Flavylium-Based Hypoxia-Responsive Probe for Cancer Cell Imaging
Abstract
1. Introduction
2. Results and Discussion
2.1. Probe Synthesis and Characterization
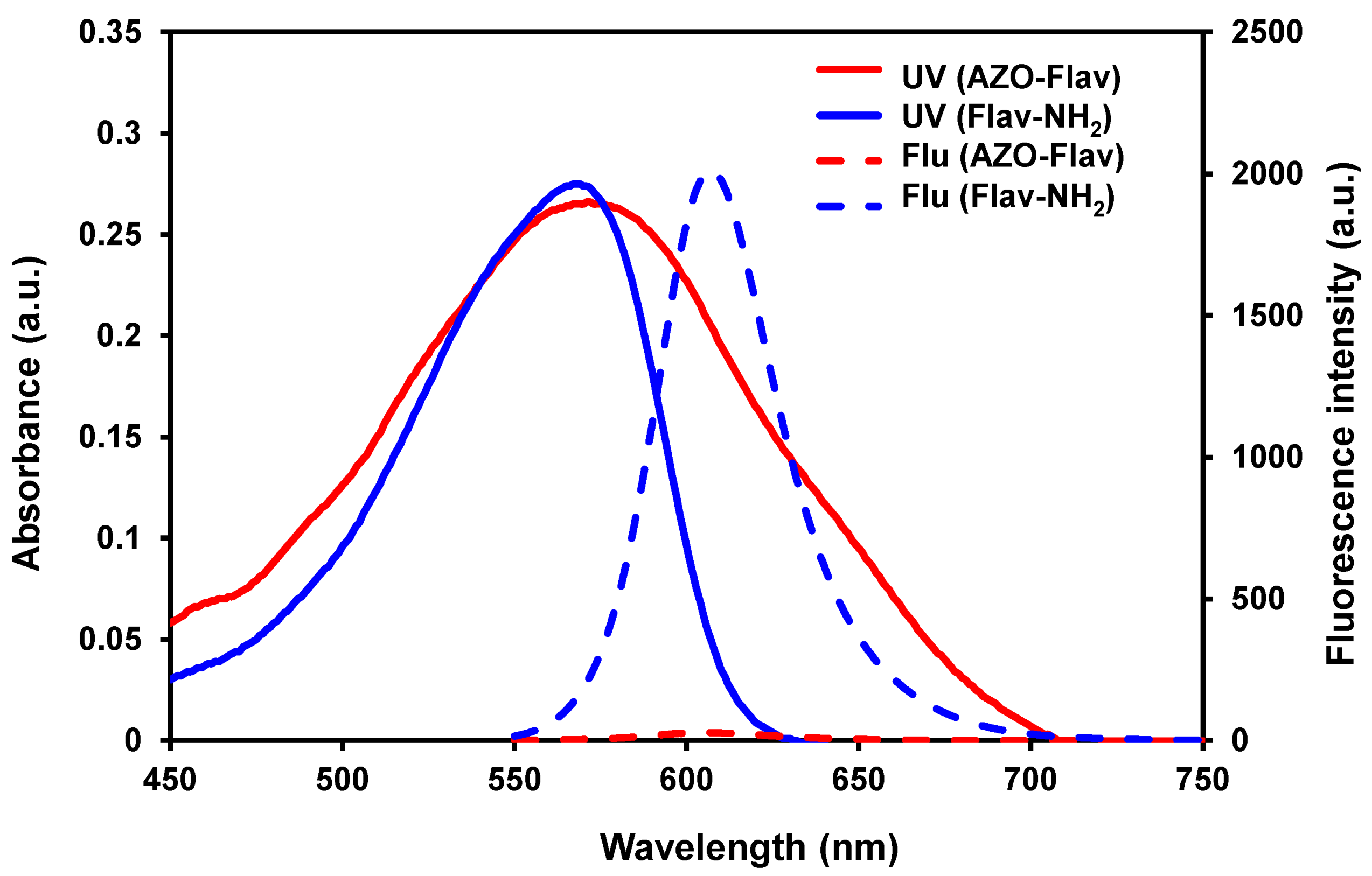
2.2. Photophysical Properties of Probe AZO-Flav and Fluorophore Flav-NH2
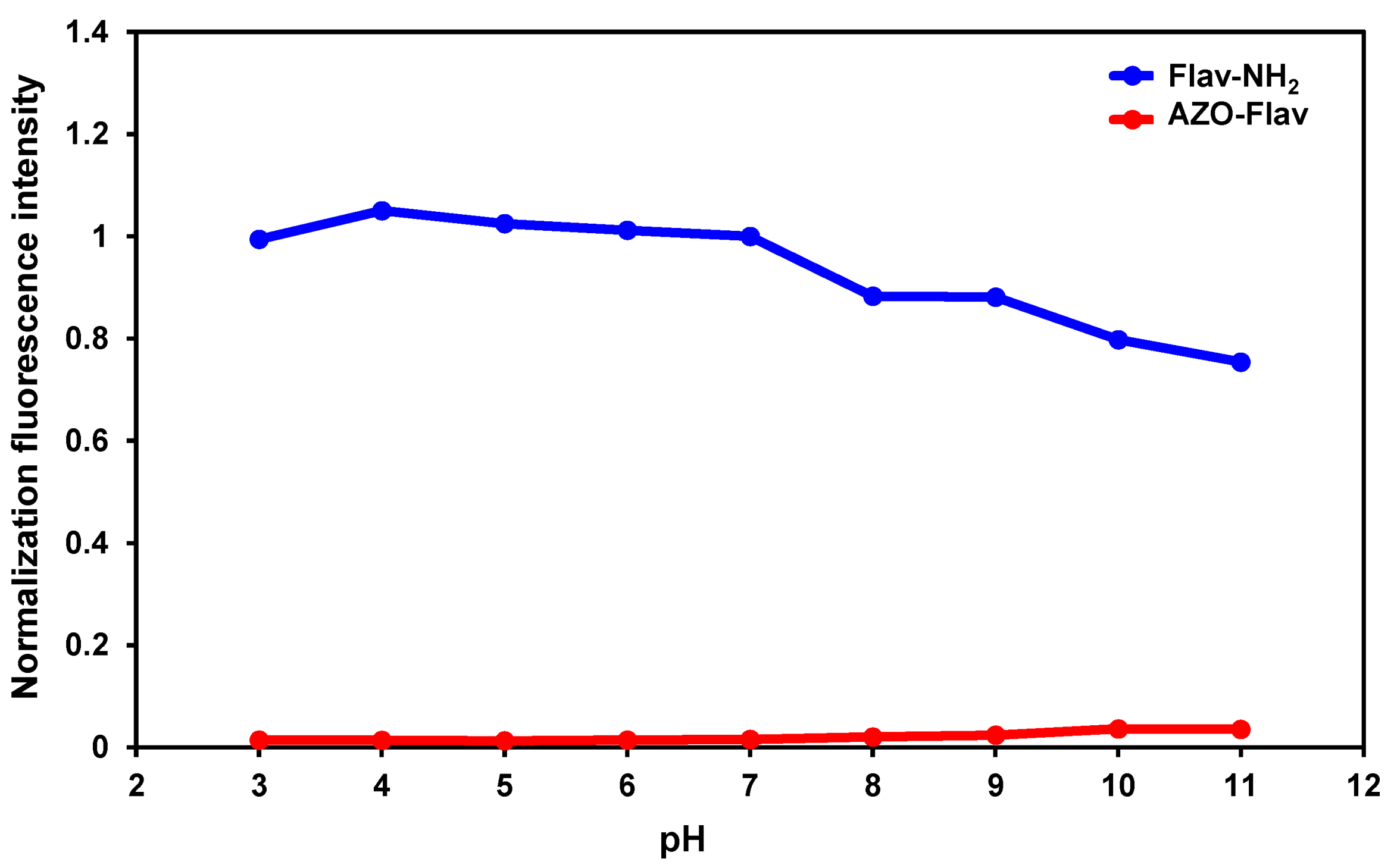
2.3. Fluorescence Stability towards pH Changes
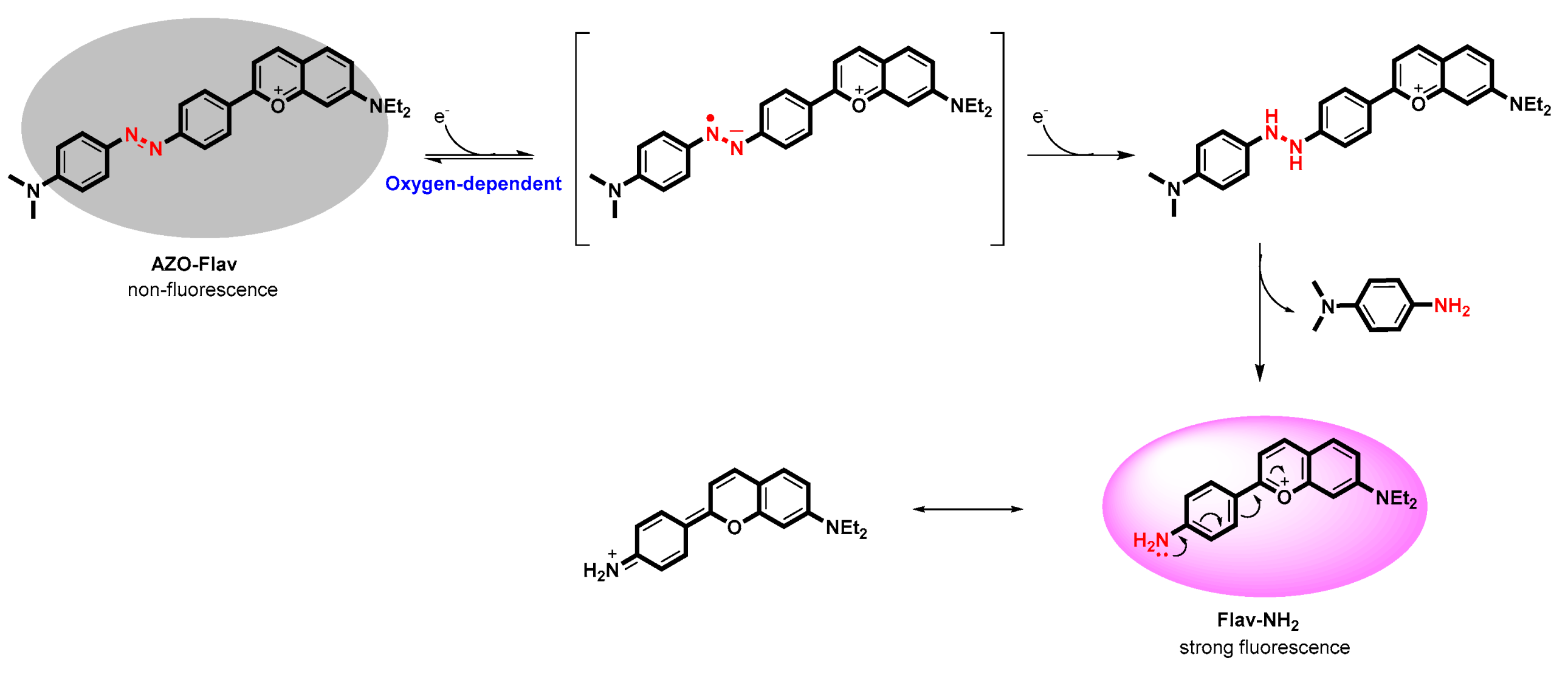
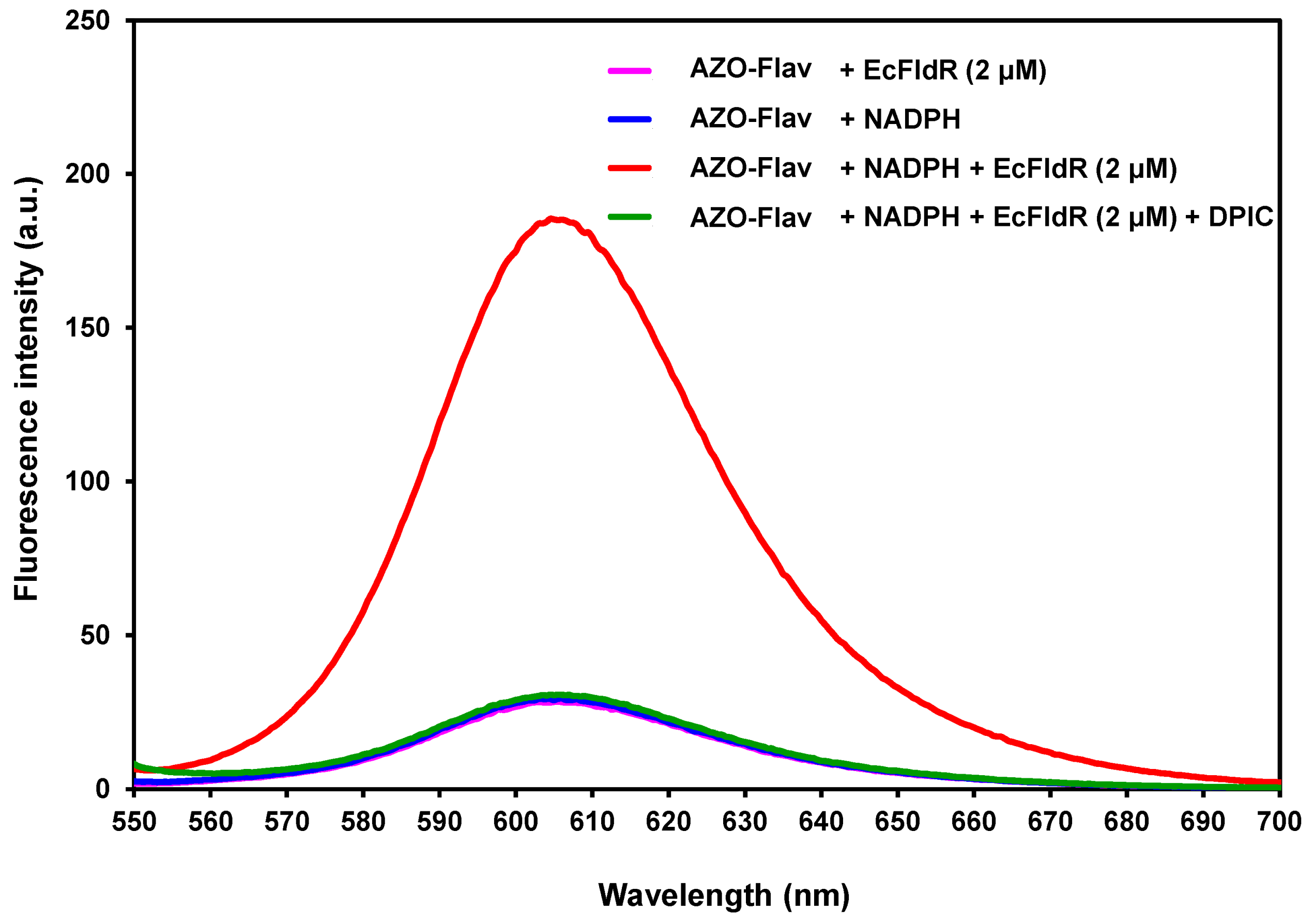
2.4. In Vitro Reduction of AZO-Flav by E. coli Flavodoxin Reductase (EcFldR)
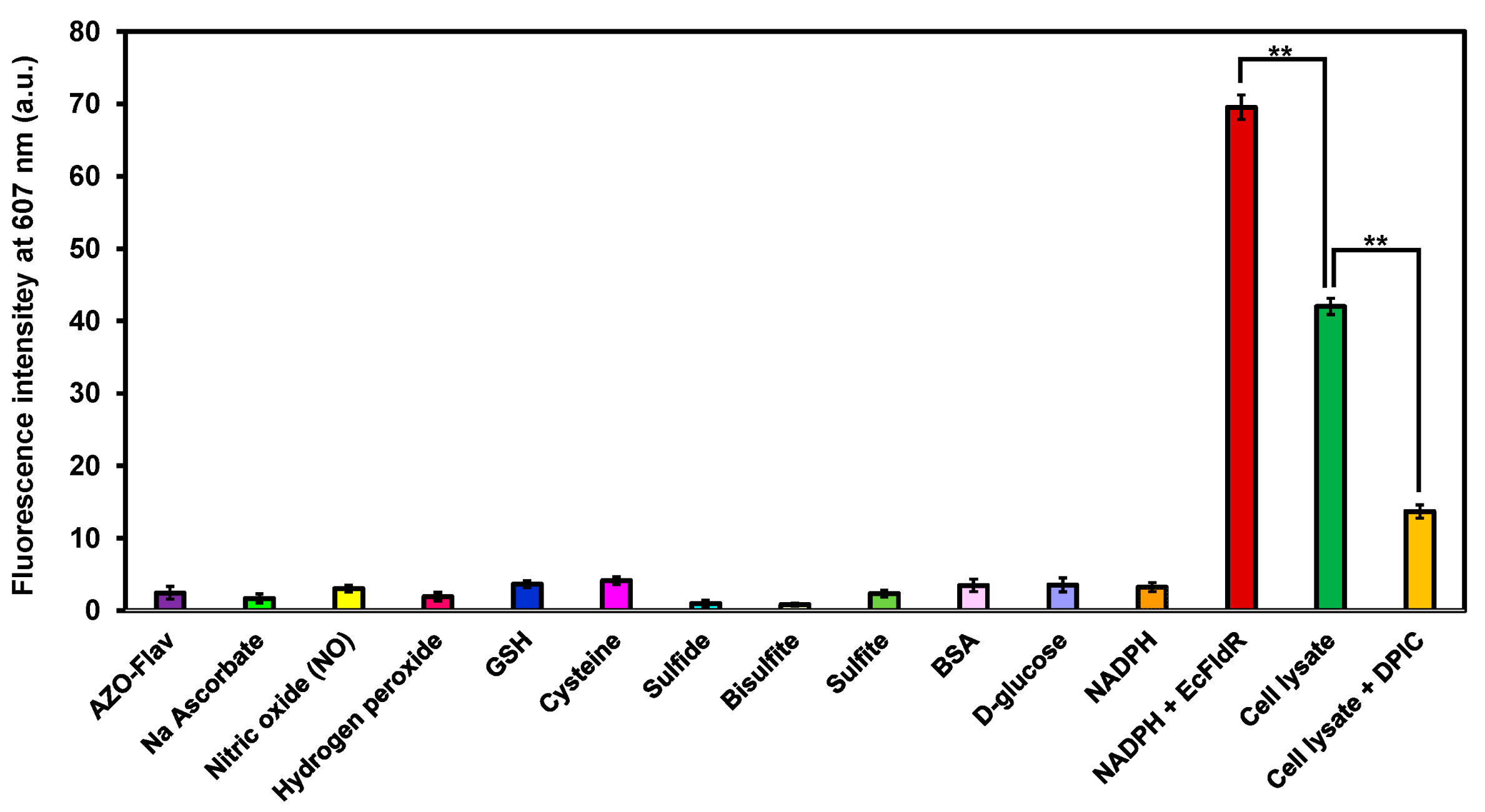
2.5. Specificity of AZO-Flav Reduction
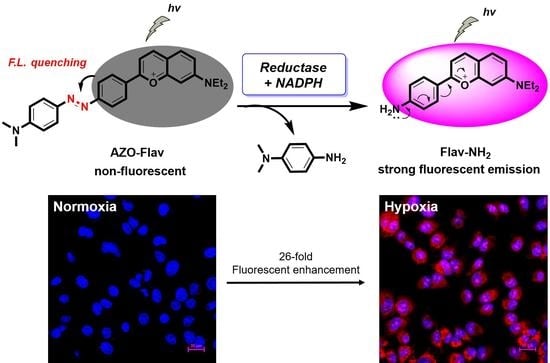
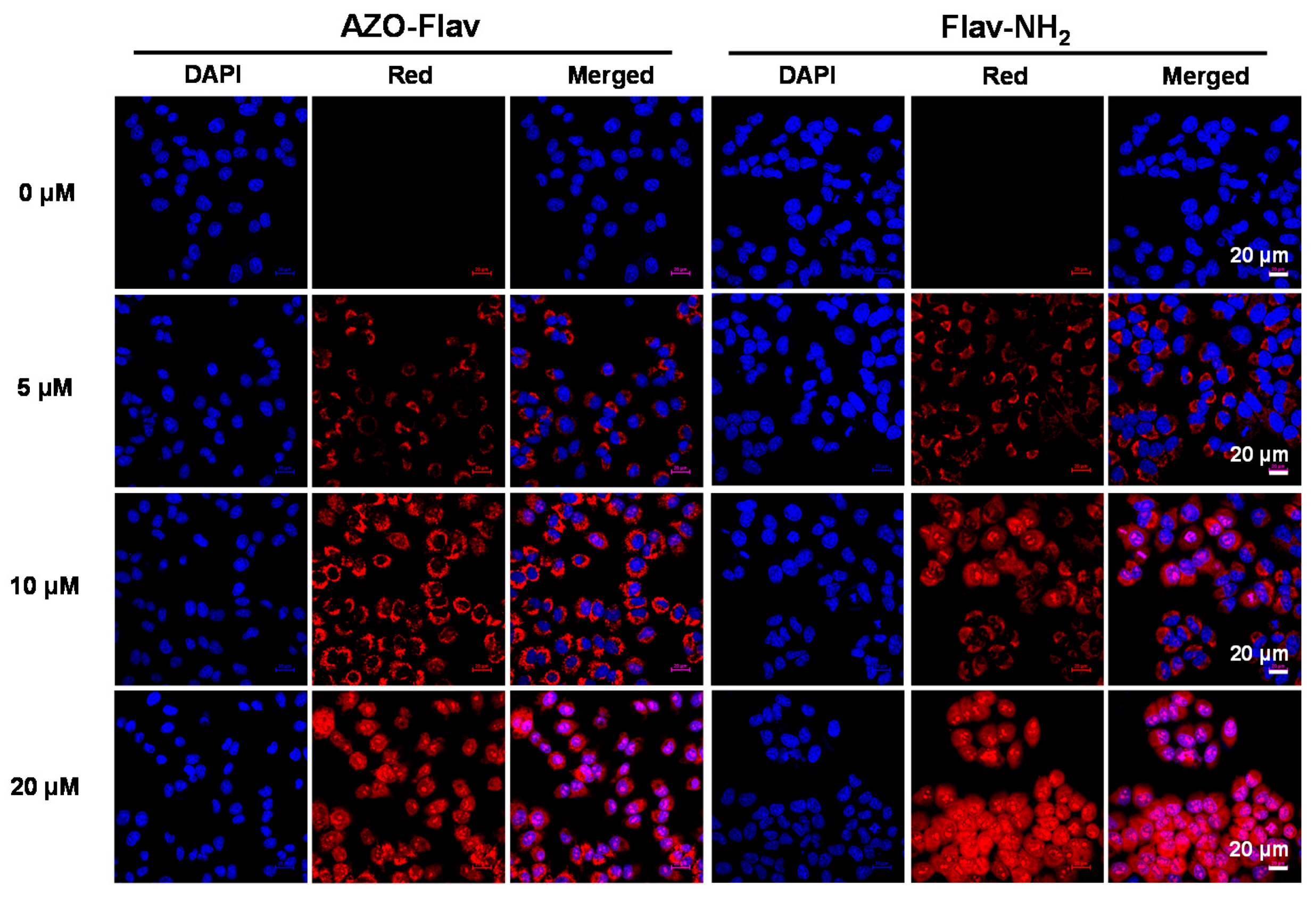
2.6. Hypoxic Cell Imaging
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Synthesis of AZO-Flav and Flav-NH2
3.3. Spectroscopic Materials and Methods
The Study of pH Effect
3.4. EcFld Reductase Assay
3.4.1. Overexpression and Purification of Escherichia coli Flavodoxin Reductase (EcFldR)
Plasmid Construction of pET30-EcFldR
Overexpression and Purification of EcFldR
3.4.2. Response towards EcFld Reductase
3.4.3. Selectivity towards EcFld Reductase
3.4.4. Limit of Detection (LOD) of AZO-Flav Reduction toward EcFldR
3.4.5. HPLC for AZO-Flav with EcFldR
3.5. Cell Culture and Confocal Imaging
3.5.1. Cell Culture
3.5.2. Cell Imaging
3.5.3. Hypoxia Inhibitory Effect
3.5.4. Cell Viability Assay of AZO-Flav and Flav-NH2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Luo, S.; Liu, Y.; Wang, F.; Fei, Q.; Shi, B.; An, J.; Zhao, C.; Tung, C.-H. A fluorescent turn-on probe for visualizing lysosomes in hypoxic tumor cells. Analyst 2016, 141, 2879–2882. [Google Scholar] [CrossRef]
- Wheeler, K.T.; Wang, L.-M.; A Wallen, C.; Childers, S.R.; Cline, J.M.; Keng, P.C.; Mach, R.H. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br. J. Cancer 2000, 82, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M.; Vaupel, P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. J. Natl. Cancer Inst. 2001, 93, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Al Tameemi, W.; Dale, T.P.; Al-Jumaily, R.M.K.; Forsyth, N.R. Hypoxia-Modified Cancer Cell Metabolism. Front. Cell Dev. Biol. 2019, 7, 4. [Google Scholar] [CrossRef]
- Dutta, B.; Yan, R.; Lim, S.K.; Tam, J.P.; Sze, S.K. Quantitative Profiling of Chromatome Dynamics Reveals a Novel Role for HP1BP3 in Hypoxia-induced Oncogenesis. Mol. Cell. Proteom. 2014, 13, 3236–3249. [Google Scholar] [CrossRef]
- Rademakers, S.E.; Span, P.; Kaanders, J.H.; Sweep, F.; Van Der Kogel, A.J.; Bussink, J. Molecular aspects of tumour hypoxia. Mol. Oncol. 2008, 2, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, S.; Huang, J.; Cui, L.; Hu, J.; Tan, S. Novel designed azo substituted semi-cyanine fluorescent probe for cytochrome P450 reductase detection and hypoxia imaging in cancer cells. RSC Adv. 2019, 9, 21572–21577. [Google Scholar] [CrossRef]
- Cai, Q.; Yu, T.; Zhu, W.; Xu, Y.; Qian, X. A turn-on fluorescent probe for tumor hypoxia imaging in living cells. Chem. Commun. 2015, 51, 14739–14741. [Google Scholar] [CrossRef]
- Kiyose, K.; Hanaoka, K.; Oushiki, D.; Nakamura, T.; Kajimura, M.; Suematsu, M.; Nishimatsu, H.; Yamane, T.; Terai, T.; Hirata, Y.; et al. Hypoxia-Sensitive Fluorescent Probes for in Vivo Real-Time Fluorescence Imaging of Acute Ischemia. J. Am. Chem. Soc. 2010, 132, 15846–15848. [Google Scholar] [CrossRef]
- Luo, S.; Zou, R.; Wu, J.; Landry, M.P. A Probe for the Detection of Hypoxic Cancer Cells. ACS Sensors 2017, 2, 1139–1145. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S.; Kumar, N.A. Azodyes as markers for tumor hypoxia imaging and therapy: An up-to-date review. Chem. Interactions 2019, 307, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Fradette, C.; Du Souich, P. Effect of hypoxia on cytochrome P450 activity and expression. Curr. Drug Metab. 2004, 5, 257–271. [Google Scholar] [CrossRef]
- Patterson, A.; Saunders, M.P.; Chinje, E.C.; Talbot, D.C.; Harris, A.; Strafford, I.J. Overexpression of human NADPH:cytochrome c (P450) reductase confers enhanced sensitivity to both tirapazamine (SR 4233) and RSU 1069. Br. J. Cancer 1997, 76, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Cowen, R.L.; Stratford, I.J. Hypoxia and oxidative stress. Tumour hypoxia--therapeutic considerations. Breast Cancer Res. 2001, 3, 328–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, A.; Song, J.; Cravatt, B.F. A Suite of Activity-Based Probes for Human Cytochrome P450 Enzymes. J. Am. Chem. Soc. 2009, 131, 10692–10700. [Google Scholar] [CrossRef]
- Huttunen, K.M.; Mähönen, N.; Raunio, H.; Rautio, J. Cytochrome P450-activated prodrugs: Targeted drug delivery. Curr. Med. Chem. 2008, 15, 2346–2365. [Google Scholar] [CrossRef]
- Rodriguez-Antona, C.; Ingelmansundberg, M. Cytochrome P450 pharmacogenetics and cancer. Oncogene 2006, 25, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Scripture, C.D.; Sparreboom, A.; Figg, W.D. Modulation of cytochrome P450 activity: Implications for cancer therapy. Lancet Oncol. 2005, 6, 780–789. [Google Scholar] [CrossRef]
- McFadyen, M.C.E.; Melvin, W.T.; I Murray, G. Cytochrome P450 enzymes: Novel options for cancer therapeutics. Mol. Cancer Ther. 2004, 3, 363–371. [Google Scholar]
- Wu, J.; Guan, X.; Dai, Z.; He, R.; Ding, X.; Yang, L.; Ge, G. Molecular probes for human cytochrome P450 enzymes: Recent progress and future perspectives. Coord. Chem. Rev. 2021, 427, 213600. [Google Scholar] [CrossRef]
- Feng, L.; Ning, J.; Tian, X.; Wang, C.; Yu, Z.; Huo, X.; Xie, T.; Zhang, B.; James, T.D.; Ma, X. Fluorescent probes for the detection and imaging of Cytochrome P450. Coord. Chem. Rev. 2021, 437, 213740. [Google Scholar] [CrossRef]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew. Chem. Int. Ed. Engl. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Shi, Y.; Zhang, S.; Yan, L.; Zhang, H.; Tian, Z.; Gu, Y.; Guo, T.; Huang, J. A NIR turn-on fluorescent probe applied in cytochrome P450 reductase detection and hypoxia imaging in tumor cells. Dye. Pigment. 2017, 139, 587–592. [Google Scholar] [CrossRef]
- Pina, F.; Petrov, V.; Laia, C.A.T. Photochromism of flavylium systems. An overview of a versatile multistate system. Dyes Pigm. 2012, 92, 877–889. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Yoon, J.; Yoon, S.; Lee, M. Ratiometric Fluorescence Assay for Nitroreductase Activity: Locked-Flavylium Fluorophore as a NTR-Sensitive Molecular Probe. Molecules 2021, 26, 1088. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yang, X.-F.; Zhong, Y.; Chen, H.; Li, Z. A flavylium-based turn-on fluorescent probe for imaging hydrogen polysulfides in living cells. RSC Adv. 2016, 6, 88519–88525. [Google Scholar] [CrossRef]
- Bandara, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef]
- Chevalier, A.; Renard, P.-Y.; Romieu, A. Azo-Based Fluorogenic Probes for Biosensing and Bioimaging: Recent Advances and Upcoming Challenges. Chem. Asian J. 2017, 12, 2008–2028. [Google Scholar] [CrossRef]
- Ren, T.-B.; Xu, W.; Jin, F.; Cheng, D.; Zhang, L.; Yuan, L.; Zhang, X. Rational Engineering of Bioinspired Anthocyanidin Fluorophores with Excellent Two-Photon Properties for Sensing and Imaging. Anal. Chem. 2017, 89, 11427–11434. [Google Scholar] [CrossRef]
- Thews, O.; Riemann, A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metastasis Rev. 2019, 38, 113–129. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Waterman, M.R. NADPH-Flavodoxin Reductase and Flavodoxin from Escherichia coli: Characteristics as a Soluble Microsomal P450 Reductase. Biochemistry 1998, 37, 6106–6113. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Waterman, M.R. Flavodoxin and NADPH-flavodoxin reductase from Escherichia coli support bovine cytochrome P450c17 hydroxylase activities. J. Biol. Chem. 1994, 269, 27401–27408. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Waterman, M.R. Flavodoxin As A Model for The P450-Interacting Domain of Nadph Cytochrome P450 Reductase. Drug Metab. Rev. 1999, 31, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Begleiter, A.; Leith, M.K.; Patel, D.; Hasinoff, B.B. Role of NADPH cytochrome P450 reductase in activation of RH1. Cancer Chemother. Pharmacol. 2007, 60, 713–723. [Google Scholar] [CrossRef]
- Kleniewska, P.; Piechota-Polanczyk, A.; Skibska, B.; Gorąca, A. The NADPH Oxidase Family and its Inhibitors. Arch. Immunol. Ther. Exp. 2012, 60, 277–294. [Google Scholar] [CrossRef]
- Panday, A.; Sahoo, M.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Crnolatac, I.; Giestas, L.; Horvat, G.; Parola, A.J.; Piantanida, I. Flavylium Dye as pH-Tunable Fluorescent and CD Probe for Double-Stranded DNA and RNA. Chemosensors 2020, 8, 129. [Google Scholar] [CrossRef]
- Sarma, A.D.; Sharma, R. Anthocyanin-DNA copigmentation complex: Mutual protection against oxidative damage. Phytochemistry 1999, 52, 1313–1318. [Google Scholar] [CrossRef]
- Koch, C.J. [1] Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol. 2002, 352, 3–31. [Google Scholar] [CrossRef]
- Iglesias, P.; Penas, C.; Barral-Cagiao, L.; Pazos, E.; Costoya, J.A. A Bio-inspired Hypoxia Sensor using HIF1a-Oxygen-Dependent Degradation Domain. Sci. Rep. 2019, 9, 7117. [Google Scholar] [CrossRef] [PubMed]
- Uddin, I.; Evans, S.M.; Craft, J.R.; Marnett, L.J.; Uddin, J.; Jayagopal, A. Applications of Azo-Based Probes for Imaging Retinal Hypoxia. ACS Med. Chem. Lett. 2015, 6, 445–449. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Jiang, W.-L.; Zhou, D.-Y.; Fei, J.; Li, C.-Y. In-Situ Imaging of Azoreductase Activity in the Acute and Chronic Ulcerative Colitis Mice by a Near-Infrared Fluorescent Probe. Anal. Chem. 2019, 91, 10901–10907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Maiti, M.; Sharma, A.; Won, M.; Yu, L.; Miao, L.X.; Shin, J.; Podder, A.; Bobba, K.N.; Han, J.; et al. Azo-based small molecular hypoxia responsive theranostic for tumor-specific imaging and therapy. J. Control. Release 2018, 288, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.; Piao, W.; Hanaoka, K.; Nagano, T.; Renard, P.-Y.; Romieu, A. Azobenzene-caged sulforhodamine dyes: A novel class of ‘turn-on’ reactive probes for hypoxic tumor cell imaging. Methods Appl. Fluoresc. 2015, 3, 44004. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Y.; Zeng, F.; Wu, S. An Activatable Near-Infrared Chromophore for Multispectral Optoacoustic Imaging of Tumor Hypoxia and for Tumor Inhibition. Theranostics 2019, 9, 7313–7324. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pewklang, T.; Wet-osot, S.; Wangngae, S.; Ngivprom, U.; Chansaenpak, K.; Duangkamol, C.; Lai, R.-Y.; Noisa, P.; Sukwattanasinitt, M.; Kamkaew, A. Flavylium-Based Hypoxia-Responsive Probe for Cancer Cell Imaging. Molecules 2021, 26, 4938. https://doi.org/10.3390/molecules26164938
Pewklang T, Wet-osot S, Wangngae S, Ngivprom U, Chansaenpak K, Duangkamol C, Lai R-Y, Noisa P, Sukwattanasinitt M, Kamkaew A. Flavylium-Based Hypoxia-Responsive Probe for Cancer Cell Imaging. Molecules. 2021; 26(16):4938. https://doi.org/10.3390/molecules26164938
Chicago/Turabian StylePewklang, Thitima, Sirawit Wet-osot, Sirilak Wangngae, Utumporn Ngivprom, Kantapat Chansaenpak, Chuthamat Duangkamol, Rung-Yi Lai, Parinya Noisa, Mongkol Sukwattanasinitt, and Anyanee Kamkaew. 2021. "Flavylium-Based Hypoxia-Responsive Probe for Cancer Cell Imaging" Molecules 26, no. 16: 4938. https://doi.org/10.3390/molecules26164938
APA StylePewklang, T., Wet-osot, S., Wangngae, S., Ngivprom, U., Chansaenpak, K., Duangkamol, C., Lai, R.-Y., Noisa, P., Sukwattanasinitt, M., & Kamkaew, A. (2021). Flavylium-Based Hypoxia-Responsive Probe for Cancer Cell Imaging. Molecules, 26(16), 4938. https://doi.org/10.3390/molecules26164938