Capillary Electrophoresis as a Monitoring Tool for Flow Composition Determination
Abstract
1. Introduction
2. Coupling FIA to CE Online
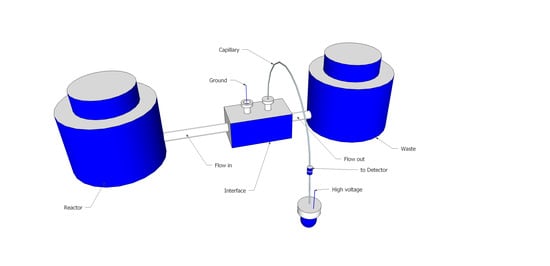
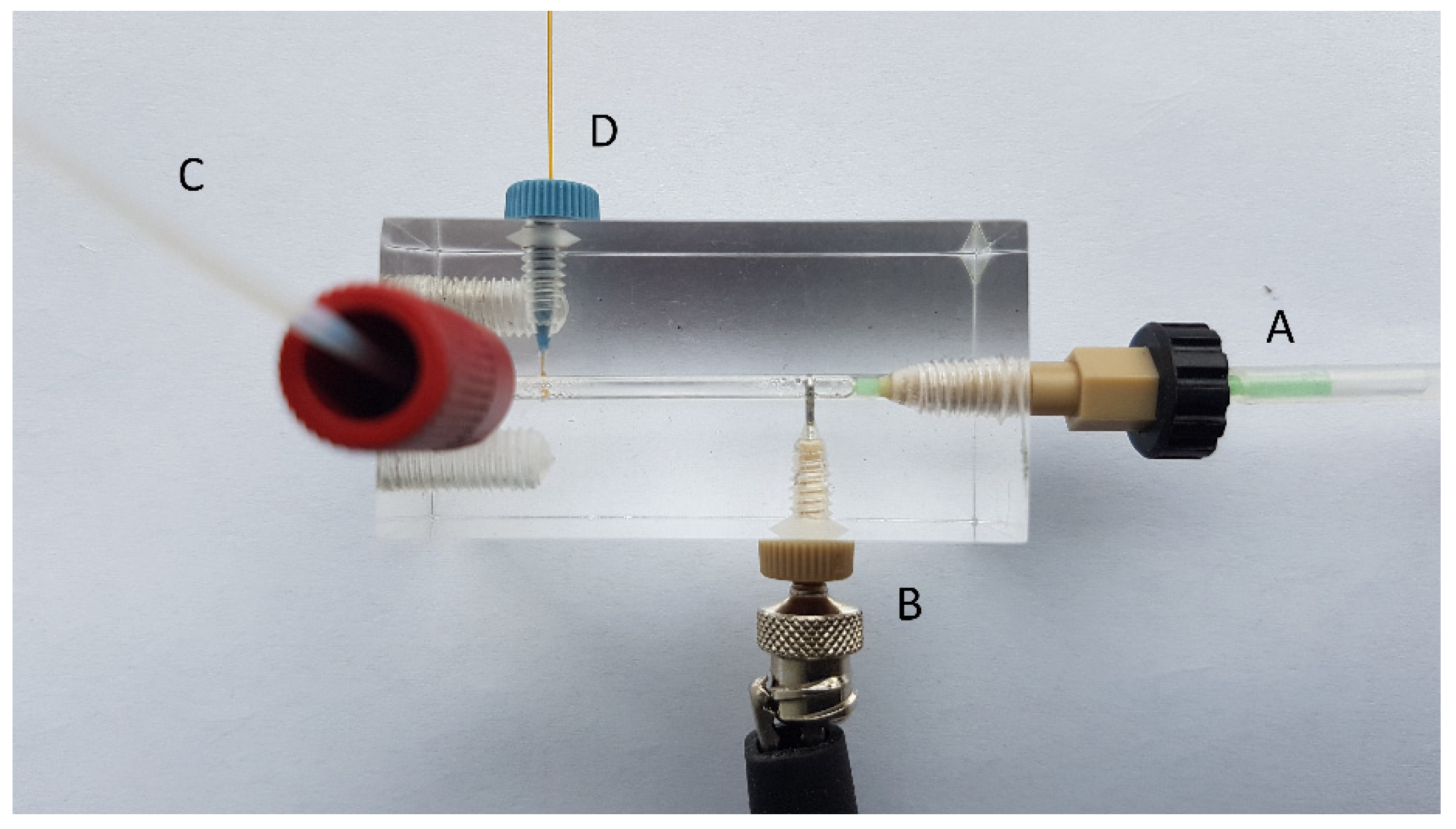
2.1. Direct Insertion of a Capillary into the Flow Channel
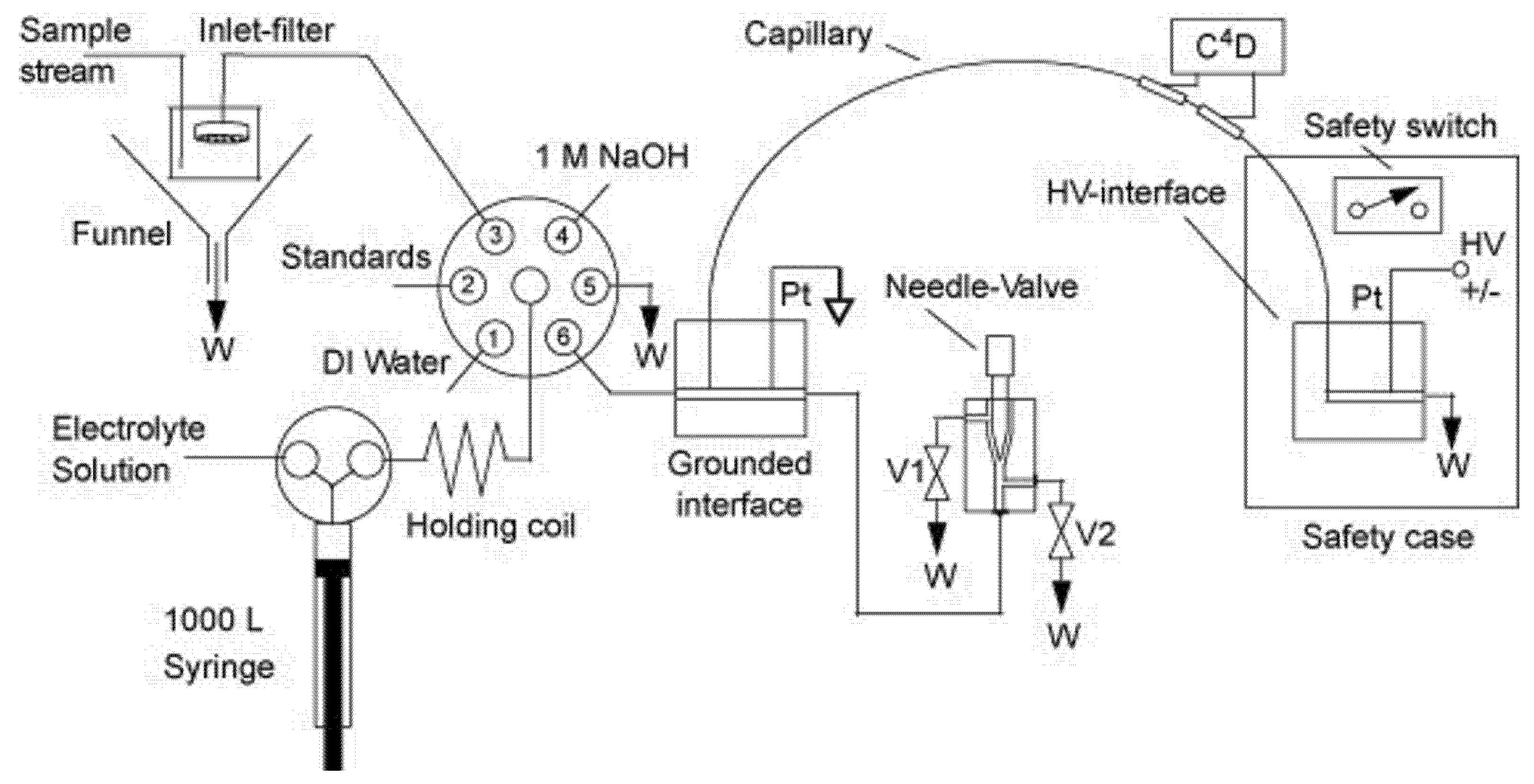
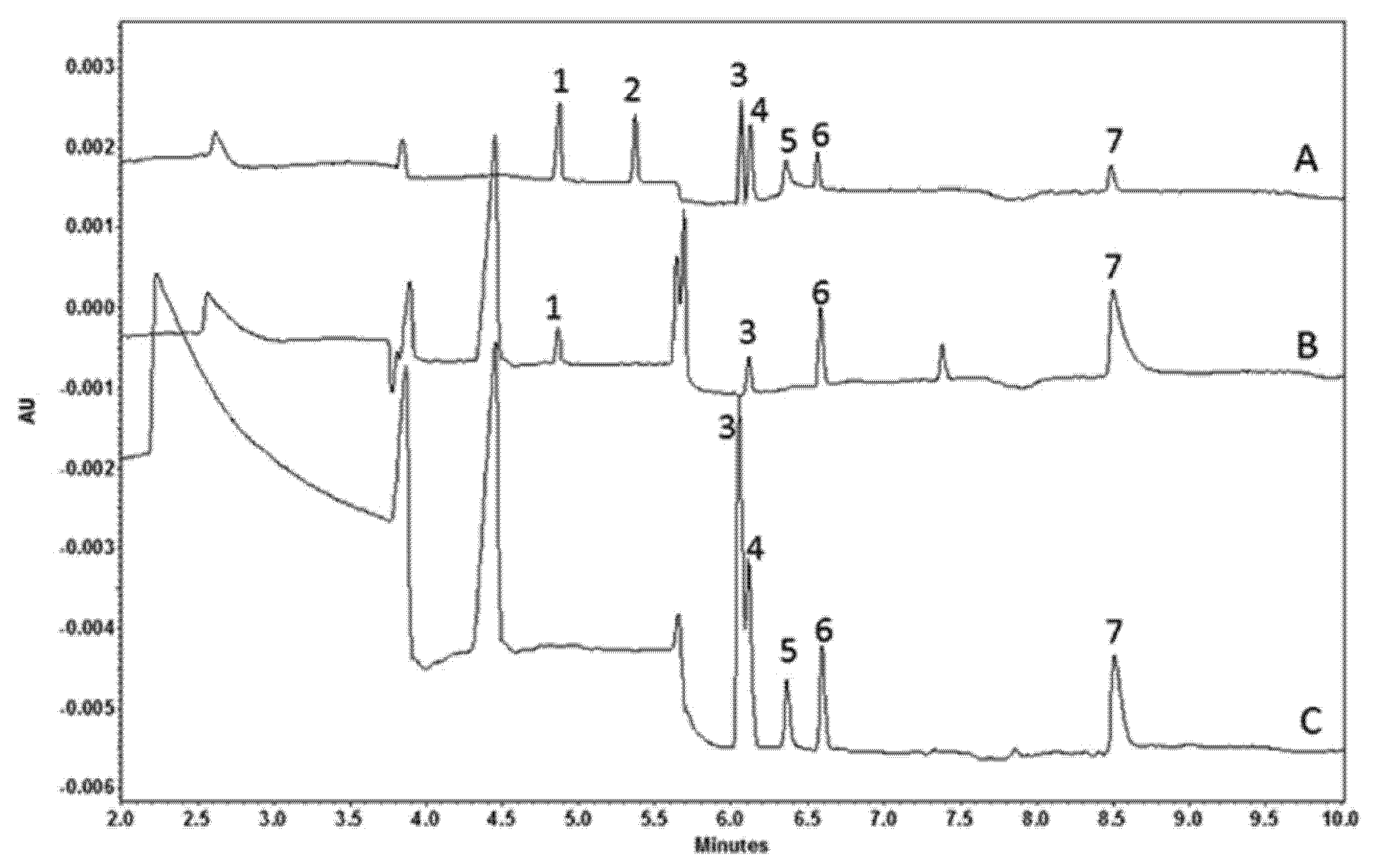
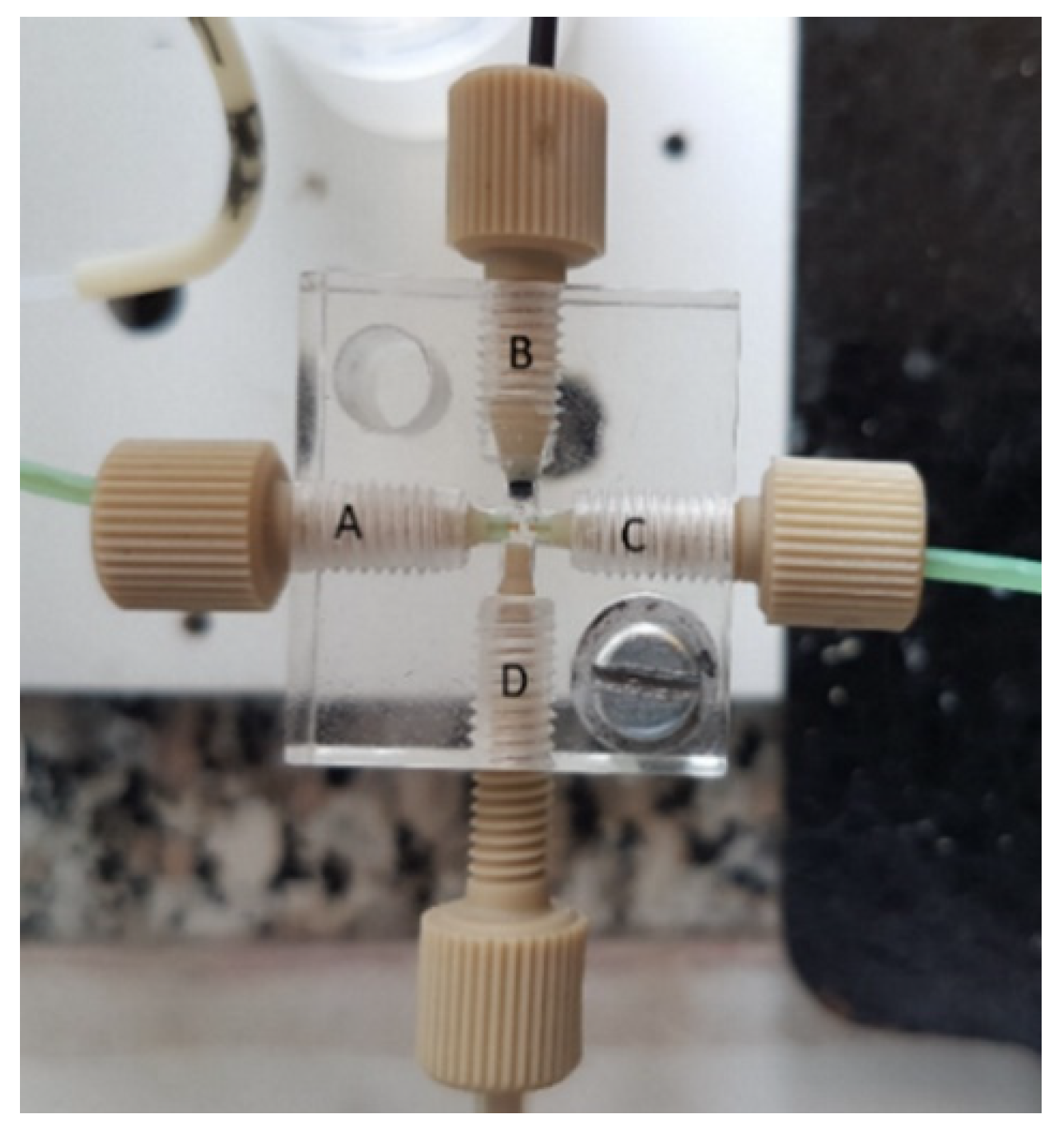
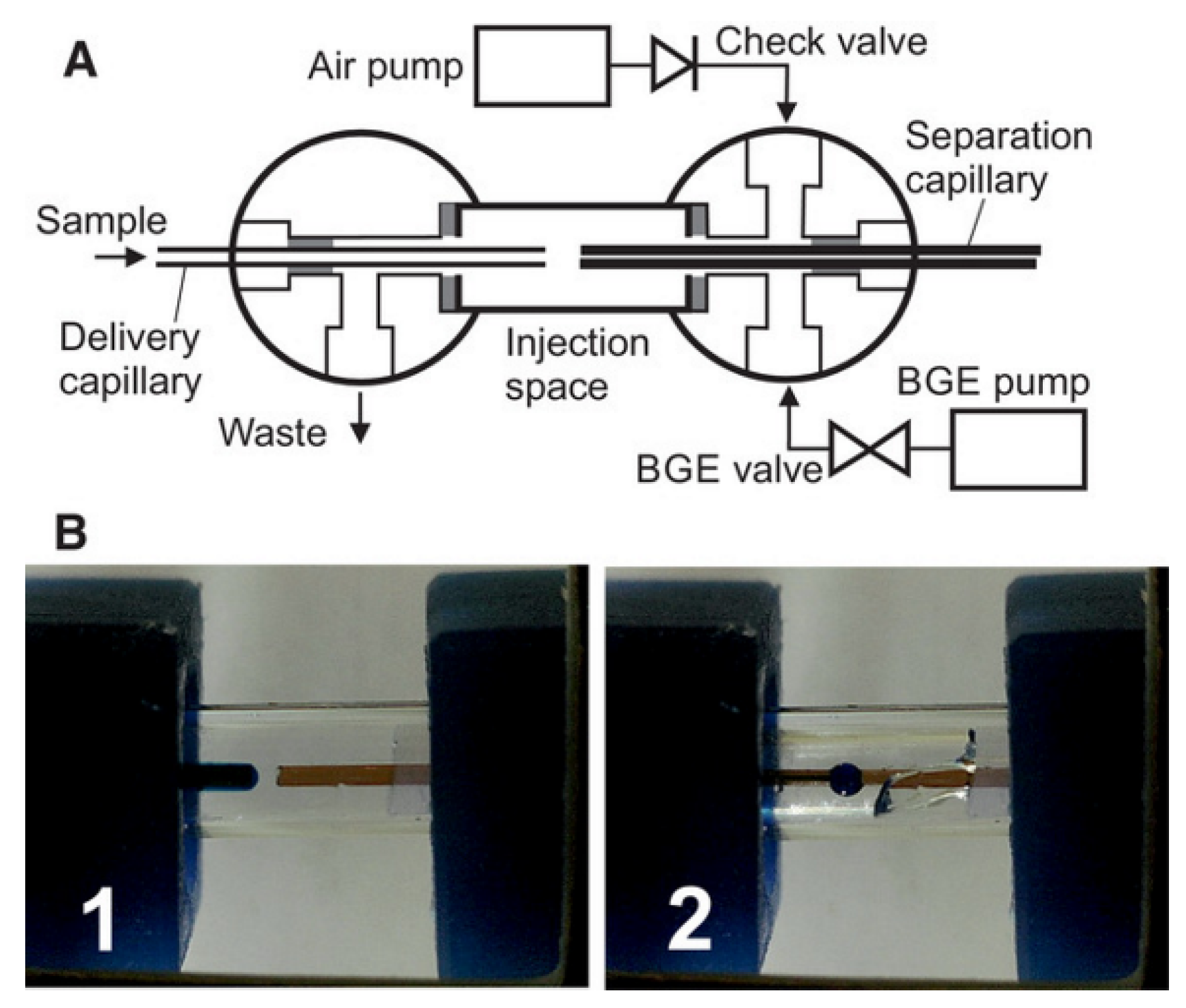
2.2. Flow Gating Interfaces
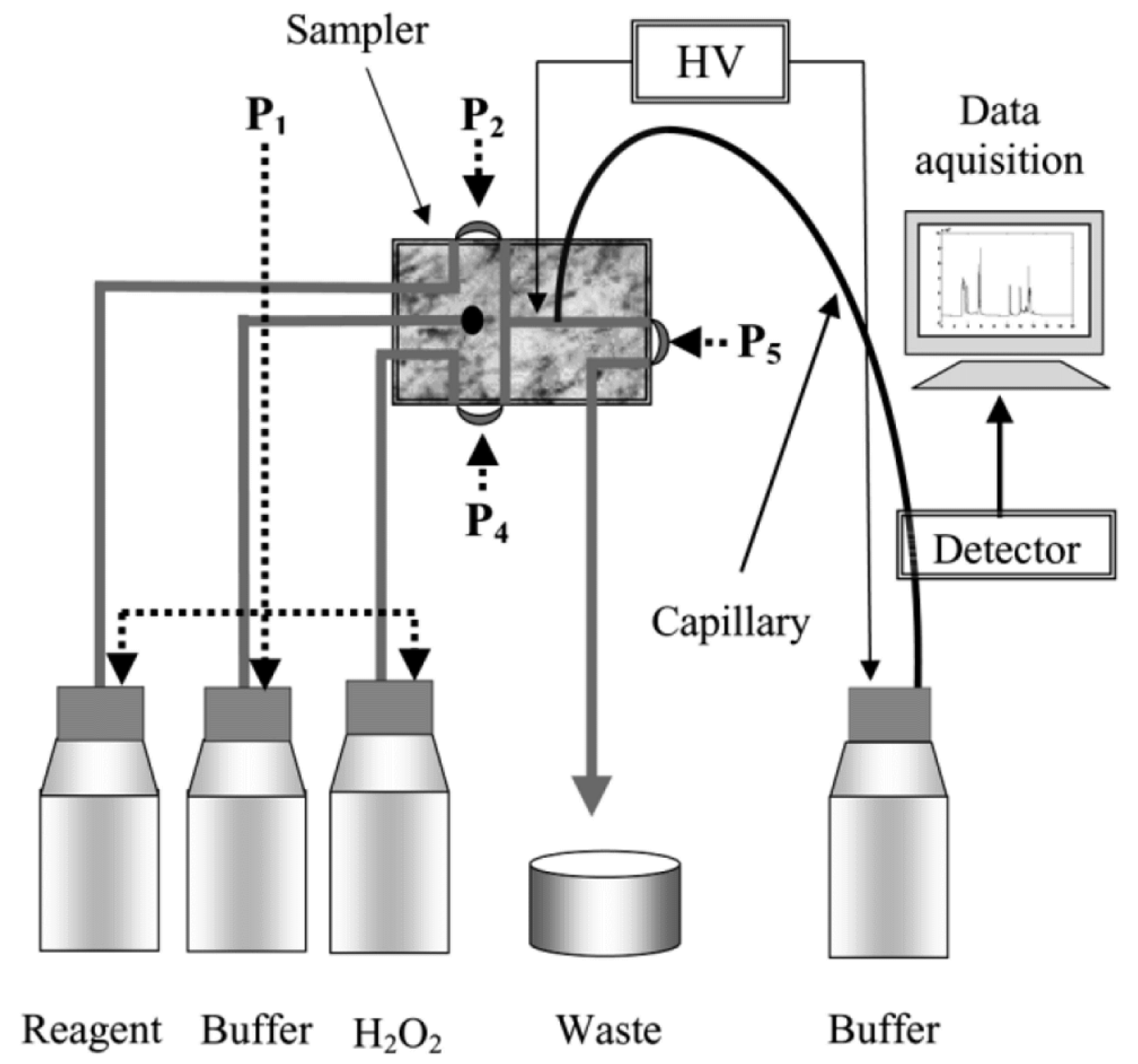
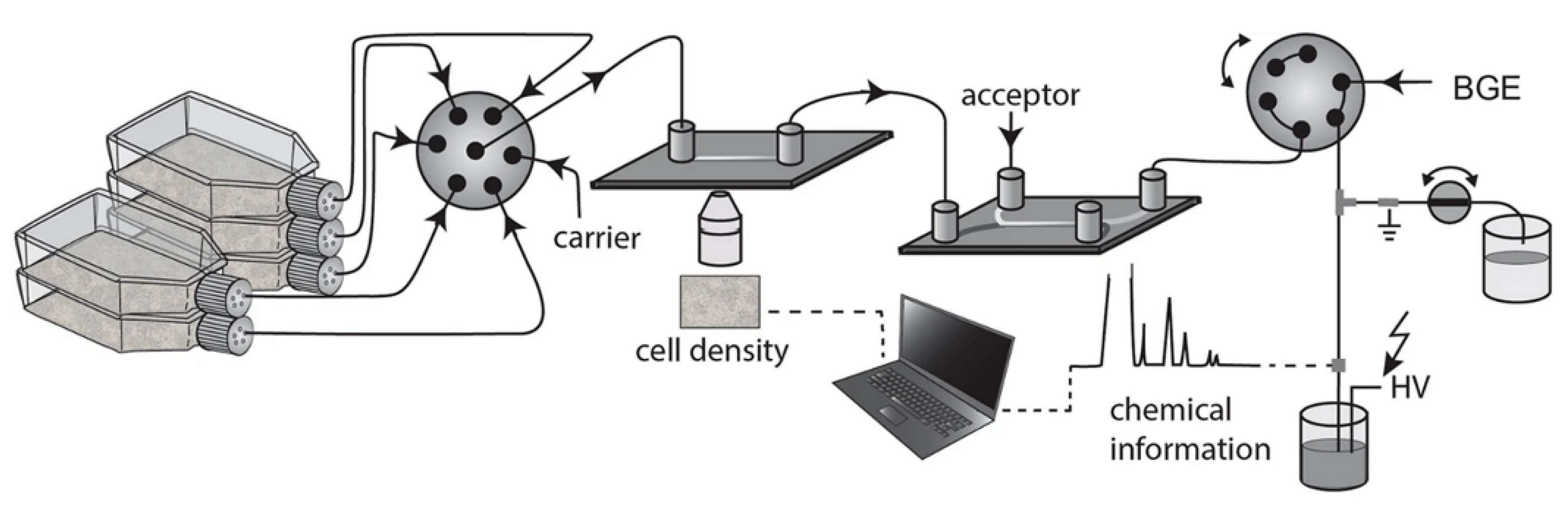
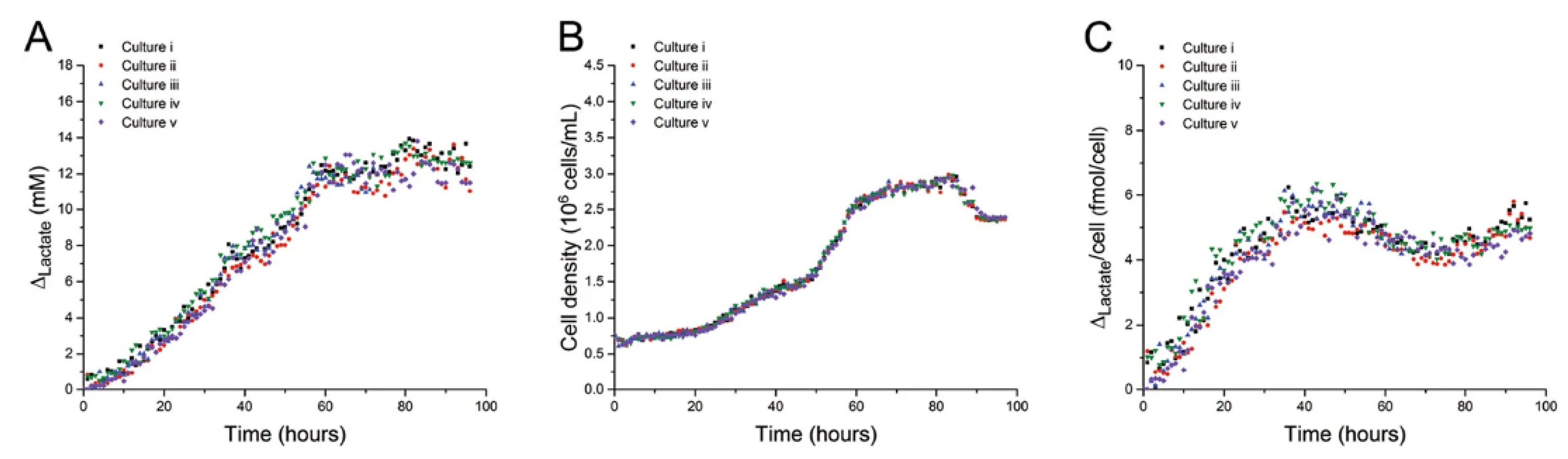
3. Open Access CE Instrumentation for Monitoring
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zagatto, E.A.G.; Vida, A.C.F.; Worsfold, P.J. Flow Analysis | Overview. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 213–219. [Google Scholar] [CrossRef]
- Horstkotte, B.; Cerdà, V. Coupling of Flow Techniques with Capillary Electrophoresis: Review of Operation Principles, Challenges, Potentials, and Applications. J. Chromatogr. Sci. 2009, 47, 636–647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saylor, R.A.; Lunte, S.M. A review of microdialysis coupled to microchip electrophoresis for monitoring biological events. J. Chromatogr. A 2015, 1382, 48–64. [Google Scholar] [CrossRef]
- Kaljurand, M.; Ebber, A.; Sõmer, T. An automatic sampling device for capillary zone electrophoresis. J. Separ. Sci. 1995, 18, 263–267. [Google Scholar] [CrossRef]
- Kuban, P.; Engström, A.; Olsson, J.C.; Thorsén, G.; Tryzell, R.; Karlberg, B. New interface for coupling flow-injection and capillary electrophoresis. Anal. Chim. Acta 1997, 337, 117–120. [Google Scholar] [CrossRef]
- Kuban, P.; Karlberg, B. On-line coupling of gas diffusion to capillary electrophoresis. Talanta 1998, 45, 477–484. [Google Scholar] [CrossRef]
- Vaher, M.; Ehala, S.; Kaljurand, M. On-column capillary electrophoretic monitoring of rapid reaction kinetics for determination of the antioxidative potential of various bioactive phenols. Electrophoresis 2005, 26, 990–1000. [Google Scholar] [CrossRef]
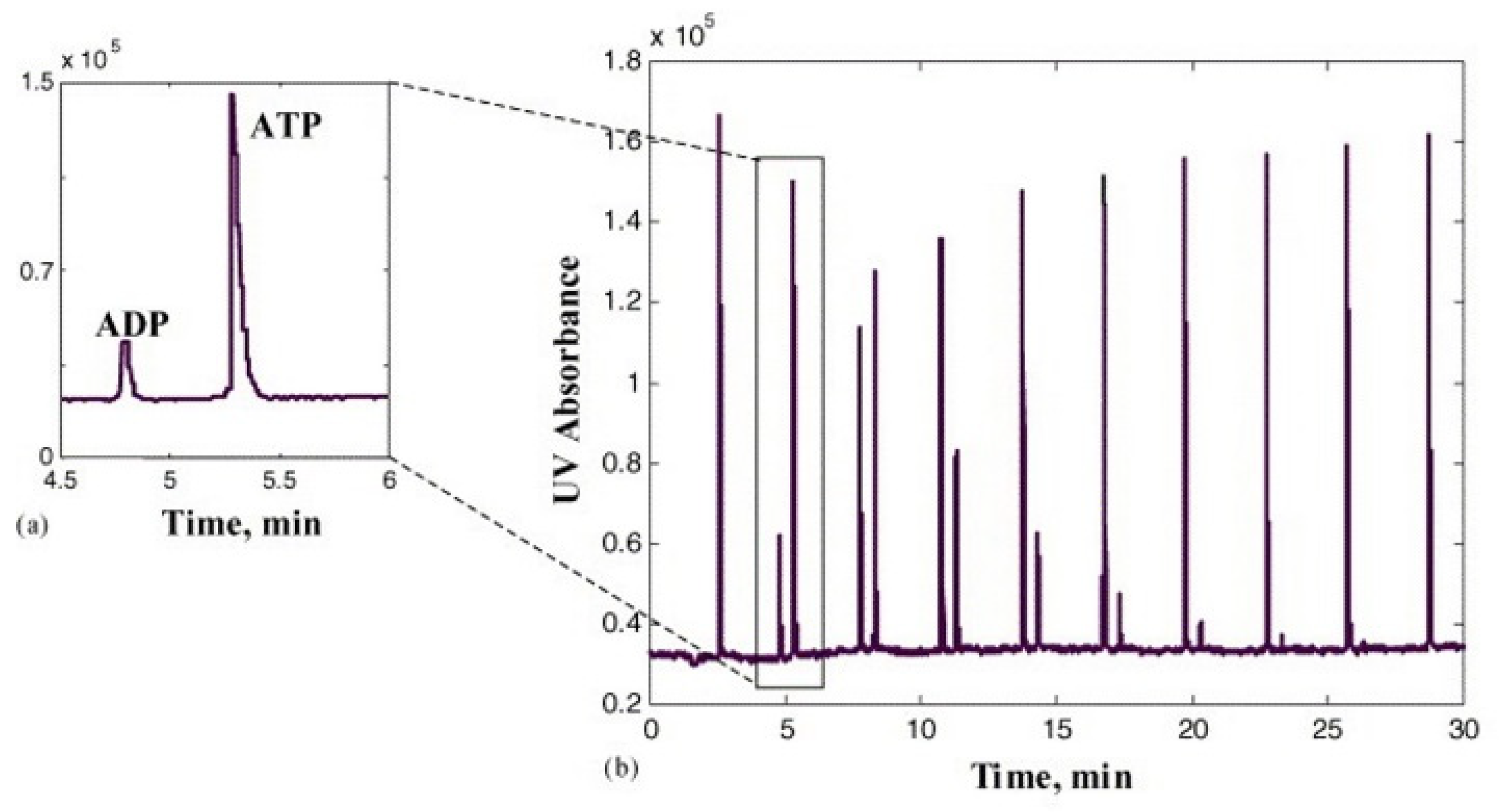
- Kulp, M.; Kaljurand, M. On-line monitoring of enzymatic conversion of adenosine triphosphate to adenosine diphosphate by micellar electrokinetic chromatography. J. Chromatogr. A 2004, 1032, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kulp, M.; Kaljurand, M.; Käämbre, T.; Sikk, P.; Saks, V. In situ monitoring of kinetics of metabolic conversion of ATP to ADP catalyzed by MgATPases of muscle Gastrocnemius skinned fibers using micellar electrokinetic chromatography. Electrophoresis 2004, 25, 2996–3002. [Google Scholar] [CrossRef]
- Kulp, M.; Vassiljeva, I.; Vilu, R.; Kaljurand, M. Monitoring of the degradation of phenols by Rhodococcus bacteria by using micellar electrokinetic chromatography. J. Sep. Sci. 2002, 25, 1129–1135. [Google Scholar] [CrossRef]
- Tahkoniemi, H.; Helmja, K.; Menert, A.; Kaljurand, M. Fermentation reactor coupled with capillary electrophoresis for on-line bioprocess monitoring. J. Pharm. Biomed. Anal. 2006, 41, 1585–1591. [Google Scholar] [CrossRef]
- Alhusban, A.A.; Breadmore, M.C.; Guijt, R.M. Capillary electrophoresis for monitoring bioprocesses. Electrophoresis 2013, 34, 1465–1482. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. Capillary electrophoresis for automated on-line monitoring of suspension cultures: Correlating cell density, nutrients and metabolites in near real-time. Anal. Chim. Acta 2016, 920, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. Time-Resolved Pharmacological Studies using Automated, On-line Monitoring of Five Parallel Suspension Cultures. Sci. Rep. 2017, 7, 10337. [Google Scholar] [CrossRef]
- Duc Mai, T.; Schmid, S.; Müller, B.; Hauser, P.C. Capillary electrophoresis with contactless conductivity detection coupled to a sequential injection analysis manifold for extended automated monitoring applications. Anal. Chim. Acta 2010, 665, 1–6. [Google Scholar]
- Turkia, H.; Sirén, H.; Pitkänen, J.P.; Wiebe, M.; Penttilä, M. Capillary electrophoresis for the monitoring of carboxylic acid production by Gluconobacter oxydans. J. Chromatogr. A 2011, 217, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Turkia, H.; Sirén, H.; Penttilä, M.; Pitkänen, J.P. Capillary electrophoresis with laser-induced fluorescence detection for studying amino acid uptake by yeast during beer fermentation. Talanta 2015, 131, 366–371. [Google Scholar] [CrossRef]
- Turkia, H.; Sirén, H.; Penttilä, M.; Pitkänen, J.P. Capillary electrophoresis for the monitoring of phenolic compounds in bioprocesses. J. Chromatogr. A 2013, 1278, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Turkia, H.; Holmström, S.; Paasikallio, T.; Sirén, H.; Penttilä, M.; Pitkänen, J.P. Online capillary electrophoresis for monitoring carboxylic acid production by yeast during bioreactor cultivations. Anal. Chem. 2013, 85, 9705–9712. [Google Scholar] [CrossRef]
- Hooker, T.F.; Jorgenson, J.W. A transparent flow gating interface for the coupling of microcolumn LC with CZE in a comprehensive two-dimensional system. Anal. Chem. 1997, 69, 4134–4142. [Google Scholar] [CrossRef]
- Weng, Q.; Fu, L.; Li, X.; Ran, A.; Li, J. A sheath flow gating interface for the on-line coupling of solid-phase extraction with capillary electrophoresis. Anal. Chim. Acta 2015, 857, 46–52. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M. Prototyping of poly(dimethylsiloxane) interfaces for flow gating, reagent mixing, and tubing connection in capillary electrophoresis. J. Chromatogr. A 2014, 1324, 231–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Opekar, F.; Tůma, P. An air-assisted flow-gating interface for capillary electrophoresis. Electrophoresis 2019, 40, 587–590. [Google Scholar] [CrossRef]
- Open Source Hardware Association. Definition. Available online: https://www.oshwa.org/definition/ (accessed on 10 August 2021).
- Prabhu, G.R.D.; Urban, P.L. Elevating chemistry research with a modern electronics toolkit. Chem. Rev. 2020, 120, 9482–9553. [Google Scholar] [CrossRef]
- Davis, J.J.; Foster, S.W.; Grinias, J.P. Low-cost and open-source strategies for chemical separations. J. Chromatogr. A 2021, 1638, 461820. [Google Scholar] [CrossRef]
- Kubáň, P.; Foret, F.; Erny, G. Open source capillary electrophoresis. Electrophoresis 2019, 40, 65–78. [Google Scholar] [CrossRef]
- Liénard, T.; Furter, J.S.; Taverna, M.; Pham, H.V.; Hauser, P.C.; Mai, T.D. Modular instrumentation for capillary electrophoresis with laser induced fluorescence detection using plug-and-play microfluidic, electrophoretic and optic modules. Anal. Chim. Acta 2020, 1135, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Furter, J.S.; Boillat, M.-A.; Hauser, P.C. Low-cost automated capillary electrophoresis instrument assembled from commercially available parts. Electrophoresis 2020, 41, 2075–2082. [Google Scholar] [CrossRef]
- Fully Automatic Minature Peristaltic Pump RP-Q1 Series by Takasago, Japan. Available online: https://www.indiamart.com/proddetail/minature-peristaltic-pump-rp-q1-series-by-takasago-japan-9955768273.html (accessed on 15 July 2018).
- OpenC4D. Available online: https://github.com/claudimir-lago/openC4D (accessed on 10 August 2021).
- Prikryl, J.; Foret, F. Fluorescence detector for capillary separations fabricated by 3D printing. Anal. Chem. 2014, 86, 11951–11956. [Google Scholar] [CrossRef]
- Kaljurand, M.; Gorbatšova, J.; Mazina-Šinkar, J. A gas chromatograph for citizen science. Microchem. J. 2021, 165, 106195. [Google Scholar] [CrossRef]



| Application/Compound | Temporal Resolution |
|---|---|
| Neurotransmitters | Milliseconds to seconds |
| Drug transport and metabolism | Minutes to hours |
| Energy biomarkers (e.g., glucose and lactate) | Minutes to hours |
| Peptides | Minutes |
| Bioreactor monitoring | Minutes to hours |
| Reactive oxygen and nitrogen species | Minutes |
| Antioxidants (e.g., glutathione and ascorbic acid) | Minutes to hours |
| Environmental monitoring | Hours to days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaljurand, M.; Saar-Reismaa, P.; Vaher, M.; Gorbatsova, J.; Mazina-Šinkar, J. Capillary Electrophoresis as a Monitoring Tool for Flow Composition Determination. Molecules 2021, 26, 4918. https://doi.org/10.3390/molecules26164918
Kaljurand M, Saar-Reismaa P, Vaher M, Gorbatsova J, Mazina-Šinkar J. Capillary Electrophoresis as a Monitoring Tool for Flow Composition Determination. Molecules. 2021; 26(16):4918. https://doi.org/10.3390/molecules26164918
Chicago/Turabian StyleKaljurand, Mihkel, Piret Saar-Reismaa, Merike Vaher, Jelena Gorbatsova, and Jekaterina Mazina-Šinkar. 2021. "Capillary Electrophoresis as a Monitoring Tool for Flow Composition Determination" Molecules 26, no. 16: 4918. https://doi.org/10.3390/molecules26164918
APA StyleKaljurand, M., Saar-Reismaa, P., Vaher, M., Gorbatsova, J., & Mazina-Šinkar, J. (2021). Capillary Electrophoresis as a Monitoring Tool for Flow Composition Determination. Molecules, 26(16), 4918. https://doi.org/10.3390/molecules26164918