Hierarchical Zeolites as Catalysts for Biodiesel Production from Waste Frying Oils to Overcome Mass Transfer Limitations
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalysts Synthesis
2.2. Esterification Reaction Procedure
2.3. Chemical Analysis
3. Results and Discussion
3.1. Characterization of the Catalysts
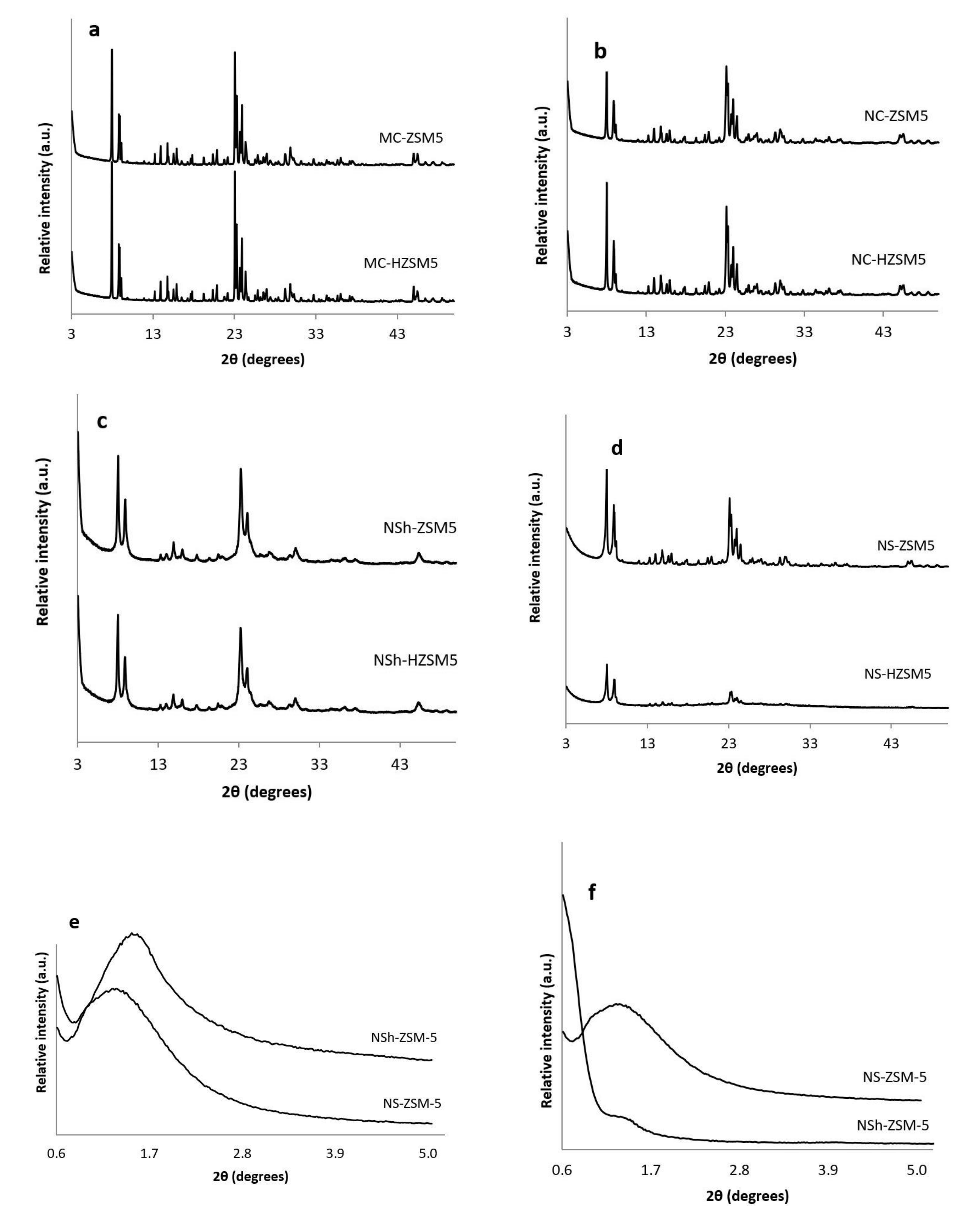
3.1.1. XRD
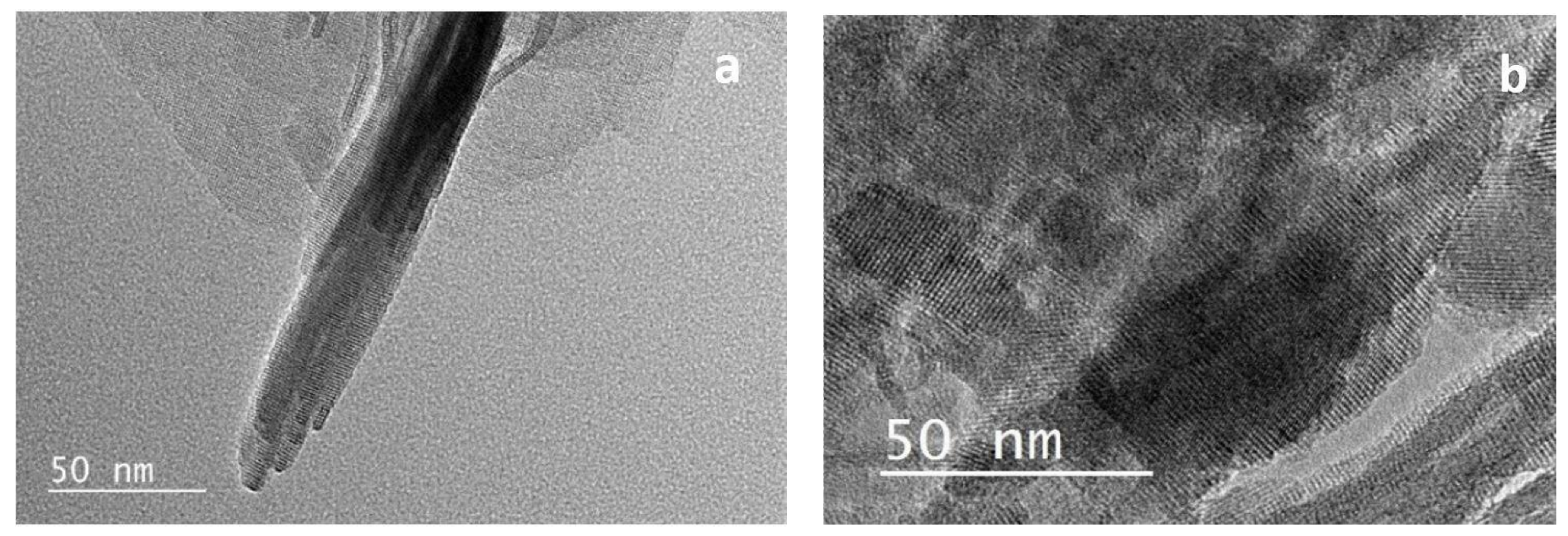
3.1.2. SEM and TEM
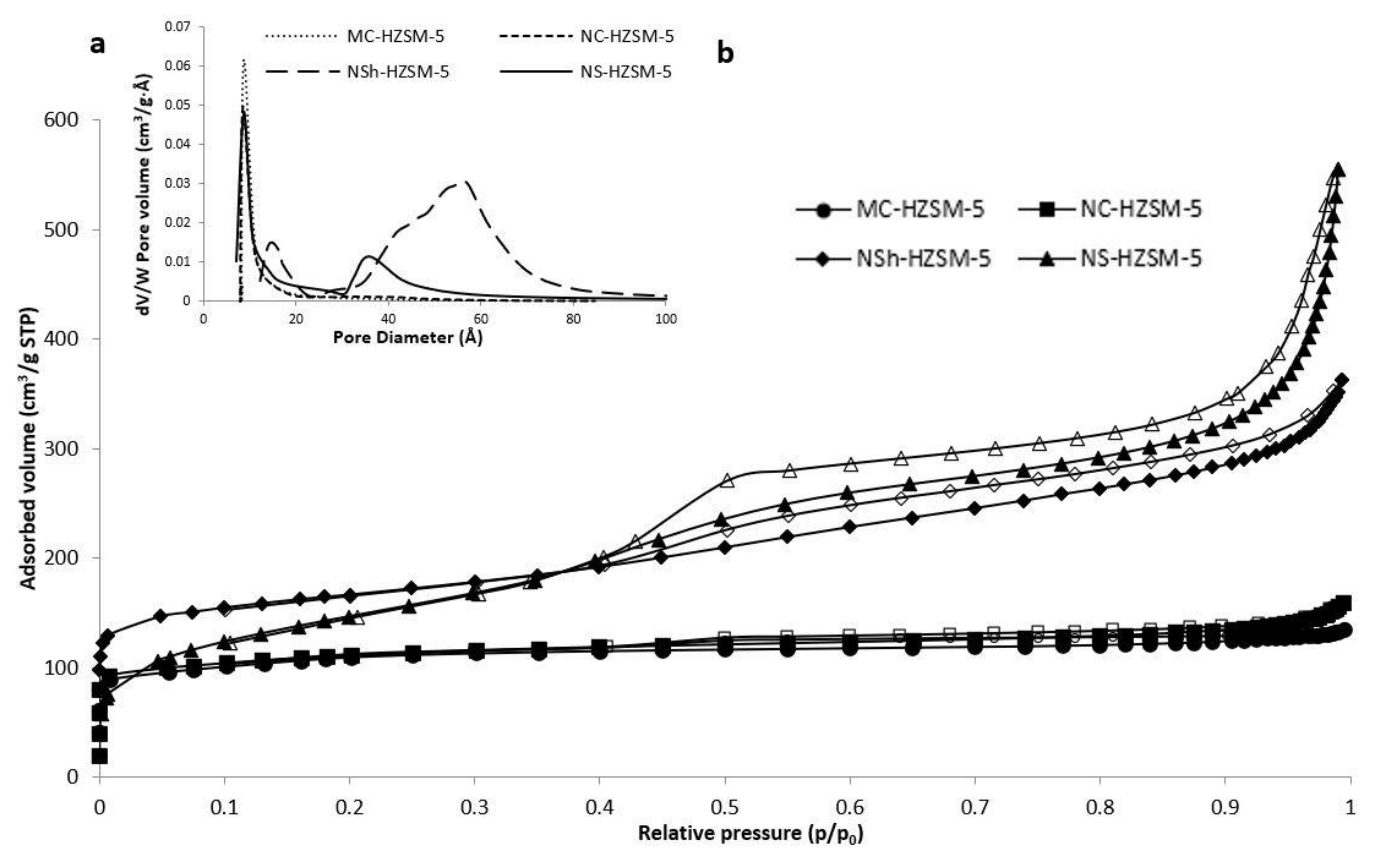
3.1.3. Nitrogen Adsorption–Desorption
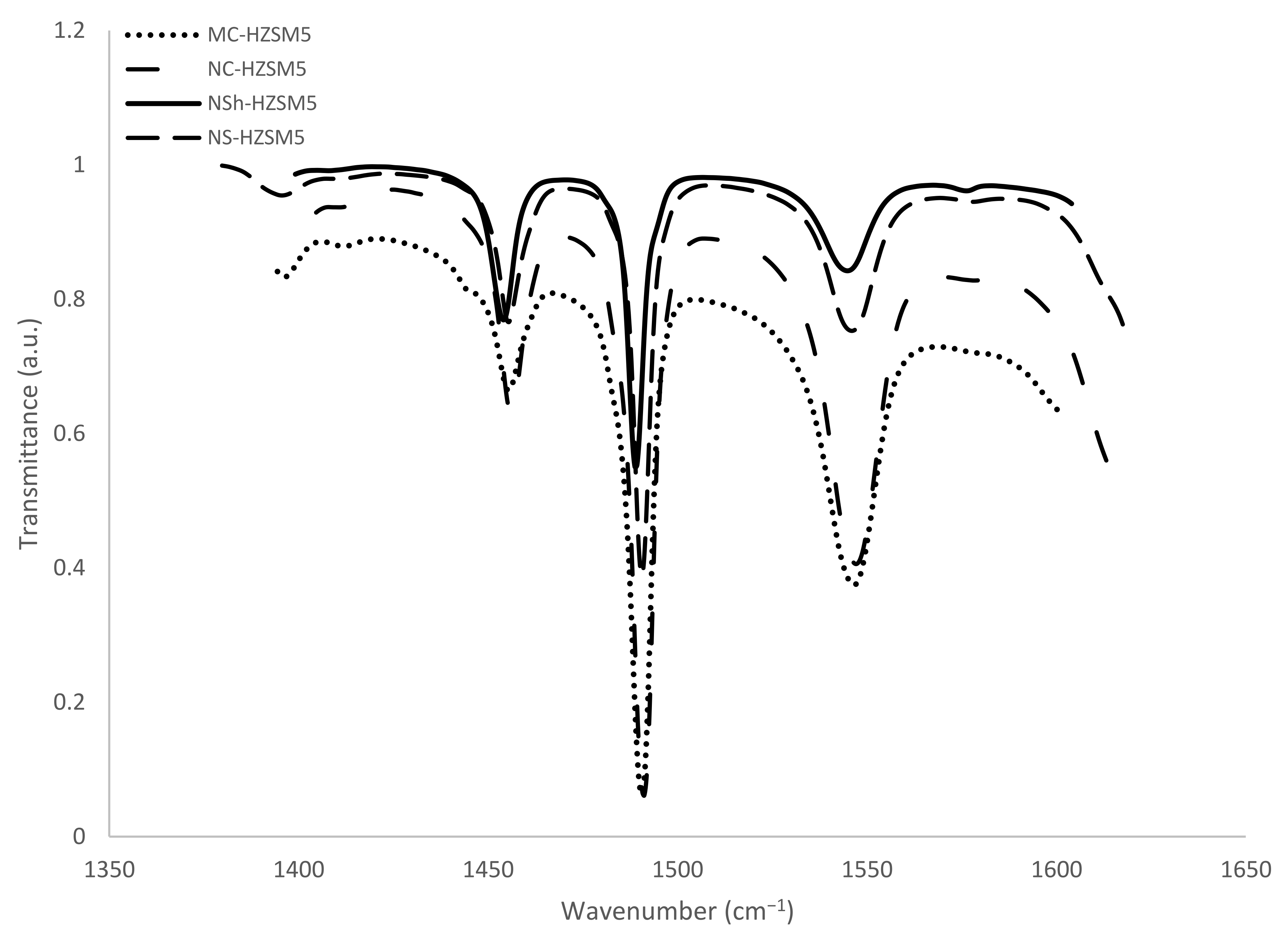
3.1.4. FTIR and 27Al MAS NMR
3.2. Optimized Reaction Conditions of Transesterification
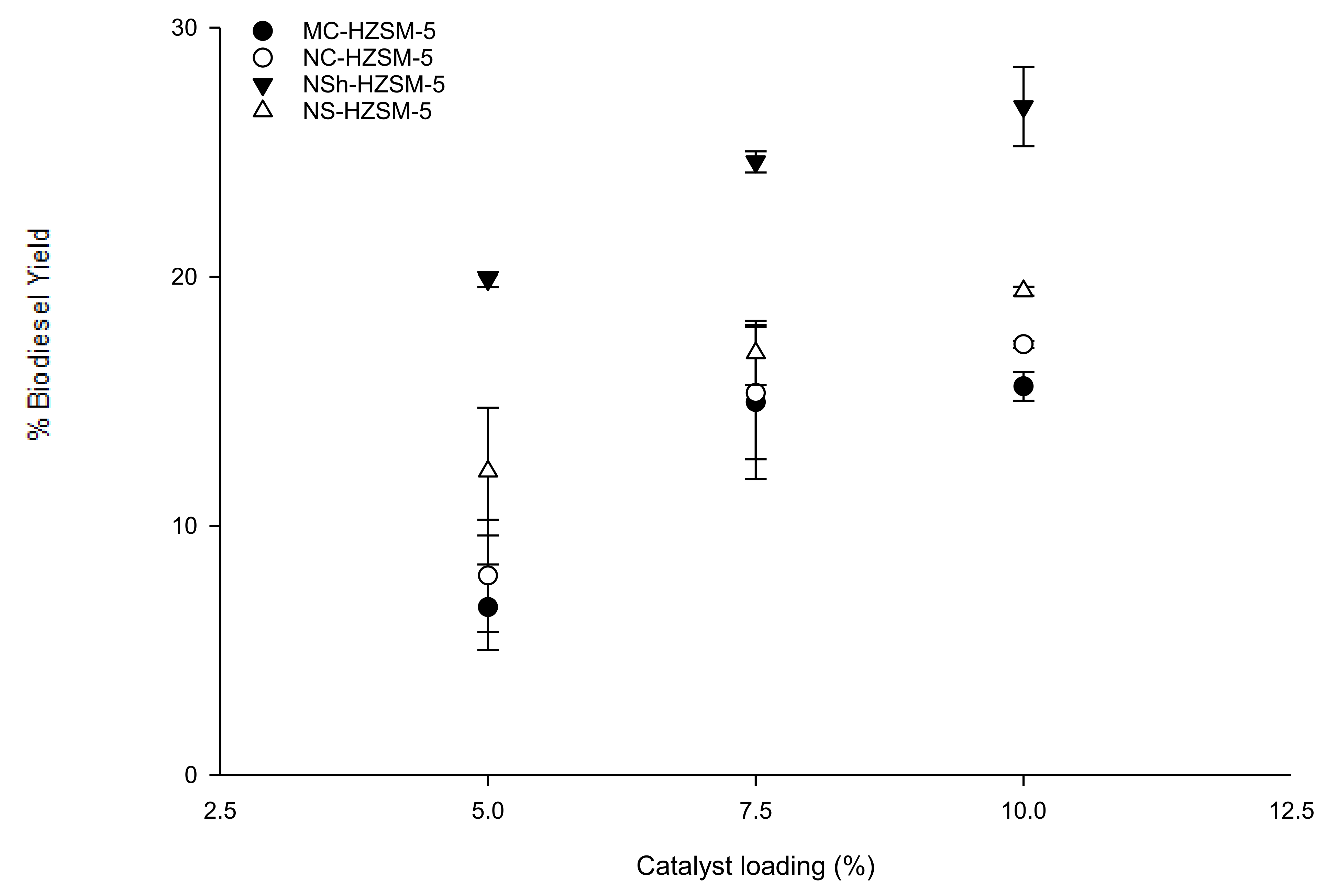
3.2.1. Catalyst Loading Effect
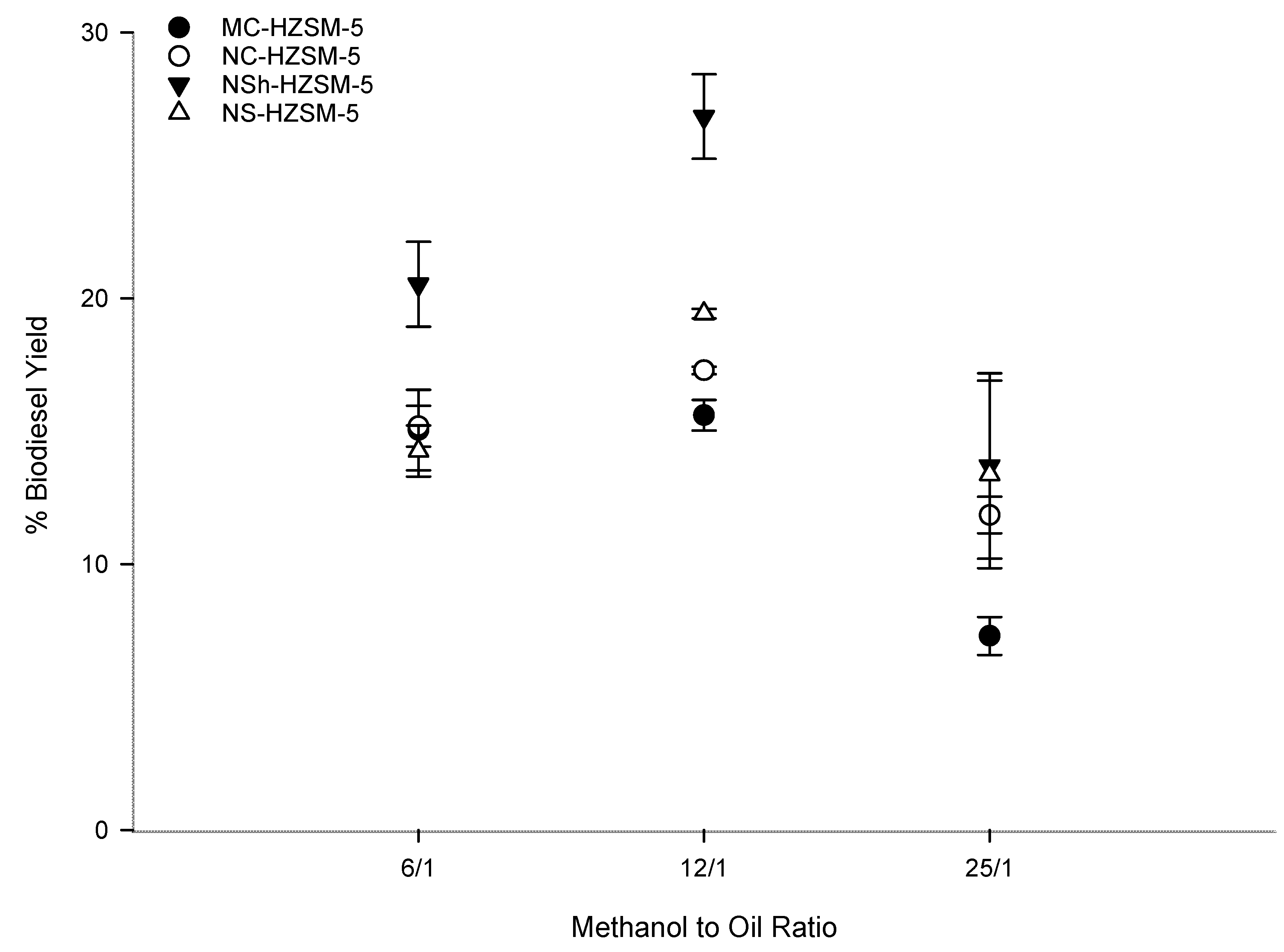
3.2.2. Methanol to WFO Ratio Effect on Transesterification
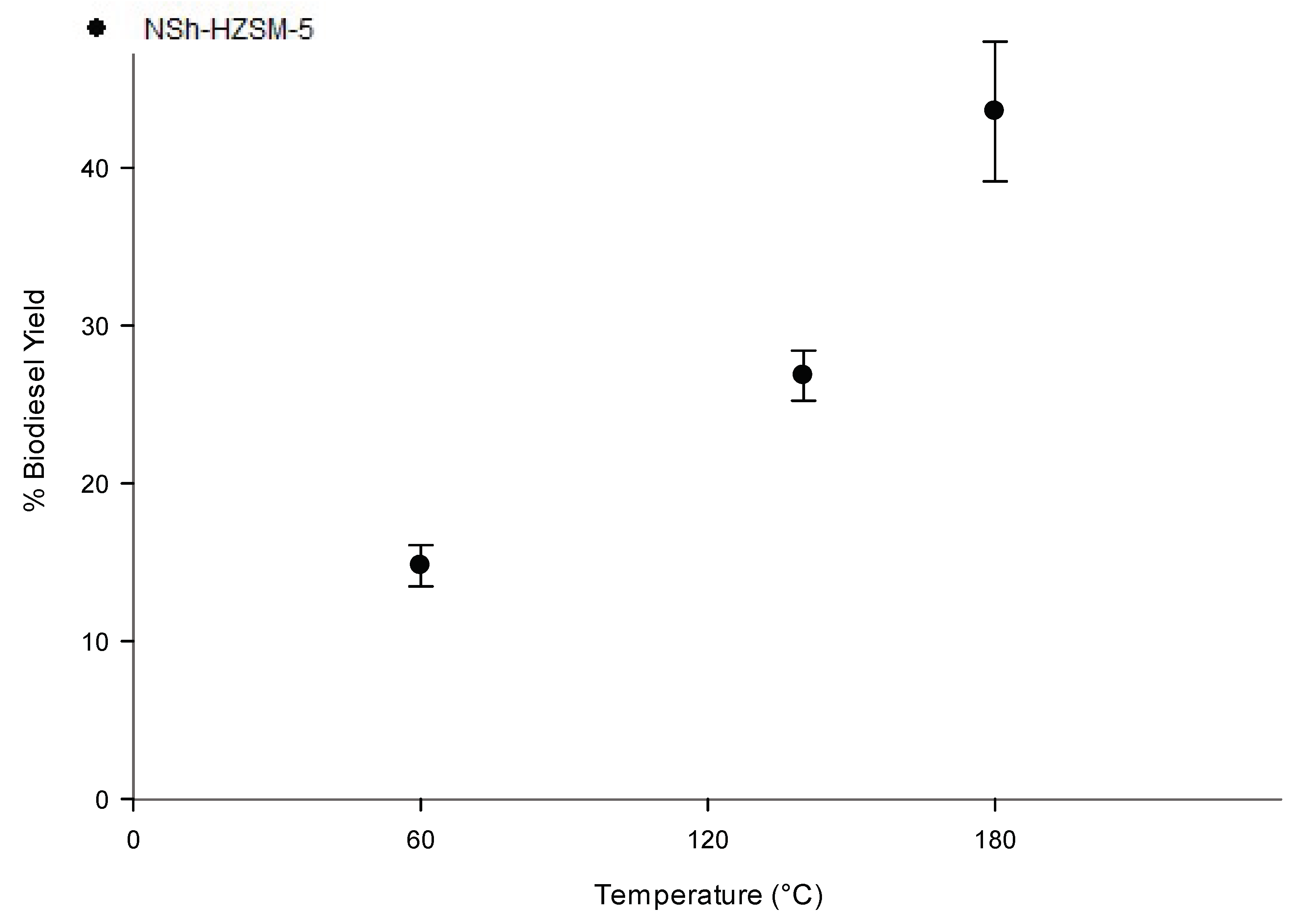
3.2.3. Reaction Temperature Effect on Transesterification
3.2.4. Reaction Time Effect on Transesterification
3.2.5. Mass Transfer Assessment of the Synthesized Catalysts on the Esterification Reaction
3.2.6. Catalyst Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Živković, S.; Veljković, M.V.; Banković-Ilić, I.; Krstić, I.M.; Konstantinović, S.S.; Ilić, S.B.; Avramović, J.M.; Stamenković, O.; Veljković, V. Technological, technical, economic, environmental, social, human health risk, toxicological and policy considerations of biodiesel production and use. Renew. Sustain. Energy Rev. 2017, 79, 222–247. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of biodiesel production from waste cooking oil and its use as fuel in compression ignition engines: 3rd generation cleaner feedstock. J. Clean. Prod. 2021, 307, 127299. [Google Scholar] [CrossRef]
- OECD Biofuels. OECD-FAO Agricultural Outlook; OECD: Paris, France, 2015; pp. 126–142. [Google Scholar] [CrossRef]
- Khan, H.M.; Iqbal, T.; Yasin, S.; Irfan, M.; Kazmi, M.; Fayaz, H.; Mujtaba, M.; Ali, C.H.; Kalam, M.; Soudagar, M.E.M.; et al. Production and utilization aspects of waste cooking oil based biodiesel in Pakistan. Alex. Eng. J. 2021, 60, 5831–5849. [Google Scholar] [CrossRef]
- Harvey, M.; Pilgrim, S. The new competition for land: Food, energy, and climate change. Food Policy 2011, 36, S40–S51. [Google Scholar] [CrossRef]
- Chakraborty, R.; Das, S.K. Optimization of Biodiesel Synthesis from Waste Frying Soybean Oil Using Fish Scale-Supported Ni Catalyst. Ind. Eng. Chem. Res. 2012, 51, 8404–8414. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste Cooking OilAn Economical Source for Biodiesel: A Review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Atadashi, I.; Aroua, M.; Aziz, A.A.; Sulaiman, N. The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem. 2013, 19, 14–26. [Google Scholar] [CrossRef]
- Feyzi, M.; Hosseini, N.; Yaghobi, N.; Ezzati, R. Preparation, characterization, kinetic and thermodynamic studies of MgO-La2O3 nanocatalysts for biodiesel production from sunflower oil. Chem. Phys. Lett. 2017, 677, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H. Synthesis of Ti(SO4)O solid acid nano-catalyst and its application for biodiesel production from used cooking oil. Appl. Catal. A Gen. 2016, 527, 81–95. [Google Scholar] [CrossRef]
- Lee, J.-S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef]
- Saravanan, K.; Tyagi, B.; Shukla, R.S.; Bajaj, H. Esterification of palmitic acid with methanol over template-assisted mesoporous sulfated zirconia solid acid catalyst. Appl. Catal. B Environ. 2015, 172–173, 108–115. [Google Scholar] [CrossRef]
- Madhuvilakku, R.; Piraman, S. Biodiesel synthesis by TiO2–ZnO mixed oxide nanocatalyst catalyzed palm oil transesterification process. Bioresour. Technol. 2013, 150, 55–59. [Google Scholar] [CrossRef]
- Feyzi, M.; Nourozi, L.; Zakarianezhad, M. Preparation and characterization of magnetic CsH 2 PW 12 O 40/Fe–SiO 2 nanocatalysts for biodiesel production. Mater. Res. Bull. 2014, 60, 412–420. [Google Scholar] [CrossRef]
- Gardy, J.; Hassanpour, A.; Lai, X.; Ahmed, M.H.; Rehan, M. Biodiesel production from used cooking oil using a novel surface functionalised TiO2 nano-catalyst. Appl. Catal. B Environ. 2017, 207, 297–310. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.; Islam, A.; Chan, E.S.; Choong, S.T.; Alharthi, N.H.; Taufiq-Yap, Y.; Awual, R. Efficient biodiesel production from Jatropha curcus using CaSO4/Fe2O3-SiO2 core-shell magnetic nanoparticles. J. Clean. Prod. 2019, 208, 816–826. [Google Scholar] [CrossRef]
- Kuniyil, M.; Kumar, J.S.; Adil, S.F.; Assal, M.E.; Shaik, M.R.; Khan, M.; Al-Warthan, A.; Siddiqui, M.R.H. Production of biodiesel from waste cooking oil using ZnCuO/N-doped graphene nanocomposite as an efficient heterogeneous catalyst. Arab. J. Chem. 2021, 14, 102982. [Google Scholar] [CrossRef]
- Chung, K.-H.; Chang, D.-R.; Park, B.-G. Removal of free fatty acid in waste frying oil by esterification with methanol on zeolite catalysts. Bioresour. Technol. 2008, 99, 7438–7443. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.; Albayati, T.; Abbas, A.; Alismaeel, Z.T. Biodiesel production by esterification of oleic acid over zeolite Y prepared from kaolin. Renew. Energy 2016, 97, 19–23. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.; Alismaeel, Z.T.; Albayati, T.; Abbas, A. High purity FAU-type zeolite catalysts from shale rock for biodiesel production. Fuel 2017, 199, 394–402. [Google Scholar] [CrossRef]
- Mowla, O.; Kennedy, E.; Stockenhuber, M. In-situ FTIR study on the mechanism of both steps of zeolite-catalysed hydroesterification reaction in the context of biodiesel manufacturing. Fuel 2018, 232, 12–26. [Google Scholar] [CrossRef]
- Astafan, A.; Benghalem, M.; Pouilloux, Y.; Patarin, J.; Bats, N.; Bouchy, C.; Daou, T.; Pinard, L. Particular properties of the coke formed on nano-sponge *BEA zeolite during ethanol-to-hydrocarbons transformation. J. Catal. 2016, 336, 1–10. [Google Scholar] [CrossRef]
- Astafan, A.; Pouilloux, Y.; Patarin, J.; Bats, N.; Bouchy, C.; Daou, T.J.; Pinard, L. Impact of extreme downsizing of *BEA-type zeolite crystals on n-hexadecane hydroisomerization. New J. Chem. 2016, 40, 4335–4343. [Google Scholar] [CrossRef]
- Kabalan, I.; Lebeau, B.; Nouali, H.; Toufaily, J.; Hamieh, T.; Koubaissy, B.; Bellat, J.-P.; Daou, T.J. New Generation of Zeolite Materials for Environmental Applications. J. Phys. Chem. C 2016, 120, 2688–2697. [Google Scholar] [CrossRef]
- Losch, P.; Kolb, J.F.; Astafan, A.; Daou, T.J.; Pinard, L.; Pale, P.; Louis, B. Eco-compatible zeolite-catalysed continuous halogenation of aromatics. Green Chem. 2016, 18, 4714–4724. [Google Scholar] [CrossRef]
- Miranda, C.; Urresta, J.; Cruchade, H.; Tran, A.; Benghalem, M.; Astafan, A.; Gaudin, P.; Daou, T.; Ramírez, A.; Pouilloux, Y.; et al. Exploring the impact of zeolite porous voids in liquid phase reactions: The case of glycerol etherification by tert-butyl alcohol. J. Catal. 2018, 365, 249–260. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, C.; Peng, H.; Peng, C.; Zhang, L.; Xu, X.; Liu, W.; Wang, Z.; Zhang, N.; Wang, X. Enhanced toluene combustion performance over Pt loaded hierarchical porous MOR zeolite. Chem. Eng. J. 2018, 334, 10–18. [Google Scholar] [CrossRef]
- Han, L.; Gao, M.; Feng, C.; Shi, L.; Zhang, D. Fe2O3–CeO2@Al2O3 Nanoarrays on Al-Mesh as SO2-Tolerant Monolith Catalysts for NOx Reduction by NH. Environ. Sci. Technol. 2019, 53, 5946–5956. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, M.; Hasegawa, J.-Y.; Li, S.; Shen, Y.; Li, H.; Shi, L.; Zhang, D. SO2-Tolerant Selective Catalytic Reduction of NOx over Meso-TiO2@Fe2O3@Al2O3 Metal-Based Monolith Catalysts. Environ. Sci. Technol. 2019, 53, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, N.M.; El Naga, A.O.A.; Younis, S.A.; Shaban, S.A.; El Torgoman, A.M.; El Kady, F.Y. Process optimization of biodiesel production via esterification of oleic acid using sulfonated hierarchical mesoporous ZSM-5 as an efficient heterogeneous catalyst. J. Environ. Chem. Eng. 2021, 9, 105035. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Park, W.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Pillared MFI Zeolite Nanosheets of a Single-Unit-Cell Thickness. J. Am. Chem. Soc. 2010, 132, 4169–4177. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.; Chmelka, B.F.; Ryoo, R. Directing Zeolite Structures into Hierarchically Nanoporous Architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Dhainaut, J.; Daou, T.J.; Bidal, Y.; Bats, N.; Harbuzaru, B.; Lapisardi, G.; Chaumeil, H.; Defoin, A.; Rouleau, L.; Patarin, J. One-pot structural conversion of magadiite into MFI zeolite nanosheets using mononitrogen surfactants as structure and shape-directing agents. CrystEngComm 2013, 15, 3009–3015. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A.; Pinard, L.; Daou, T.J. Study on the catalytic performance of different crystal morphologies of HZSM-5 zeolites for the production of biodiesel: A strategy to increase catalyst effectiveness. Catal. Sci. Technol. 2019, 9, 5456–5471. [Google Scholar] [CrossRef]
- Gratzfeld-Hüsgen, A.; Schuster, R. HPLC for Food Analysis: A Primer; Agilent Technologies 136: Santa Clara, CA, USA, 2001. [Google Scholar]
- Danaher, P.J.; Medino, C.; Shevchuk, H.; Zhang, E.M. Testing the Stability of Cation Exchanged Zeolite ZSM-5 in Hot Liquid Water; 2017. [Google Scholar]
- Kabalan, I.; Rioland, G.; Nouali, H.; Lebeau, B.; Rigolet, S.; Fadlallah, M.-B.; Toufaily, J.; Hamiyeh, T.; Daou, T.J. Synthesis of purely silica MFI-type nanosheets for molecular decontamination. RSC Adv. 2014, 4, 37353–37358. [Google Scholar] [CrossRef]
- Bräuer, P.; Ng, P.L.; Situmorang, O.; Hitchcock, I.; D’Agostino, C. Effect of Al content on number and location of hydroxyl acid species in zeolites: A DRIFTS quantitative protocol without the need for molar extinction coefficients. RSC Adv. 2017, 7, 52604–52613. [Google Scholar] [CrossRef] [Green Version]
- Guisnet, M.; Ayrault, P.; Datka, J. Acid properties of dealuminated mordenites studied by IR spectroscopy. 2. Concentration, acid strength and heterogeneity of OH groups. Pol. J. Chem. 1997, 71, 1455–1461. [Google Scholar]
- Liu, W.; Yin, P.; Zhang, J.; Tang, Q.; Qu, R. Biodiesel production from esterification of free fatty acid over PA/NaY solid catalyst. Energy Convers. Manag. 2014, 82, 83–91. [Google Scholar] [CrossRef]
- Shu, Q.; Yang, B.; Yuan, H.; Qing, S.; Zhu, G. Synthesis of biodiesel from soybean oil and methanol catalyzed by zeolite beta modified with La3+. Catal. Commun. 2007, 8, 2159–2165. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Heterogeneous solid base nanocatalyst: Preparation, characterization and application in biodiesel production. Bioresour. Technol. 2011, 102, 4150–4156. [Google Scholar] [CrossRef]
- Sun, K.; Lu, J.; Ma, L.; Han, Y.; Fu, Z.; Ding, J. A comparative study on the catalytic performance of different types of zeolites for biodiesel production. Fuel 2015, 158, 848–854. [Google Scholar] [CrossRef]
- Borges, L.D.; Moura, N.N.; Costa, A.A.; Braga, P.R.; Dias, J.A.; Dias, S.C.; Macedo, J.; Ghesti, G.F. Investigation of biodiesel production by HUSY and Ce/HUSY zeolites: Influence of structural and acidity parameters. Appl. Catal. A Gen. 2013, 450, 114–119. [Google Scholar] [CrossRef]
- Pacey, P.D. Changing conceptions of activation energy. J. Chem. Educ. 1981, 58, 612. [Google Scholar] [CrossRef]
- Laidler, K.J. The development of the Arrhenius equation. J. Chem. Educ. 1984, 61, 494. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Wang, H.; Frenklach, M. Transport properties of polycyclic aromatic hydrocarbons for flame modeling. Combust. Flame 1994, 96, 163–170. [Google Scholar] [CrossRef]
- Sánchez, A.; Maceiras, R.; Cancela, A.; Rodríguez, M. Influence of n-Hexane on in Situ Transesterification of Marine Macroalgae. Energies 2012, 5, 243–257. [Google Scholar] [CrossRef]
- Amara, S.; Patin, A.; Giuffrida, F.; Wooster, T.J.; Thakkar, S.K.; Bénarouche, A.; Poncin, I.; Robert, S.; Point, V.; Molinari, S. In vitro digestion of citric acid esters of mono-and diglycerides (CITREM) and CITREM-containing infant formula/emulsions. Food Funct. 2014, 5, 1409–1421. [Google Scholar] [CrossRef]
- Carberry, J.J. Chemical and Catalytic Reaction Engineering; Courier Corporation: North Chelmsford, MA, USA, 2001. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Boltz, M.; Losch, P.; Louis, B.; Rioland, G.; Tzanis, L.; Daou, T.J. MFI-type zeolite nanosheets for gas-phase aromatics chlorination: A strategy to overcome mass transfer limitations. RSC Adv. 2014, 4, 27242–27249. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’connell, J.P. Properties of Gases and Liquids; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Khalid, M.; Joly, G.; Renaud, A.A.; Magnoux, P. Removal of Phenol from Water by Adsorption Using Zeolites. Ind. Eng. Chem. Res. 2004, 43, 5275–5280. [Google Scholar] [CrossRef]



| Type of HZSM-5 | Si/Al Ratio | S BET (m2/g) | S External (m2·g−1) | S Micropore (m2·g−1) | Total Porous Volume (cm3/g) | Microporous Volume (cm3/g) | Mesoporous Volume (cm3/g) |
|---|---|---|---|---|---|---|---|
| MC-HZSM5 | 45 | 395 | 22 | 374 | 0.17 | 0.17 | - |
| NC-HZSM5 | 25 | 411 | 34 | 377 | 0.17 | 0.17 | - |
| NSh-HZSM5 | 44 | 521 | 175 | 346 | 0.32 | 0.19 | 0.13 |
| NS-HZSM5 | 23 | 613 | 205 | 398 | 0.62 | 0.27 | 0.35 |
| Type of HZSM5 | Si/Al Ratio a | Si/Al of the Framework b | [PyrH+] c (µmol·g−1) | [PyrL] d (µmol·g−1) |
|---|---|---|---|---|
| MC-HZSM5 | 45 | 53 | 351 | 44 |
| NC-HZSM5 | 25 | 30 | 301 | 76 |
| NSh-HZSM5 | 44 | 55 | 103 | 80 |
| NS-HZSM5 | 23 | 33 | 218 | 103 |
| MC-HZSM5 | NC-HZSM5 | NSh-HZSM5 | NS-HZSM5 | |
|---|---|---|---|---|
| (cm3·g−1) | 0.165 | 0.166 | 0.323 | 0.624 |
| (m2·s−1) | 1.28 × 10−7 | 1.29 × 10−7 | 2.51 × 10−7 | 4.85 × 10−7 |
| (m2·h−1) | 0.075 | 0.082 | 0.125 | 0.097 |
| 0.1603 | 0.0050 | 0.0004 | 0.0033 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawaz, E.G.; Salam, D.A.; S. Rigolet, S.; Daou, T.J. Hierarchical Zeolites as Catalysts for Biodiesel Production from Waste Frying Oils to Overcome Mass Transfer Limitations. Molecules 2021, 26, 4879. https://doi.org/10.3390/molecules26164879
Fawaz EG, Salam DA, S. Rigolet S, Daou TJ. Hierarchical Zeolites as Catalysts for Biodiesel Production from Waste Frying Oils to Overcome Mass Transfer Limitations. Molecules. 2021; 26(16):4879. https://doi.org/10.3390/molecules26164879
Chicago/Turabian StyleFawaz, Elyssa G., Darine A. Salam, Severinne S. Rigolet, and T. Jean Daou. 2021. "Hierarchical Zeolites as Catalysts for Biodiesel Production from Waste Frying Oils to Overcome Mass Transfer Limitations" Molecules 26, no. 16: 4879. https://doi.org/10.3390/molecules26164879
APA StyleFawaz, E. G., Salam, D. A., S. Rigolet, S., & Daou, T. J. (2021). Hierarchical Zeolites as Catalysts for Biodiesel Production from Waste Frying Oils to Overcome Mass Transfer Limitations. Molecules, 26(16), 4879. https://doi.org/10.3390/molecules26164879







