Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives
Abstract
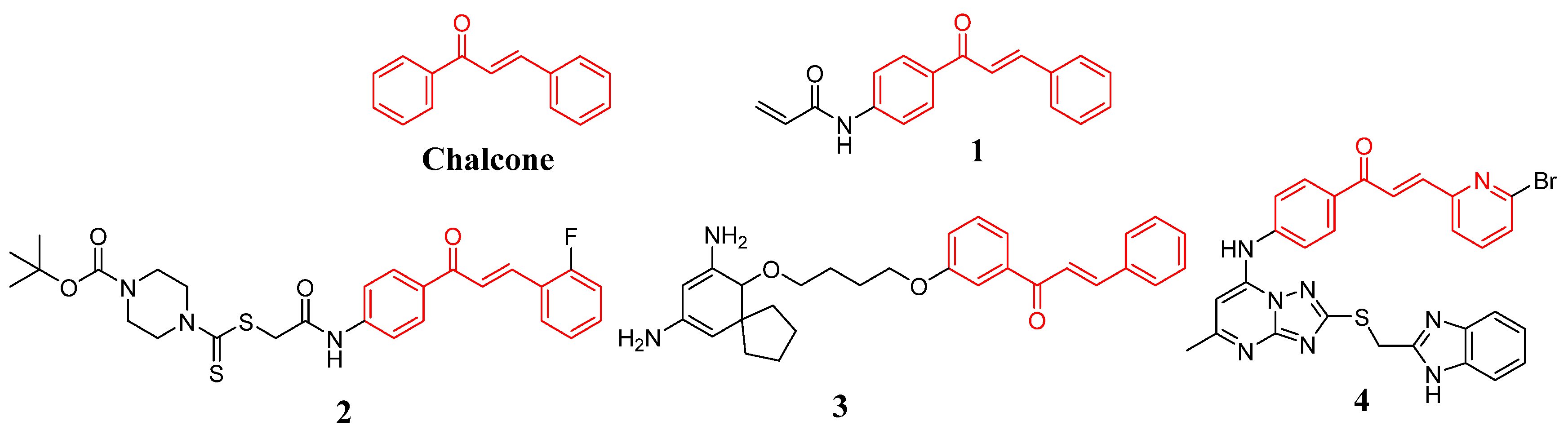
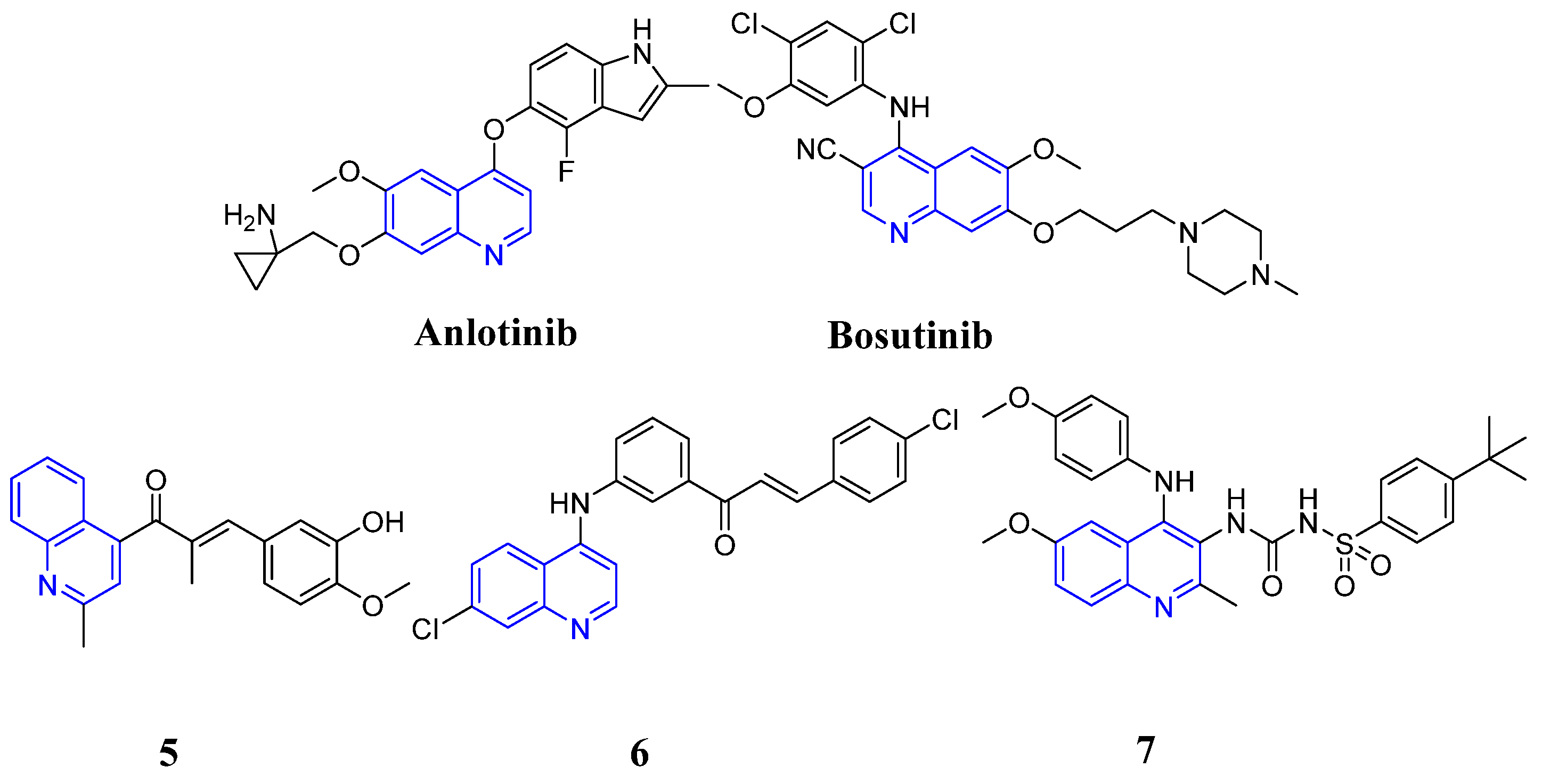
:1. Introduction
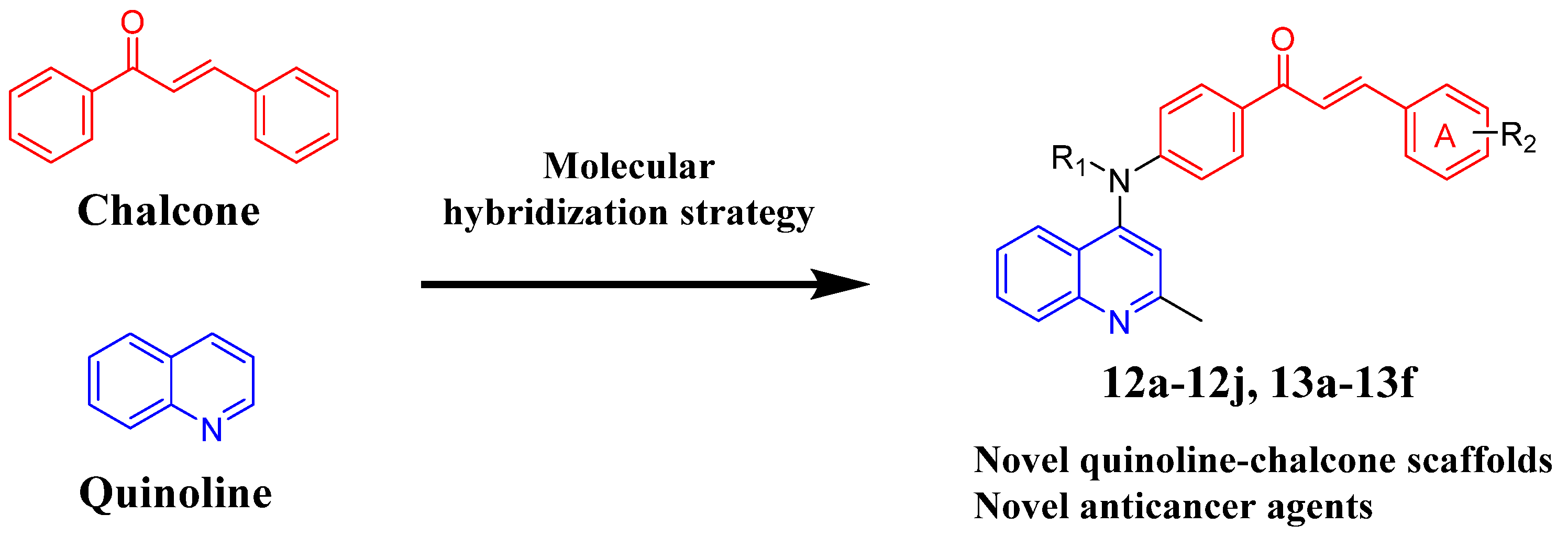
2. Results and Discussion
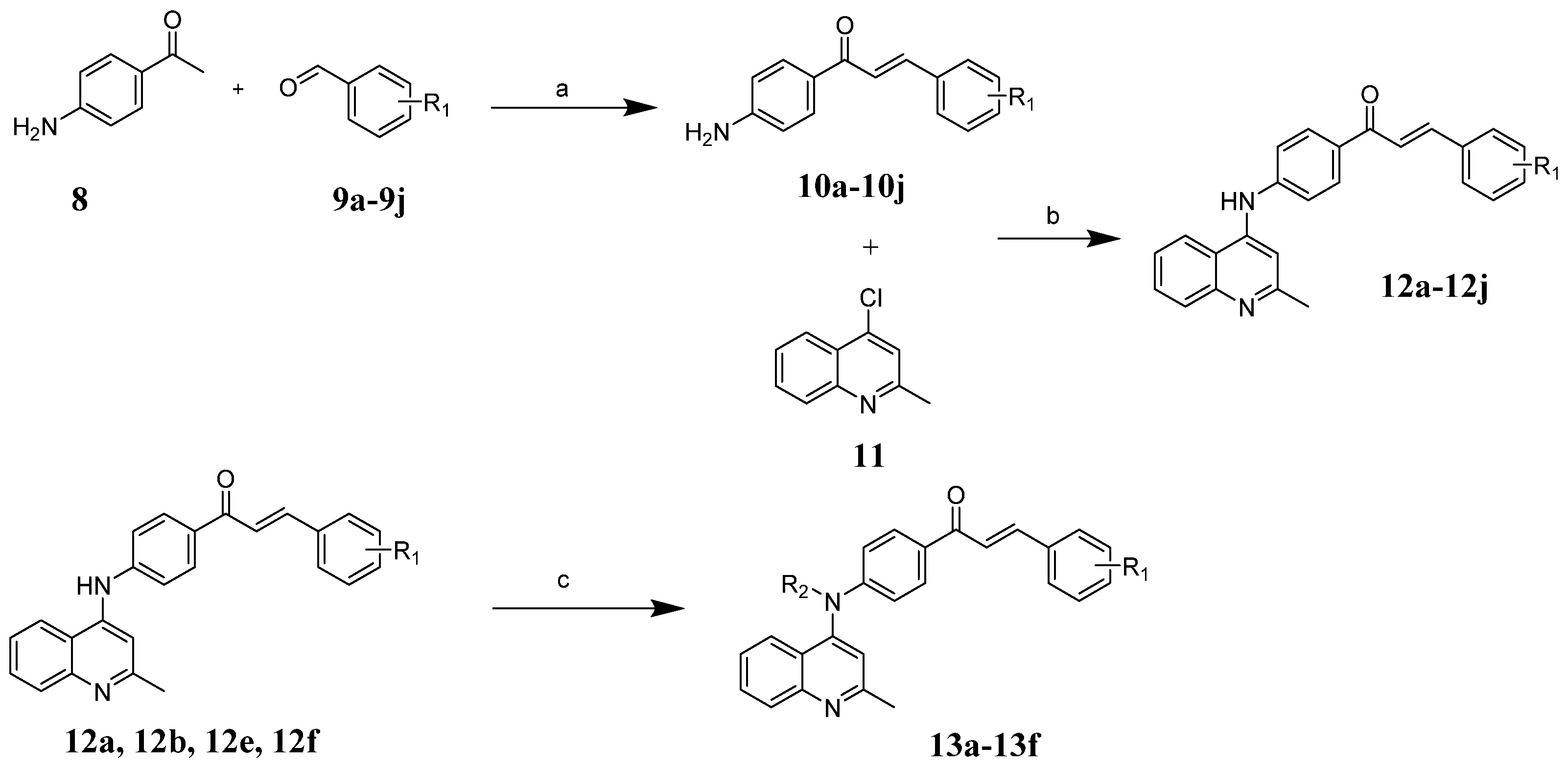
2.1. Chemistry
2.2. Antiproliferative Activity and Structure Activity Relationship Analysis
2.3. Compound 12e Inhibited Gastric Cancer Cells
2.4. Compound 12e Inhibited Gastric Cancer Cells through the Generation of Reactive Oxygen Species (ROS)
3. Materials and Methods
3.1. Synthesis of Compounds 10a–10j
3.2. Synthesis of Compounds 12a–12j and 13a–13f
3.3. Cell Culture
3.4. MTT Assay
3.5. Western Blotting Analysis
3.6. General Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, G.; Xiao, J. Chalcone hybrids as potential anticancer agents: Current development, mechanism of action, and structure-activity relationship. Med. Res. Rev. 2020, 40, 2049–2084. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Anand, A.; Kumar, V. Recent developments in biological activities of chalcones: A mini review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Mahapatra, D.K.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Johnson, T.E.; Lad, R.; Xing, C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem. 2009, 52, 7228–7235. [Google Scholar] [CrossRef]
- Irfan, R.; Mousavi, S.; Alazmi, M.; Saleem, R.S.Z. A Comprehensive Review of Aminochalcones. Molecules 2020, 25, 5381. [Google Scholar] [CrossRef]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnere, C.; Sol, V.; et al. Synthesis and biological evaluation of chalcone-polyamine conjugates as novel vectorized agents in colorectal and prostate cancer chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef]
- Fu, D.J.; Li, J.H.; Yang, J.J.; Li, P.; Zhang, Y.B.; Liu, S.; Li, Z.R.; Zhang, S.Y. Discovery of novel chalcone-dithiocarbamates as ROS-mediated apoptosis inducers by inhibiting catalase. Bioorg. Chem. 2019, 86, 375–385. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Fu, D.J.; Yue, X.X.; Liu, Y.C.; Song, J.; Sun, H.H.; Liu, H.M.; Zhang, Y.B. Design, Synthesis and Structure-Activity Relationships of Novel Chalcone-1,2,3-triazole-azole Derivates as Antiproliferative Agents. Molecules 2016, 21, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Yue, X.-X.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Zhang, Y.-B.; et al. Design, synthesis and antiproliferative activity studies of 1,2,3-triazole–chalcones. MedChemComm 2016, 7, 1664–1671. [Google Scholar] [CrossRef]
- Lu, C.F.; Wang, S.H.; Pang, X.J.; Zhu, T.; Li, H.L.; Li, Q.R.; Li, Q.Y.; Gu, Y.F.; Mu, Z.Y.; Jin, M.J.; et al. Synthesis and Biological Evaluation of Amino Chalcone Derivatives as Antiproliferative Agents. Molecules 2020, 25, 5530. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Xie, J.; Chen, L.; Wei, X.; Jiang, X.; Bao, M.; Qiu, Y.; Chen, Q.; Li, W.; et al. Design, synthesis, and evaluation of chalcone analogues incorporate alpha,beta-Unsaturated ketone functionality as anti-lung cancer agents via evoking ROS to induce pyroptosis. Eur. J. Med. Chem. 2018, 157, 1395–1405. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Zhou, Y.; Li, X.Y.; Zhang, H.; Zhang, G.J. Discovery of orally active chalcones as histone lysine specific demethylase 1 inhibitors for the treatment of leukaemia. J. Enzym. Inhib. Med. Chem. 2021, 36, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.L.; Ma, X.; Chew, E.H.; Chui, W.K. Design, Synthesis, and Biological Evaluation of Coupled Bioactive Scaffolds as Potential Anticancer Agents for Dual Targeting of Dihydrofolate Reductase and Thioredoxin Reductase. J. Med. Chem. 2017, 60, 1734–1745. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109, 104639. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Wang, S.H.; Guan, Y.F.; Liu, X.J.; Yuan, X.Y.; Yu, G.X.; Li, Y.R.; Zhang, Y.B.; Song, J.; Li, W.; Zhang, S.Y. Design, Synthesis and Anticancer Activity Studies of Novel Quinoline-Indole Derivatives. Chin. J. Org. Chem. [CrossRef]
- Solomon, V.R.; Lee, H. Quinoline as a privileged scaffold in cancer drug discovery. Curr. Med. Chem. 2011, 18, 1488–1508. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chandra, V.; Jain, P.K.; Pathak, K.; Pathak, D.; Vaidya, A. Comprehensive review on current developments of quinoline-based anticancer agents. Arab. J. Chem. 2019, 12, 4920–4946. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.W.; Cui, X.X.; Zhu, T.; Wang, S.H.; Lu, C.F.; Wang, J.J.; Dang, H.X.; Zhang, S.Y.; Ding, L.N.; Jin, C.Y. Design, Synthesis and Anticancer Activity Studies of Novel Trimethoxyphenyl-quinoline Derivatives. Chin. J. Org. Chem. 2020, 40, 978–987. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Lu, X.Q.; Ma, X.D.; Zhang, H.J.; Ji, Y.Y.; Ding, W.J.; Shen, L. Design, Synthesis and Biological Evaluation of Novel (Quinolinyl-3-pyridinyl)benzenesulfonamide-Based Hydroxamic Acids as PI3K and HDAC Dual Targeting Inhibitors. Chin. J. Org. Chem. 2020, 40, 95–107. [Google Scholar] [CrossRef]
- Zhong, C.C.; Chen, F.; Yang, J.L.; Jia, W.W.; Li, L.; Cheng, C.; Du, F.F.; Zhang, S.P.; Xie, C.Y.; Zhang, N.T.; et al. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol. Sin. 2018, 39, 1048–1063. [Google Scholar] [CrossRef] [Green Version]
- Golas, J.M.; Arndt, K.; Etienne, C.; Lucas, J.; Nardin, D.; Gibbons, J.; Frost, P.; Ye, F.; Boschelli, D.H.; Boschelli, F. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003, 63, 375–381. [Google Scholar] [PubMed]
- Li, W.; Xu, F.; Shuai, W.; Sun, H.; Yao, H.; Ma, C.; Xu, S.; Yao, H.; Zhu, Z.; Yang, D.H.; et al. Discovery of Novel Quinoline-Chalcone Derivatives as Potent Antitumor Agents with Microtubule Polymerization Inhibitory Activity. J. Med. Chem. 2019, 62, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- Tussaint, M.; Martinez, G.; Sanctis, J.D.; Monasterios, M.; Rojas, H. Antimalarial, antiproliferative, and apoptotic activity of quinoline-chalcone and quinoline-pyrazoline hybrids. A dual action. Med. Chem. Res. 2019, 28, 2050–2066. [Google Scholar]
- Zhao, B.B.; Lei, F.; Wang, C.L.; Zhang, B.L.; Yang, Z.H.; Li, W.; Zhu, W.F.; Xu, S. Design, Synthesis and Biological Evaluation of Novel Phenylsulfonylurea Derivatives as PI3K/mTOR Dual Inhibitors. Molecules 2018, 23, 1553. [Google Scholar] [CrossRef] [Green Version]
- Viegas-Junior, C.; Danuello, A.; Bolzani, V.D.; Barreir, E.J.; Fraga, C.A.M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jian, S.; Gao, Q.L.; Wu, B.W.; Li, D.; Shi, L.; Zhu, T.; Lou, J.F.; Jin, C.Y.; Zhang, Y.B.; Zhang, S.Y.; et al. Novel tertiary sulfonamide derivatives containing benzimidazole moiety as potent anti-gastric cancer agents: Design, synthesis and SAR studies. Eur. J. Med. Chem. 2019, 183, 111731. [Google Scholar] [CrossRef]
- Song, J.; Gao, Q.L.; Wu, B.W.; Zhu, T.; Cui, X.X.; Jin, C.J.; Wang, S.Y.; Wang, S.H.; Fu, D.J.; Liu, H.M.; et al. Discovery of tertiary amide derivatives incorporating benzothiazole moiety as anti-gastric cancer agents in vitro via inhibiting tubulin polymerization and activating the Hippo signaling pathway. Eur. J. Med. Chem. 2020, 203, 112618. [Google Scholar] [CrossRef]
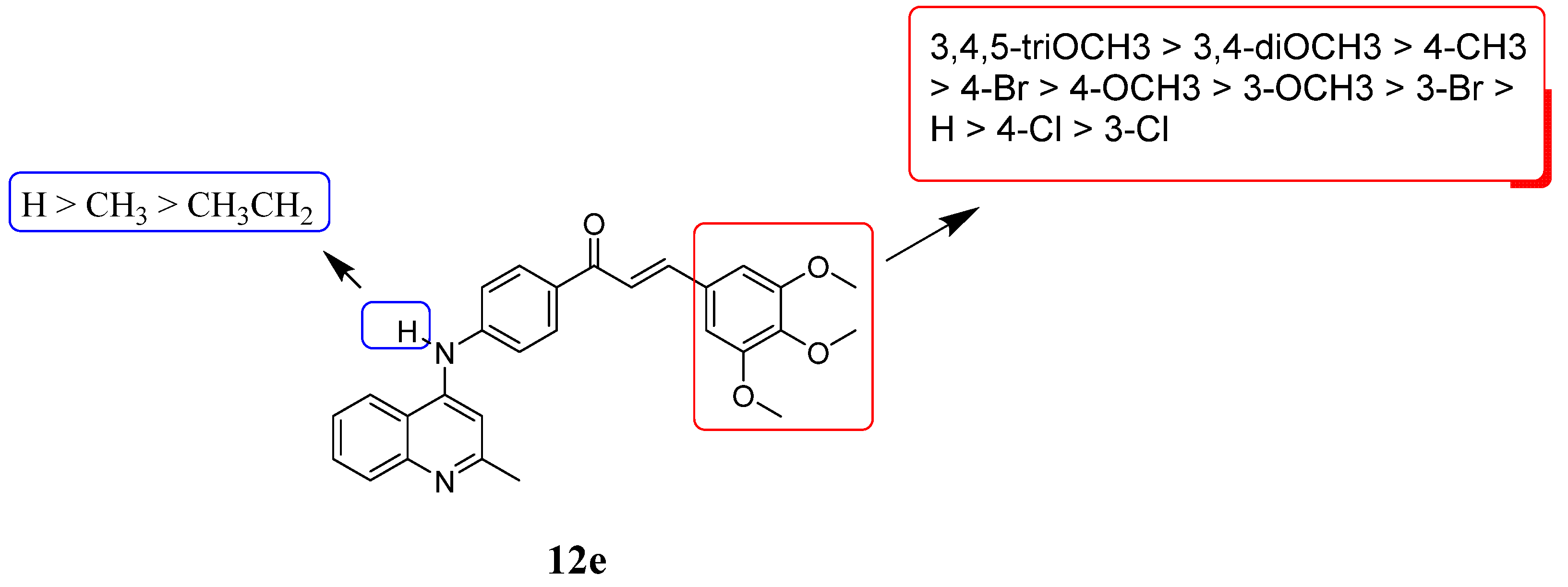


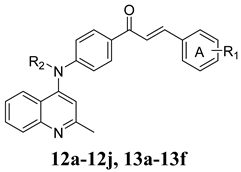
| Compounds | R1 | R2 | IC50 (μM) a | ||
|---|---|---|---|---|---|
| MGC-803 | HCT-116 | MCF-7 | |||
| 12a | 4-CH3 | H | 1.86 ± 0.23 | 7.22 ± 1.28 | 6.86 ± 0.41 |
| 12b | 4-OCH3 | H | 2.39 ± 0.36 | 8.61 ± 1.02 | 6.32 ± 0.47 |
| 12c | 3-OCH3 | H | 2.63 ± 0.18 | 6.41 ± 0.78 | 4.43 ± 0.22 |
| 12d | 3,4-diOCH3 | H | 1.62 ± 0.11 | 5.62 ± 0.41 | 7.86 ± 1.02 |
| 12e | 3,4,5-triOCH3 | H | 1.38 ± 0.21 | 5.34 ± 0.39 | 5.21 ± 0.51 |
| 12f | H | H | 3.24 ± 0.43 | 7.25 ± 0.82 | 8.23 ± 1.07 |
| 12g | 4-Cl | H | 3.82 ± 0.18 | 13.2 ± 1.39 | 7.41 ± 0.82 |
| 12h | 4-Br | H | 2.28 ± 0. 32 | 9.22 ± 0.82 | 7.82 ± 1.03 |
| 12i | 3-Cl | H | 4.25 ± 0.41 | 8.77 ± 0.74 | 10.21 ± 1.24 |
| 12j | 3-Br | H | 3.22 ± 0.19 | 16.2 ± 1.55 | 8.23 ± 1.06 |
| 13a | H | CH3 | 8.26 ± 1.48 | >20 | >20 |
| 13b | 4-CH3 | CH3 | 4.63 ± 0.52 | 10.2 ± 1.06 | 7.41 ± 0.56 |
| 13c | 4-OCH3 | CH3 | 4.54 ± 0.44 | 11.7 ± 1.12 | 7.82 ± 0.48 |
| 13d | 4-Cl | CH3 | 8.25 ± 1.03 | 18.4 ± 1.47 | 15.2 ± 1.44 |
| 13e | 3,4,5-triOCH3 | CH3 | 3.73 ± 0.28 | >20 | 10.7 ± 1.01 |
| 13f | 3,4,5-triOCH3 | CH3CH2 | 10.21 ± 1.21 | >20 | >20 |
| 5-Fu | - | - | 6.22 ± 1.12 | 10.4 ± 1.23 | 11.1 ± 1.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.-F.; Liu, X.-J.; Yuan, X.-Y.; Liu, W.-B.; Li, Y.-R.; Yu, G.-X.; Tian, X.-Y.; Zhang, Y.-B.; Song, J.; Li, W.; et al. Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives. Molecules 2021, 26, 4899. https://doi.org/10.3390/molecules26164899
Guan Y-F, Liu X-J, Yuan X-Y, Liu W-B, Li Y-R, Yu G-X, Tian X-Y, Zhang Y-B, Song J, Li W, et al. Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives. Molecules. 2021; 26(16):4899. https://doi.org/10.3390/molecules26164899
Chicago/Turabian StyleGuan, Yong-Feng, Xiu-Juan Liu, Xin-Ying Yuan, Wen-Bo Liu, Yin-Ru Li, Guang-Xi Yu, Xin-Yi Tian, Yan-Bing Zhang, Jian Song, Wen Li, and et al. 2021. "Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives" Molecules 26, no. 16: 4899. https://doi.org/10.3390/molecules26164899
APA StyleGuan, Y.-F., Liu, X.-J., Yuan, X.-Y., Liu, W.-B., Li, Y.-R., Yu, G.-X., Tian, X.-Y., Zhang, Y.-B., Song, J., Li, W., & Zhang, S.-Y. (2021). Design, Synthesis, and Anticancer Activity Studies of Novel Quinoline-Chalcone Derivatives. Molecules, 26(16), 4899. https://doi.org/10.3390/molecules26164899






