. Paeonol effect on cholesterol efflux and cholesterol accumulation was inhibited by siRNA knockdown of liver X receptor α (LXRα). Paeonol stimulated the activity of nuclear translocation of LXRα. Furthermore, paeonol reduced atherosclerotic lesions, hyperlipidemia, and systemic inflammation as well as increased ABCA1 protein expression in aortas of paeonol-treated ApoE−/− mice. These suggest that reduced the foam cells formation by stimulating LXRα-ABCA1–dependent cholesterol efflux in ApoE−/− mice [64].
In summary, the active compound paeonol has considerable research evidence to be targeted as a therapeutic agent in atherosclerosis management which in consequence reduces the risk of CVD.
3.3. Regulation of Vascular Tone
The endothelium embodies a wide range of homeostatic functions fundamental for the regulation of vascular tone and structure by synthesizing and secreting a broad spectrum of substances including (a) endothelium-derived relaxing factors (EDRFs) [NO, prostacyclin and endothelium-derived hyperpolarizing factor (EDHF)], (b) endothelium-derived contracting factors (EDCF) [endothelin-1 and angiotensin II], (c) pro-inflammatory and pro-thrombolytic mediators and (d) growth factors [65]. NO is the primary EDRF released in most vascular beds and serves as a major endogenous local control of vascular tone. NO is formed from the conversion of a semi-essential amino acid, L-arginine to L-citrulline, by an enzyme called nitric oxide synthase (NOS) [66]. Once NO is released, it diffuses into smooth muscle cells of blood vessels and activates soluble guanylyl cyclase (sGC) to convert guanosine monophosphate (GMP) to cyclic GMP. cGMP is known to have a function in activating cGMP-dependent protein kinase through which it regulates several pathways involved in Ca2+ homeostasis, i.e., reduction in intracellular Ca2+ available for contraction and a decrease in the sensitivity of contractile proteins to Ca2+ which ultimately leads to vasodilatation [67].
Evidence from clinical and experimental studies has demonstrated that impairment of the endothelial function either initiates or is associated with the development and progression of cardiovascular diseases [68]. Endothelial dysfunction is characterized by a significant reduction in the bioavailability of vasodilator substances, in particular NO, and/or elevation in endothelium-derived contracting factors. This results in a dysfunctional endothelium, whereby it is the first step in the cascade of events leading to atherosclerosis and coronary diseases [69].
Numerous studies point to the loss of NO biological activity as a central mechanism of endothelial dysfunction. Endothelial dysfunction associated with eNOS uncoupling which leads to the production of ROS has been reported both in animal models such as angiotensin II-induced hypertension [70], streptozotocin (STZ)-induced diabetes [71], and hypertension-induced heart failure [72] as well as in patients with diabetes, hypertension, hypercholesterolemia, and atherosclerosis [73]. Besides eNOS uncoupling, enhanced EDCFs also increase the production of superoxide anion and cause cellular oxidative damage [74]. Disruption in the endothelium triggers a number of downstream signaling cascades that converge on the underlying vascular smooth muscle cells, thus disrupting the vasomotor function [75].
Studies have demonstrated that paeonol possesses a vasodilatory effect in the isolated aortic ring [13,76]. These studies have demonstrated the involvement of endothelium-dependent and independent mechanisms for its potent vasodilatory effect. The eNOS/NO was shown as the endothelium-dependent mechanism and inhibition of voltage-dependent and receptor-operated Ca2+ channel, as well as intracellular Ca2+ release as the endothelium-independent mechanism for its vasodilatory effect in rat aorta [13] and rat mesenteric artery [77].
In recent years, more studies have illustrated the protective role of paeonol in regulating vascular tone against dysfunction induced by exogenous stressors. In 2016, Choy et al. investigated the protective mechanism of paeonol against tunicamycin-induced endoplasmic reticulum stress in isolated mouse aortas. The study showed ex vivo treatment with 1 μM paeonol for 16 h reversed the impaired endothelium-dependent relaxations induced by tunicamycin in C57BJ/6J and PPARδ wild type (WT) mouse aortas. Moreover, the elevated ER stress markers, oxidative stress, and reduction of NO bioavailability induced by tunicamycin in both mouse aortas were reversed by paeonol treatment. However, these beneficial effects of paeonol were diminished in PPARδ knockout (KO) mouse aortas. Paeonol also was shown to increase the expression of 5’ adenosine monophosphate-activated protein kinase (AMPK) and PPARδ expression and activity while restoring the decreased phosphorylation of eNOS. Thus, paeonol protects against tunicamycin-induced vascular endothelial dysfunction by reducing endoplasmic reticulum stress-associated ROS levels and enhancing eNOS-induced NO production via the AMPK/PPARδ signaling pathway [78].
In 2017, the same group demonstrated the vascular protective effects of chronic treatment with paeonol on ER stress-induced endothelial dysfunction in mice [79]. Paeonol treatment of 20 mg/kg/day significantly ameliorated the impairment of endothelium-dependent relaxations of the aorta. Furthermore, paeonol reduced ROS levels in the mouse aorta and improved NO bioavailability in tunicamycin treated mice, thus further proving paeonol preserved endothelial function impaired by tunicamycin in vivo through the inhibition of ER stress-associated ROS.
Lipopolysaccharide (LPS) which is a major constituent of outer membranes of bacteria triggers inflammation, microvascular leaking, endothelial cells detachment, and eventually apoptosis leading to sepsis and its complication [80,81]. The protective effect of paeonol on vascular function was also demonstrated in endothelial dysfunction induced by lipopolysaccharides (LPSs) inflammatory conditions ex vivo and in vivo [82]. Ex vivo treatment with 1 μM paeonol reversed the impaired relaxation in response to the endothelium-dependent vasodilator acetylcholine in mouse aortae after exposure to LPS. Similarly, chronic co-treatment with 20 mg/kg/day paeonol reversed lipopolysaccharide-induced endothelial dysfunction. Furthermore, paeonol treatment downregulated the toll-like receptor 4 and bone-morphogenic protein 4 protein and its downstream signaling protein including iNOS and decreased ROS production which subsequently leads to an increase in NO bioavailability [82]. These studies concede that NO is an endothelial cell-protecting factor capable of suppressing apoptosis-related activities and elevating cellular survival.
In a recent study, paeonol was shown to decrease elevated blood pressure and increased cerebral blood flow velocity as well as decrease vascular endothelium injury in Spontaneously Hypertensive Rats [83]. They related the improvement in vascular function to the fact that paeonol reduced blood viscosity and oxidative stress and improved antioxidant capacity.
3.4. Anti-Apoptotic Mechanism
Apoptosis or programmed cell death occurs via activation of specific signaling pathways which requires a high level of energy leading to cell death [84]. Apoptosis plays a key role in multiple physiological processes such as embryogenesis, normal tissue homeostasis, and aging [85]. Recently, numerous reports in the literature have shown the involvement of apoptosis in the pathogenesis of various cardiovascular diseases. For instance, acute and chronic apoptosis of the myocytes occurs in acute myocardial infarction [86]. Animal and human studies showed that apoptosis occurs at the peri-infarcted region in the initial phase, proposing the role of apoptosis in acute myocardial loss after myocardial infarction [87]. Patients with heart failure are also associated with elevated rates of apoptosis which influence the infarction size and extend of left ventricle remodeling [88]. Apoptosis of endothelial cells triggers atherosclerosis while activated macrophages may kill the smooth muscle cells which disturb the stability of plaque [89].
Cardiovascular diseases can be induced via apoptotic mechanisms such as caspase-dependent or caspase-independent apoptosis [90]. In general, the caspase-dependent mechanisms can be subdivided into either extrinsic (involving death receptors) or intrinsic (mitochondria-mediated) pathways [91,92]. In the extrinsic apoptotic pathway, the binding of death ligands such as Fas ligand (FasL) or TNF-α to a membrane-bound death receptor initiates the death receptor-mediated pathway. This consequently activates caspase-8 followed by the downstream effector caspases [93]. Various literature reported that the extrinsic apoptotic pathway leads to the pathogenesis of heart failure [94,95,96]. In the intrinsic apoptotic pathway, the mitochondria are induced to release cytochrome c into the cytosol and form an activation complex called apoptotic protein activating factor-1 (Apaf-1) and caspase 9 [97,98]. Both extrinsic and intrinsic apoptotic pathways eventually merge into a common effector caspase called caspase-3 to complete the final biochemical and morphology apoptotic changes [99]. Meanwhile, the caspase-independent mechanism is related to the release of apoptotic factors including apoptosis-inducing factor (AIF) from mitochondria to the cytosol and subsequently nucleus translocation, leading to deoxyribonucleic acid (DNA) fragmentation without activation of caspase [100,101].
Cardiomyopathy induced by epirubicin, an antrum antibiotic remains a major worry as it led to significant side effects such as irreversible left ventricular dysfunction and congestive heart failure [102,103]. Wu et al. had discovered that paeonol protects against epirubicin-induced rat cardiac myocytes H9C2 and mice cardiomyocytes LH-1 apoptosis with a significant increase in viability rates and the number of apoptotic cells from the LTT assay and flow cytometry [104]. In TUNEL-stained mice heart sections, treatment with paeonol significantly reversed the apoptosis of cardiomyocytes induced by epirubicin from 78.19% to 36.83% [104]. Compared to the epirubicin-treated mice group, paeonol treated mice showed a decrease in cleaved caspase-3 and Bax as well as an increase in Bcl-2. Furthermore, paeonol-treatment reversed cardiac dysfunction induced by epirubicin which subsequently improved various physiological indexes such as reduction of left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic diameter (LVESD), myocardial enzymes, inflammatory markers, and normalization of heart tissue morphology [104]. Paeonol also downregulated the protein expression of phosphoinositide 3-kinases (PI3K), protein kinase B (AKT), and mTOR in H9C2 and LH-1. These suggest that paeonol improved epirubicin-induced cardiomyopathy and cardiac dysfunction by reducing apoptosis via suppression of PI3K/AKT/mTOR signaling pathways [104]. In addition, in an arteriosclerotic in vivo model using ApoE−/− mice, paeonol showed anti-atherosclerotic and anti-proliferation effects on vascular smooth muscle cells (VSMCs) by stimulating AMPK phosphorylation which reduced reduction p-mTOR/mTOR as well as induced conversion LC3II and therefore, inhibits the proliferation of cell in VSMCs [105].
Myocardial ischemia or reperfusion (I/R) injury can be caused by cellular apoptosis, leading to major cardiac injury [106,107]. The Notch1 signaling regulates the proliferation and differentiation of the cardiomyocytes in the heart [108,109]. In I/R-induced apoptosis in H9c2 embryonic rat myocardium-derived cells, pre-treatment with paeonol reduced the percentage of H9c2 cells apoptosis [110]. The anti-apoptotic effects of paeonol were associated with the downregulation of pro-apoptotic proteins such as cleaved-caspase-3 and Bax, as well as up-regulation of anti-apoptotic protein Bcl-2 [110]. In addition, paeonol up-regulated the Notch1 level which was reduced in I/R-induced apoptosis in H9c2, suggesting that paeonol protects SIR-induced H9c2 cells apoptosis via the Notch1 signaling pathway [110].
Ischemic heart disease (IHD) is a condition characterized by an imbalance between the demand of myocardial and oxygen supply from the coronary artery, leading to myocardium necrosis [111]. This results in various changes in pathophysiology and biochemistry, for instance, lipid peroxidation, hyperglycemia, or hyperlipidemia [111]. The protective effects and mechanism of paeonol were investigated in isoproterenol (ISO)-induced myocardial infarction in rats [112]. The results demonstrated that paeonol significantly suppressed ISO-induced apoptosis in the myocardial tissue as evidenced by a reduction in TUNEL-positive myocytes compared to the ISO-induced group in the myocardial tissue [112]. Paeonol treated group also showed reduced apoptosis signaling molecules namely Fas, TNF-α, Bax, caspase-3, caspase-8, caspase-9, cytochrome c while increased the anti-apoptotic protein such as Bcl-2 in myocardial tissue compared to the ISO-induced group. In addition, the paeonol-treated rat group showed lowered the myocardial injury markers level serum myocardial injury markers such as serum levels of serum creatine kinase-MB (CK-MB), cardiac troponin I (cTnI), and cardiac troponin T (cTnT), normalized morphological changes and mitigates lipid peroxidation in the myocardial heart tissue, suggesting that paeonol prevented myocardial injury induced by ISO [112]. During the exploration of underlying signaling pathways by paeonol, treatment with paeonol showed increased translocation of nuclear factor erythroid 2–related factor 2 (Nrf2) as well as enhanced phosphorylated PI3K and Akt which may lead to activation of its anti-oxidative and anti-apoptotic signaling in rats [112]. Collectively, the results suggest that the cardioprotective and anti-apoptotic effects of paeonol against ISO-induced myocardial infarction in rats were mediated via activation of Nrf2/PI3K/Akt cell survival signaling pathway [112].
One of the cardiovascular complications in type 2 diabetes mellitus is diabetic cardiomyopathy which is defined as changes in morphology and function of the myocardial leading to cardiac dysfunction and eventually heart failure [113]. Activation of cell death pathways is reported as one of the pathophysiologies of diabetic cardiomyopathy [114]. Li et al. investigated the effects of paeonol on diabetic cardiomyopathy [115]. The result revealed that rats treated with paeonol attenuated apoptosis of myocardial tissues followed by improvement of cardiac function and myocardial morphology compared to the diabetes group. In addition, paeonol treated group showed decreased glucose serum level, ROS, inflammatory mediators (TNF-α, IL-6, and IL-1β), and reduced collagen deposition in the cardiac tissue. The cardioprotective effect of paeonol was achieved via modulation of PI3K, p-Akt, glycogen synthase kinase-3β, and glycogen synthase followed by down-regulation of apoptotic and inflammatory signaling protein such as protease-activated receptor-1, caspase-3, TNF-α, NF-κB p65, and p-Iκ-Bα expressions. The result suggests that paeonol protects against diabetic cardiomyopathy and improves myocardial function by reducing apoptosis, inflammation, and activation of the PI3K/Akt-GSK-3β signaling pathway [115].
The effects of paeonol against cardiovascular diseases and the related mechanisms are discussed in the subsections below (Table 1).

Table 1.
Mechanism of action of Paeonol in cardiovascular diseases.
4. Conclusions
In conclusion, paeonol, a naturally occurring bioactive compound in Cortex Moutan, has been shown to be a promising therapeutic agent for the treatment of cardiovascular diseases. These cardioprotective effects are attributed to its multifactorial actions such as anti-inflammatory, anti-oxidant, anti-apoptotic, and regulation of vascular tone. Paeonol’s pleiotropic pharmacological effects suggest that it has a lot of potential for clinical use in atherosclerosis prevention and treatment. In terms of paeonol’s undesirable physical properties, there have been reports that several paeonol-loaded carriers have overcome the shortcomings of poor solubility and stability, improving the bioavailability and residence of paeonol in vivo, and thus providing reliable technical support for paeonol in practice. However, scientific investigations examining paeonol’s therapeutic effects have been uncommon in recent years. To assess the efficacy and safety of the treatment, large randomized, controlled, and double-blind trials are urgently needed.
Author Contributions
C.K.W. designed the flow of the review, planned the manuscript as well as supervised the entire work. C.K.W., S.V., D.M., R.A., A.A. and W.Y.S. collected the data, wrote the manuscript and made the table and figure for the sub-section of the manuscript. S.V. revised the format of the manuscript. All the co-authors revised the final draft. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank Geran Penyelidikan Khas: 600-RMC/GPK 5/3 (269/2020) from Universiti Teknologi MARA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
We confirm that there are no conflict of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Abbreviations
| Ac-H3 K14 | histone H3 acetylation at lysine 14 |
| Ac-H4 K16 | histone H4 acetylation at lysine 16 |
| AIF | apoptosis inducing factor |
| ApoE−/− | apoliprotein E-deficient |
| BrdU | Bromodeoxyuridine/5-bromo-2’-deoxyuridine |
| CD36 | cluster of differentiation 36 |
| c-Jun-AP-1 | c-JUN-Activator protein-1 |
| CK | creatinine kinase |
| CK-MB | creatine kinase-MB |
| EDCF | endothelium-derived contracting factors |
| EDHF | endothelium-derived hyperpolarizing factor |
| EDRFs | endothelium-derived relaxing factors |
| eNOS | endothelial nitric oxide synthase |
| HUVECs | human umbilical vascular endothelial cells |
| LOX-1 | lipoprotein receptor-1 |
| LTT assay | lymphocyte transformation test assay |
| LVEDD | left ventricular end-diastolic dimension |
| MCP-1 | monocyte chemoattractant protein-1 |
| PRRs | pattern recognition receptors |
| RAECs | rat aortic endothelial cells |
| SA-beta-galactosidase | Senescence-associated beta-galactosidase |
| sGC | soluble guanylyl cyclase |
| SIRT 1 | Sirtuin 1 |
| STZ | streptozotocin |
| TUNEL | deoxynucleotidyl transferase-mediated dUTP nick-end labelling |
| VECs | vascular endothelial cells |
References
- Clark, H. NCDs: A challenge to sustainable human development. Lancet 2013, 381, 510–511. [Google Scholar] [CrossRef]
- Middlemiss, D.; Watson, S.P. A medicinal chemistry case study: An account of an angiotensin II antagonist drug discovery programme. Tetrahedron 1994, 50, 13049–13080. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2016 Summary Tables: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. The Global Health Observatory; United Nations: Geneva, Switzerland, 2018. [Google Scholar]
- Alwan, A. Global Status Report on Noncommunicable Diseases 2010; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Bonita, R.; Magnusson, R.; Bovet, P.; Zhao, D.; Malta, D.C.; Geneau, R.; Suh, I.; Thankappan, K.R.; McKee, M.; Hospedales, J.; et al. Country actions to meet UN commitments on non-communicable diseases: A stepwise approach. Lancet 2013, 381, 575–584. [Google Scholar] [CrossRef]
- Sacco, R.L.; Roth, G.A.; Reddy, K.S.; Arnett, D.K.; Bonita, R.; Gaziano, T.A.; Heidenreich, P.A.; Huffman, M.; Mayosi, B.M.; Mendis, S.; et al. The heart of 25 by 25: Achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke. Circulation 2016, 133, e674–e690. [Google Scholar] [CrossRef]
- Pashkow, F.J. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int. J. Inflamm. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hajjar, D.P.; Gotto, A.M. Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. Am. J. Pathol. 2013, 182, 1474–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 2016, 21, 807. [Google Scholar] [CrossRef]
- Adki, K.M.; Kulkarni, Y.A. Chemistry, pharmacokinetics, pharmacology and recent novel drug delivery systems of paeonol. Life Sci. 2020, 250, 117544. [Google Scholar] [CrossRef] [PubMed]
- Gjertsen, F.B.; Solheim, E.; Scheline, R.R. Metabolism of aromatic plant ketones in rats: Acetovanillone and paeonol. Xenobiotica 1988, 18, 225–234. [Google Scholar] [CrossRef]
- Li, Y.-J.; Bao, J.-X.; Xu, J.-W.; Murad, F.; Bian, K. Vascular dilation by paeonol—A mechanism study. Vasc. Pharmacol. 2010, 53, 169–176. [Google Scholar] [CrossRef]
- Hirai, A.; Terano, T.; Hamazaki, T.; Sajiki, J.; Saito, H.; Tahara, K.; Tamura, Y.; Kumagai, A. Studies on the mechanism of antiaggregatory effect of moutan cortex. Thromb. Res. 1983, 31, 29–40. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Ge, N.; Zhang, Z.Y. Theoretical elucidation of activity differences of five phenolic antioxidants. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1999, 20, 363–366. [Google Scholar]
- Nizamutdinova, I.T.; Jin, Y.C.; Kim, J.S.; Yean, M.H.; Kang, S.S.; Kim, Y.S.; Lee, J.H.; Seo, H.G.; Kim, H.J.; Chang, K.C. Paeonol and paeoniflorin, the main active principles of paeonia albiflora, protect the heart from myocardial ischemia/reperfusion injury in rats. Planta Med. 2008, 74, 14–18. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Bates, S.; Gurney, A.M. The effects of paeonol on the electrophysiological properties of cardiac ventricular myocytes. Eur. J. Pharmacol. 2006, 545, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qin, Y.; Chen, C.; Guo, X. Beneficial effects exerted by paeonol in the management of atherosclerosis. Oxidative Med. Cell. Longev. 2018, 2018, 1098617. [Google Scholar] [CrossRef]
- Li, S.-S.; Wu, Q.; Yin, D.-D.; Feng, C.; Liu, Z.-A.; Wang, L.-S. Phytochemical variation among the traditional Chinese medicine Mu Dan Pi from Paeonia suffruticosa (tree peony). Phytochemistry 2018, 146, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J.; Liu, J.-L.; Wang, J.-K. Synthesis and anti-tumor activity of paeonol and its derivatives. Yao Xue Xue Bao Acta Pharm. Sin. 2012, 47, 72–76. [Google Scholar]
- Jung, E.-H.; Hwang, J.-S.; Kwon, M.-Y.; Kim, K.-H.; Cho, H.; Lyoo, I.K.; Shin, S.; Park, J.-H.; Han, I.-O. A tryptamine-paeonol hybridization compound inhibits LPS-mediated inflammation in BV2 cells. Neurochem. Int. 2016, 100, 35–43. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, B.; Yang, Y.; Gong, X.; Chen, Z.; Wang, Z.; Zhang, P.; Zhang, Q. Synthesis and anti-inflammatory activity of paeonol analogues in the murine model of complete Freund’s adjuvant induced arthritis. Bioorg. Med. Chem. Lett. 2016, 26, 5218–5221. [Google Scholar] [CrossRef]
- Fu, P.-K.; Yang, C.-Y.; Huang, S.-C.; Hung, Y.-W.; Jeng, K.-C.; Huang, Y.-P.; Chuang, H.; Huang, N.-C.; Li, J.-P.; Hsu, M.-H.; et al. Evaluation of LPS-induced acute lung injury attenuation in rats by aminothiazole-paeonol derivatives. Molecules 2017, 22, 1605. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.-H.; Cao, S.-W.; Yu, Y.-Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013, 62, 589–595. [Google Scholar] [CrossRef]
- Han, F.; Zhuang, T.-T.; Chen, J.-J.; Zhu, X.-L.; Cai, Y.-F.; Lü, Y.-P. Novel derivative of Paeonol, Paeononlsilatie sodium, alleviates behavioral damage and hippocampal dendritic injury in Alzheimer’s disease concurrent with cofilin1/phosphorylated-cofilin1 and RAC1/CDC42 alterations in rats. PLoS ONE 2017, 12, e0185102. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Wu, H.; Pan, J.; Wang, X.; Li, J.; Wu, Z.; Hui, A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of alzheimer’s disease. Molecules 2015, 20, 1304–1318. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-Y.; Kapoor, M.; Huang, Y.-P.; Lin, H.-H.; Liang, Y.-C.; Lin, Y.-L.; Huang, S.-C.; Liao, W.-N.; Chen, J.-K.; Huang, J.-S.; et al. Synthesis and evaluation of aminothiazole-paeonol derivatives as potential anticancer agents. Molecules 2016, 21, 145. [Google Scholar] [CrossRef] [Green Version]
- Anh, H.L.T.; Cuc, N.T.; Tai, B.H.; Yen, P.H.; Nhiem, N.X.; Thao, D.T.; Nam, N.H.; Van Minh, C.; Van Kiem, P.; Kim, Y.H. Synthesis of chromonylthiazolidines and their cytotoxicity to human cancer cell lines. Molecules 2015, 20, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.-J.; Chuang, H.; Liang, Y.-C.; Lin, H.-H.; Horng, J.-C.; Kuo, Y.-C.; Chen, C.-W.; Tsai, F.-Y.; Yen, S.-C.; Chou, S.-C.; et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015, 90, 428–435. [Google Scholar] [CrossRef]
- Tsai, C.-H.; Hsu, M.-H.; Huang, P.-H.; Hsieh, C.-T.; Chiu, Y.-M.; Shieh, D.-C.; Lee, Y.-J.; Tsay, G.J.; Wu, Y.-Y. A paeonol derivative, YPH-PA3 promotes the differentiation of monocyte/macrophage lineage precursor cells into osteoblasts and enhances their autophagy. Eur. J. Pharmacol. 2018, 832, 104–113. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2021, 269, 113708. [Google Scholar] [CrossRef]
- Chen, X.; Lu, Y.; Du, S.; Xu, B.; Wang, S.; Zhai, Y.; Song, X.; Li, P. In Situ and In Vivo study of nasal absorption of paeonol in rats. Int. J. Mol. Sci. 2010, 11, 4882–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Xie, Y.-H.; Yang, Q.; Wang, S.-W.; Zhang, B.-L.; Wang, J.-B.; Cao, W.; Bi, L.-L.; Sun, J.-Y.; Miao, S.; et al. Cardioprotective effect of paeonol and danshensu combination on isoproterenol-induced myocardial injury in rats. PLoS ONE 2012, 7, e48872. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, S.; Zhang, B.-L.; Xie, Y.; Wang, J.; Yang, Q.; Cao, W.; Hu, J.; Duan, L. Influence of Co-Administered Danshensu on Pharmacokinetic Fate and Tissue Distribution of Paeonol in Rats. Planta Medica 2011, 78, 135–140. [Google Scholar] [CrossRef]
- Li, S.-S.; Li, G.-F.; Liu, L.; Li, H.; Jiang, X.; Li, X.-L.; Liu, Z.-G.; Zuo, T.; Weng, L.-D.; Liu, Q. Optimization of paeonol-loaded microparticle formulation by response surface methodology. J. Microencapsul. 2015, 32, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Luo, M.; Chen, J. Transdermal delivery of paeonol using cubic gel and microemulsion gel. Int. J. Nanomed. 2011, 6, 1603–1610. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of paeonol-loaded transethosomes as transdermal delivery carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Li, G.-F.; Liu, L.; Jiang, X.; Zhang, B.; Liu, Z.-G.; Li, X.-L.; Weng, L.-D.; Zuo, T.; Liu, Q. Evaluation of paeonol skin-target delivery from its microsponge formulation: In Vitro skin permeation and In Vivo microdialysis. PLoS ONE 2013, 8, e79881. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-C.; Zhu, N.; Zhu, J.-X.; Zhang, W.-J.; Zhang, H.-M.; Wang, Q.-Q.; Wu, X.-X.; Wang, X.; Zhang, J.; Hao, J.-F. Self-assembled cubic liquid crystalline nanoparticles for transdermal delivery of paeonol. Med Sci. Monit. 2015, 21, 3298–3310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Jia, F.; Hou, Z.; Ruan, S.; Lu, Q. Delivery of paeonol by nanoparticles enhances its In Vitro and In Vivo antitumor effects. Int. J. Nanomed. 2017, 12, 6605–6616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Zhang, J.; Wu, L.; Wu, H.; Dai, M. Paeonol nanoemulsion for enhanced oral bioavailability: Optimization and mechanism. Nanomedicine 2018, 13, 269–282. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, Y.; Hu, Q.; Zeng, D.; Hua, F.; Meng, W.; Wang, W.; Bao, G.-H. Optimization of paeonol-loaded poly(butyl-2-cyanoacrylate) nanocapsules by central composite design with response surface methodology together with the antibacterial properties. Eur. J. Pharm. Sci. 2017, 101, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. NISCAIR Online Period. Repos. 2009, 46, 421–440. [Google Scholar]
- Ding, Z.; Liu, S.; Wang, X.; Khaidakov, M.; Dai, Y.; Mehta, J.L. Oxidant stress in mitochondrial DNA damage, autopha-gy and inflammation in atherosclerosis. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yon, J.-Y.; Cai, H. Mechanisms and consequences of eNOS dysfunction in hypertension. J. Hypertens. 2015, 33, 1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasconcelos, E.M.; Degasperi, G.R.; de Oliveira, H.C.; Vercesi, A.E.; de Faria, E.C.; Castilho, L.N. Reactive oxygen spe-cies generation in peripheral blood monocytes and oxidized LDL are increased in hyperlipidemic patients. Clin. Biochem. 2009, 42, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.-h.; Zhang, Y.-w.; Zhou, H.-h. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-κB pathway. J. Ethnopharmacol. 2013, 146, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Jamal, J.; Mustafa, M.R.; Wong, P.-F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Yu, C.; Yang, H.; Zhang, C.; Ye, Y.; Xiao, S. Paeonol suppresses lipid accumulation in macrophages via upregulation of the ATP-binding cassette transporter A1 and downregulation of the cluster of differentiation. Int. J. Oncol. 2015, 46, 764–774. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Mozos, I.; Malainer, C.; Horbańczuk, J.; Gug, C.; Stoian, D.; Luca, C.T.; Atanasov, A.G. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 2017, 8, 1058. [Google Scholar] [CrossRef] [Green Version]
- Katsiari, C.G.; Bogdanos, D.P.; I Sakkas, L. Inflammation and cardiovascular disease. World J. Transl. Med. 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Fioranelli, M.; Bottaccioli, A.G.; Bottaccioli, F.; Bianchi, M.; Rovesti, M.; Roccia, M.G. Stress and inflammation in coronary artery disease: A review psychoneuroendocrineimmunology-based. Front. Immunol. 2018, 9, 2031. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.; Libby, P. Inflammation and atherothrombosis. Ann. N. Y. Acad. Sci. 2006, 947, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Loscalzo, J.; Ridker, P.M.; Farkouh, M.E.; Hsue, P.Y.; Fuster, V.; Hasan, A.A.; Amar, S. Inflammation, immunity, and infection in atherothrombosis. J. Am. Coll. Cardiol. 2018, 72, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Dai, M. Paeonol from Paeonia suffruticosa prevents TNF-α-induced monocytic cell adhesion to rat aortic endothelial cells by suppression of VCAM-1 expression. Phytomedicine 2009, 16, 1027–1032. [Google Scholar] [CrossRef]
- Kim, S.-A.; Lee, H.-J.; Ahn, K.S.; Lee, E.-O.; Ahn, K.-S.; Choi, S.-H.; Jung, S.-J.; Kim, J.Y.; Baek, N.; Kim, S.-H. Paeonol exerts anti-angiogenic and anti-metastatic activities through downmodulation of akt activation and inactivation of matrix metalloproteinases. Biol. Pharm. Bull. 2009, 32, 1142–1147. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Chen, J.; Dai, M. Paeonol promotes microRNA-126 expression to inhibit monocyte adhesion to ox-LDL-injured vascular endothelial cells and block the activation of the PI3K/Akt/NF-κB pathway. Int. J. Mol. Med. 2016, 38, 1871–1878. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-R.; Chen, J.-J.; Dai, M. Paeonol protects rat vascular endothelial cells from ox-LDL-induced injury in vitro via downregulating microRNA-21 expression and TNF-α release. Acta Pharmacol. Sin. 2014, 35, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, C.; Wu, H.; Xie, X.; Sun, Y.; Dai, M. Paeonol attenuated inflammatory response of endothelial cells via stimulating monocytes-derived exosomal microRNA-223. Front. Pharmacol. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Ma, L.; Chuang, C.-C.; Weng, W.; Zhao, L.; Zheng, Y.; Zhang, J.; Zuo, L. Paeonol protects rat heart by improving regional blood perfusion during no-reflow. Front. Physiol. 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-F.; Leu, S.-J.J.; Shyue, S.-K.; Su, K.-H.; Wei, J.; Lee, T.-S. Novel effect of paeonol on the formation of foam cells: Promotion of LXRα-ABCA1–dependent cholesterol efflux in macrophages. Am. J. Chin. Med. 2013, 41, 1079–1096. [Google Scholar] [CrossRef]
- Kietadisorn, R.; Juni, R.P.; Moens, A.L. Tackling endothelial dysfunction by modulating NOS uncoupling: New insights into its pathogenesis and therapeutic possibilities. Am. J. Physiol. Metab. 2012, 302, E481–E495. [Google Scholar] [CrossRef] [Green Version]
- Griffith, T.M.; Lewis, M.J.; Newby, A.C.; Henderson, A.H. Endothelium-derived relaxing factor. J. Am. Coll. Cardiol. 1988, 12, 797–806. [Google Scholar] [CrossRef] [Green Version]
- Gewaltig, M.T. Vasoprotection by nitric oxide: Mechanisms and therapeutic potential. Cardiovasc. Res. 2002, 55, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H. Endothelial Dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef] [PubMed]
- Mollnau, H.; Wendt, M.; Szoöcs, K.; Lasseègue, B.; Schulz, E.; Oelze, M.; Li, H.; Bodenschatz, M.; August, M.; Kleschyov, A.L.; et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002, 90, e58–e65. [Google Scholar] [CrossRef] [Green Version]
- Hink, U.; Li, H.; Mollnau, H.; Oelze, M.; Matheis, E.; Hartmann, M.; Skatchkov, M.; Thaiss, F.; Stahl, R.A.K.; Warnholtz, A.; et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001, 88, e14–e22. [Google Scholar] [CrossRef]
- Takimoto, E.; Champion, H.C.; Li, M.; Ren, S.; Rodriguez, E.R.; Tavazzi, B.; Lazzarino, G.; Paolocci, N.; Gabrielson, K.L.; Wang, Y.; et al. Oxidant stress from nitric oxide synthase–3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Investig. 2005, 115, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Hadi, H.A.R.; Carr, C.S.; Al Suwaidi, J. Endothelial dysfunction: Cardiovascular risk factors, therapy, and outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar] [PubMed]
- Pryor, W.A.; Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am. J. Physiol. Cell. Mol. Physiol. 1995, 268, L699–L722. [Google Scholar] [CrossRef] [PubMed]
- Thuillez, C.; Richard, V. Targeting endothelial dysfunction in hypertensive subjects. J. Hum. Hypertens. 2005, 19, S21–S25. [Google Scholar] [CrossRef]
- Goto, H.; Shimada, Y.; Akechi, Y.; Kohta, K.; Hattori, M.; Terasawa, K. Endothelium-dependent vasodilator effect of extract prepared from the roots ofpaeonia lactifloraon isolated rat aorta. Planta Med. 1996, 62, 436–439. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Cao, Y.-X.; Weng, W.-L.; Li, Y.-K.; Zhao, L. Paeonol induces vasodilatation in rat mesenteric artery via inhibiting extracellular Ca2+ influx and intracellular Ca2+ release. Chin. J. Integr. Med. 2013, 19, 510–516. [Google Scholar] [CrossRef]
- Choy, K.-W.; Mustafa, M.R.; Lau, Y.S.; Liu, J.; Murugan, D.; Lau, C.W.; Wang, L.; Zhao, L.; Huang, Y. Paeonol protects against endoplasmic reticulum stress-induced endothelial dysfunction via AMPK/PPARδ signaling pathway. Biochem. Pharmacol. 2016, 116, 51–62. [Google Scholar] [CrossRef]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Mustafa, M.R. Chronic treatment with paeonol improves endothelial function in mice through inhibition of endoplasmic reticulum stress-mediated oxidative stress. PLoS ONE 2017, 12, e0178365. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Goldblum, S.E. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L899–L914. [Google Scholar] [CrossRef] [Green Version]
- Mann, U.L.; Topkara, V.K.; Evans, S.; Barger, P.M. Innate immunity in the adult mammalian heart: For whom the cell tolls. Trans. Am. Clin. Clim. Assoc. 2010, 121, 34–51. [Google Scholar]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Vanhoutte, P.M.; Mustafa, M.R. Paeonol attenuates LPS-induced endothelial dysfunction and apoptosis by inhibiting BMP4 and TLR4 signaling simultaneously but independently. J. Pharmacol. Exp. Ther. 2018, 364, 420–432. [Google Scholar] [CrossRef] [Green Version]
- Gai, Z.; Wang, Z.; Zhang, L.; Ma, J.; Zhu, Q. Paeonol protects against hypertension in spontaneously hypertensive rats by restoring vascular endothelium. Biosci. Biotechnol. Biochem. 2019, 83, 1992–1999. [Google Scholar] [CrossRef]
- Teringova, E.; Tousek, P. Apoptosis in ischemic heart disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraste, A.; Pulkki, K.; Kallajoki, M.; Henriksen, K.; Parvinen, M.; Voipio-Pulkki, L.-M. Apoptosis in human acute myocardial infarction. Circulation 1997, 95, 320–323. [Google Scholar] [CrossRef]
- Olivetti, G. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J. Mol. Cell. Cardiol. 1996, 28, 2005–2016. [Google Scholar] [CrossRef]
- Abbate, A.; Biondi-Zoccai, G.G.; Bussani, R.; Dobrina, A.; Camilot, D.; Feroce, F.; Rossiello, R.; Baldi, F.; Silvestri, F.; Biasucci, L.M.; et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Stoneman, V.E.A.; Bennett, M. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin. Sci. 2004, 107, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.-H.; Kang, P.M. Apoptosis in cardiovascular diseases: Mechanism and clinical implications. Korean Circ. J. 2010, 40, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.M.; Haunstetter, A.; Aoki, H.; Usheva, A.; Izumo, S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ. Res. 2000, 87, 118–125. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Gores, G.J. Life and death by death receptors. FASEB J. 2009, 23, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Chiong, M.; Wang, Z.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; A Hill, J.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, K.; Whelan, R.S.; Kitsis, R.N. Mechanisms of cell death in heart disease. Arter. Thromb. Vasc. Biol. 2012, 32, 1552–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marín-García, J. Cell death in the pathogenesis and progression of heart failure. Hear. Fail. Rev. 2016, 21, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Bao, Q.; Shi, Y. Apoptosome: A platform for the activation of initiator caspases. Cell Death Differ. 2006, 14, 56–65. [Google Scholar] [CrossRef]
- Nicholson, D.W.; Thornberry, N.A. Caspases: Killer proteases. Trends Biochem. Sci. 1997, 22, 299–306. [Google Scholar] [CrossRef]
- Cregan, S.P.; Dawson, V.; Slack, R.S. Role of AIF in caspase-dependent and caspase-independent cell death. Oncogene 2004, 23, 2785–2796. [Google Scholar] [CrossRef] [Green Version]
- Penninger, J.; Kroemer, G. Mitochondria, AIF and caspases—Rivaling for cell death execution. Nat. Cell Biol. 2003, 5, 97–99. [Google Scholar] [CrossRef]
- Florescu, M.; Magda, L.S.; Enescu, O.A.; Jinga, D.; Vinereanu, D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J. Am. Soc. Echocardiogr. 2014, 27, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like doxorubicin in breast cancer? A clinical review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, C.; Wang, R.; Li, J.; Zhou, M.; Yan, M.; Xue, X.; Wang, C. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem. Interact. 2018, 286, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, A.; Hu, W.; Dai, M. The anti-atherosclerotic effect of paeonol against vascular smooth muscle cell proliferation by up-regulation of autophagy via the AMPK/mTOR signaling pathway. Front. Pharmacol. 2018, 8, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, G. Treatment of myocardial ischemia/reperfusion injury by ischemic and pharmacological postconditioning. Compr. Physiol. 2015, 5, 1123–1145. [Google Scholar] [CrossRef] [PubMed]
- Von Elverfeldt, D.; Maier, A.; Duerschmied, D.; Braig, M.; Witsch, T.; Wang, X.; Mauler, M.; Neudorfer, I.; Menza, M.; Idzko, M.; et al. Dual-contrast molecular imaging allows noninvasive characterization of myocardial ischemia/reperfusion injury after coronary vessel occlusion in mice by magnetic resonance imaging. Circulation 2014, 130, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Du, J.; Song, X.; He, L.; Zhang, Y.; Li, X.; Qiu, C.; Zhang, Y.; Hou, J.; Feng, J.; et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free. Radic. Biol. Med. 2016, 97, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Fang, Y.H.; Zhao, Y.; Zou, B.; Xu, Q.R.; Xu, H.; Rao, C.X.; Liu, J.C. The effect of Notch1 on myocardial ischemia reperfusion injury. Zhonghua Yi Xue Za Zhi 2016, 96, 1591–1596. [Google Scholar]
- Li, X.; Yang, R.; Yang, X.; Zheng, X. Paeonol protects H9C2 cardiomyocytes from ischemia/reperfusion injury by activating Notch1 signaling pathway In Vitro. Int. J. Clin. Exp. Med. 2017, 10, 2866–2873. [Google Scholar]
- Cusack, M. Recent advances in ischaemic heart disease. Postgrad. Med J. 2000, 76, 542–546. [Google Scholar] [CrossRef]
- Lin-Rui, D.; Song, F.; Duan, L.-R.; Sheng, J.-J.; Xie, Y.-H.; Yang, Q.; Chen, Y.; Dong, Q.-Q.; Zhang, B.-L.; Wang, S.-W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef]
- Sun, D.; Shen, M.; Li, J.; Li, W.; Zhang, Y.; Zhao, L.; Zhang, Z.; Yuan, Y.; Wang, H.; Cao, F. Cardioprotective effects of tanshinone IIA pretreatment via kinin B2 receptor-Akt-GSK-3beta dependent pathway in experimental diabetic cardiomyopathy. Cardiovasc. Diabetol. 2011, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frustaci, A.; Kajstura, J.; Chimenti, C.; Jakoniuk, I.; Leri, A.; Maseri, A.; Nadal-Ginard, B.; Anversa, P. Myocardial cell death in human diabetes. Circ. Res. 2000, 87, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z.-Q.; Bao, L.; Li, Z.-C.; Qu, J.-X. Protective effects of paeonol on cardiovascular complications in diabetes mellitus involves modulation of PI3K /Akt-GSK-3β signalling, regulation of protease-activated receptor-1 expressions and down-regulation of inflammatory mediators. Bangladesh J. Pharmacol. 2015, 10, 903–916. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).