Comparative Study of a Series of 99mTc(CO)3 Mannosylated Dextran Derivatives for Sentinel Lymph Node Detection
Abstract
1. Introduction
2. Results
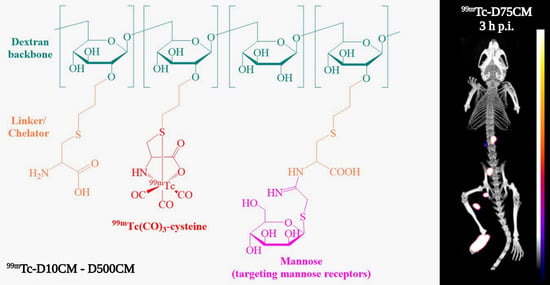
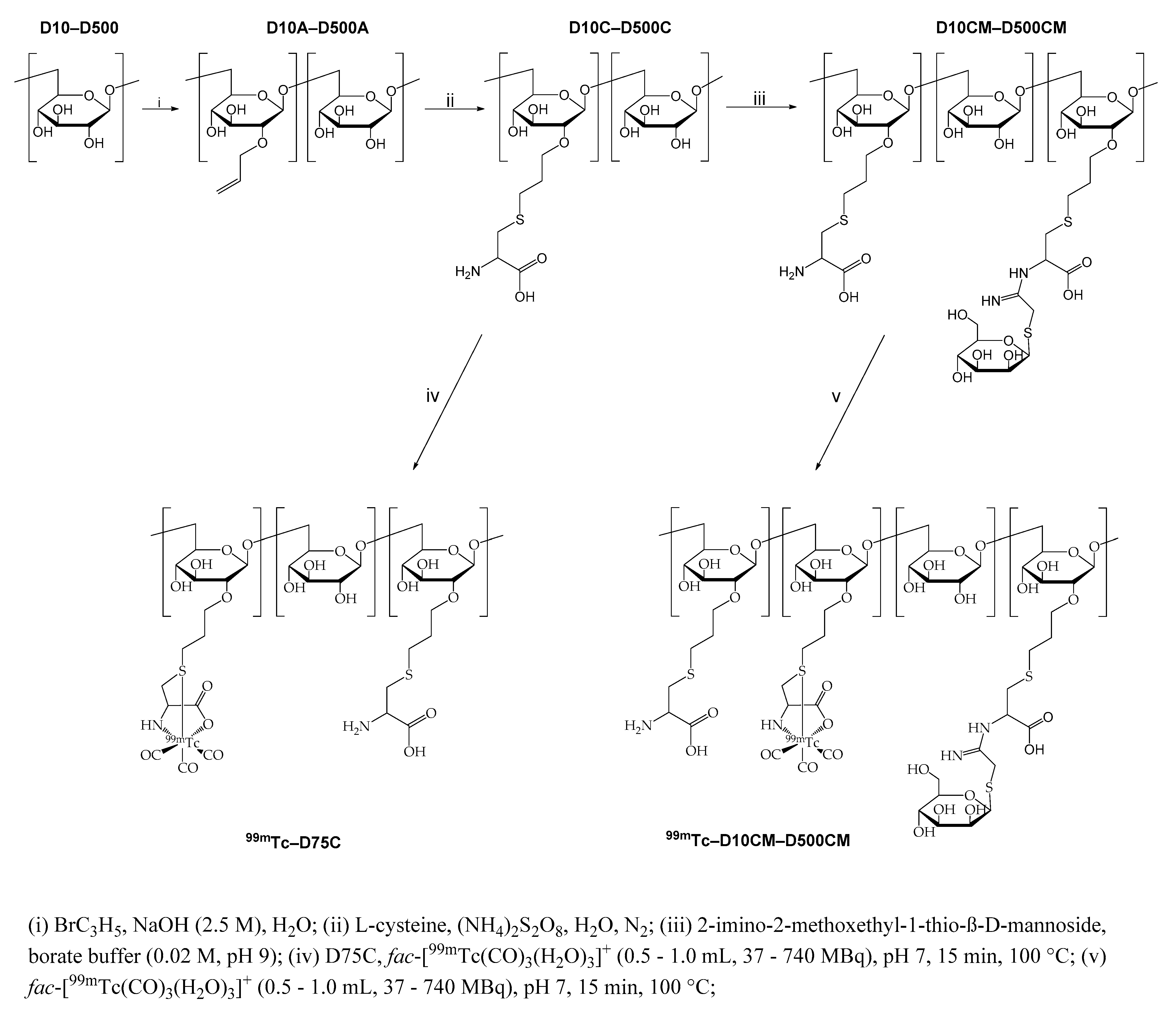
2.1. Synthesis and Characterization of the Mannosylated Dextran Derivatives
2.2. Radiolabeling
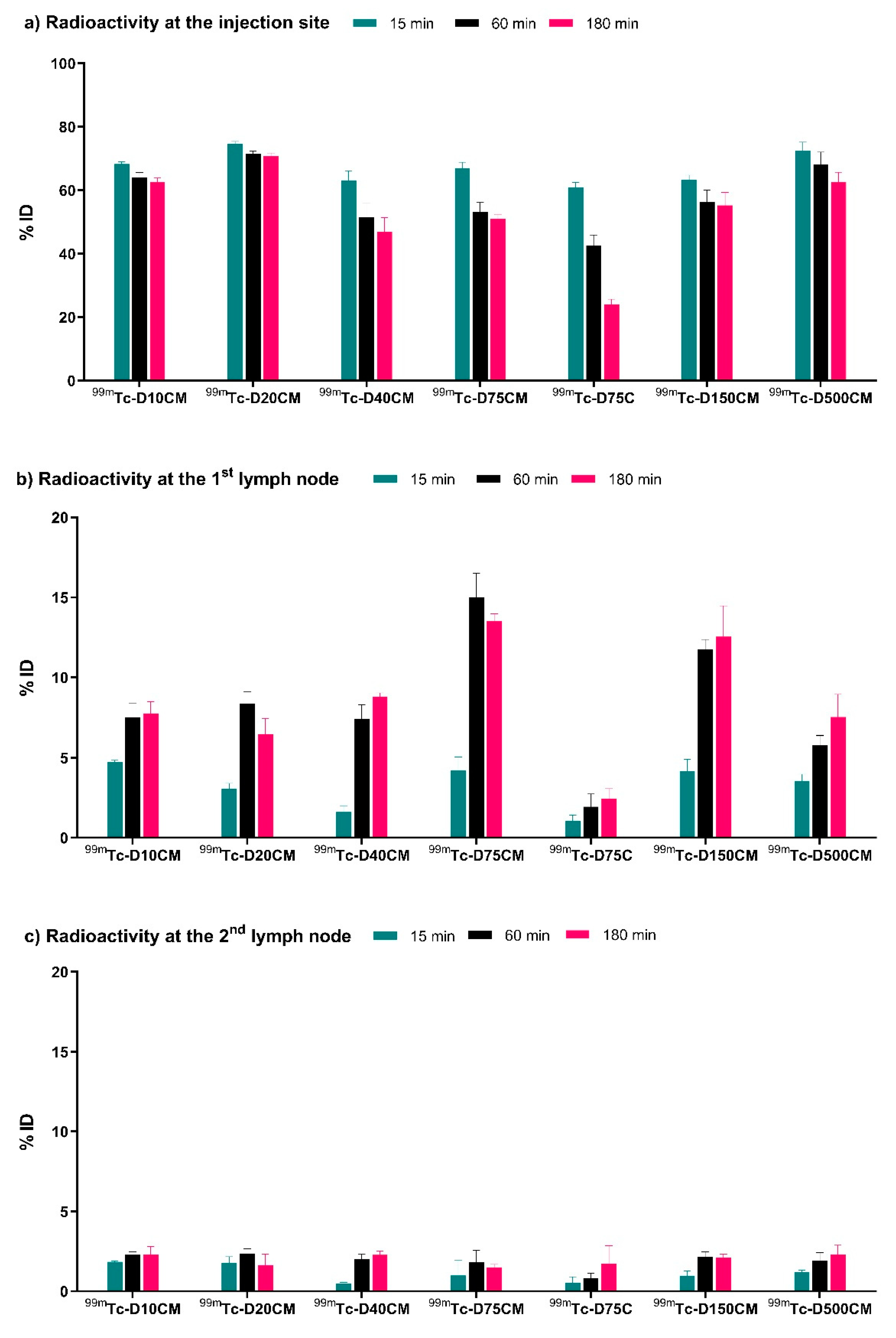
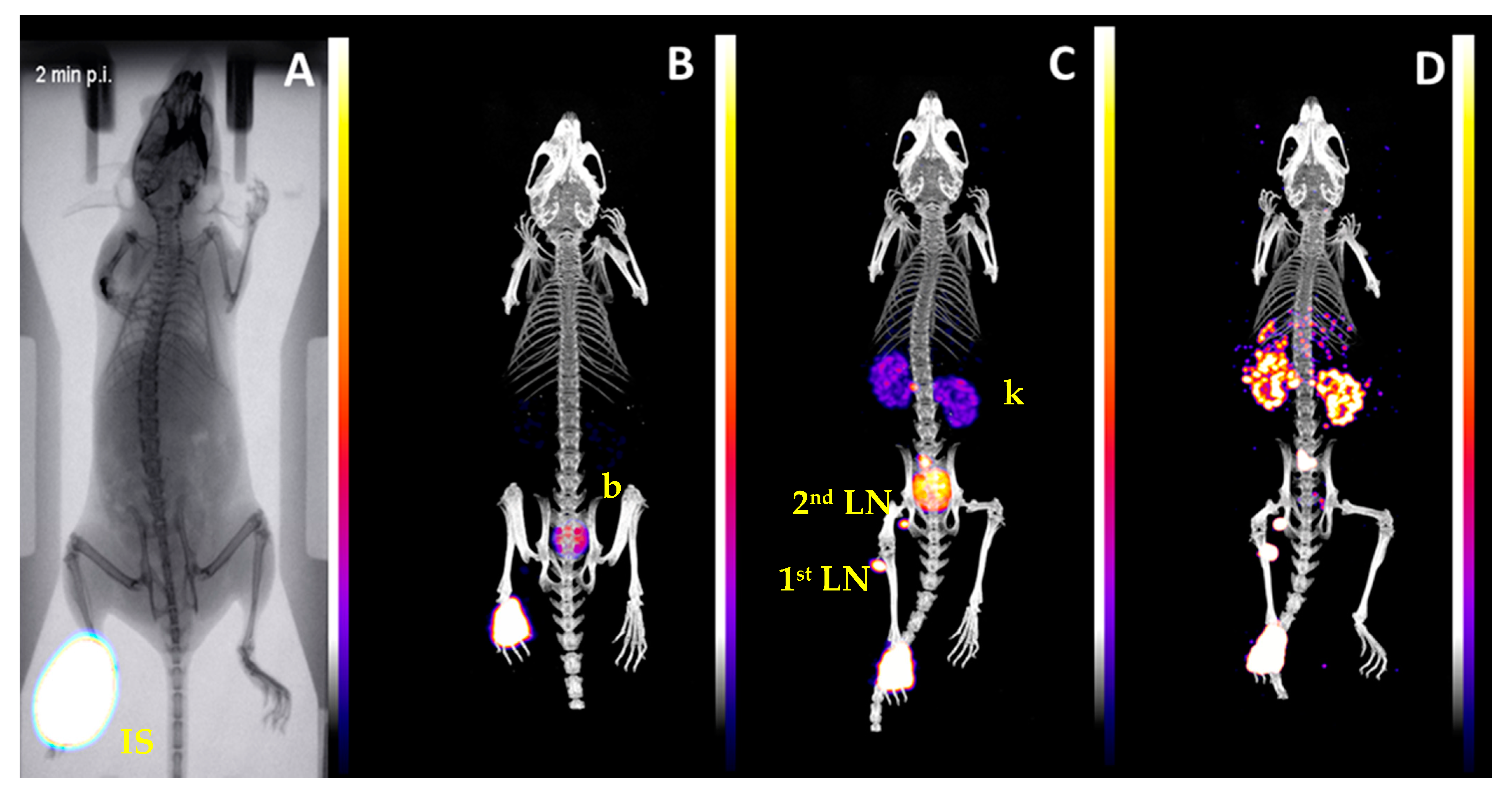
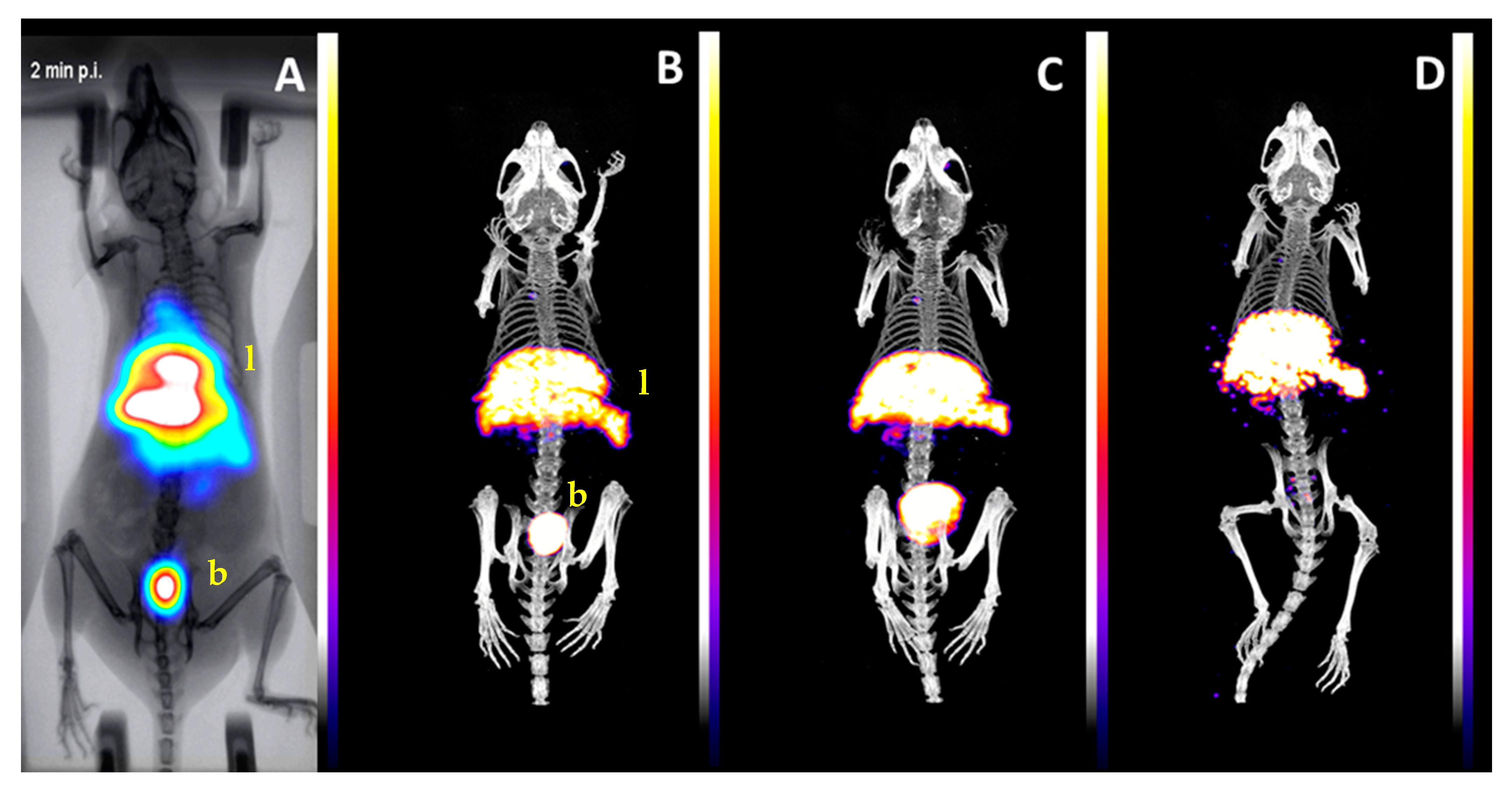
2.3. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Synthesis of Allyl-Dextran Compounds (D10A–D500A)
4.2. Synthesis of Dextran-S-Cysteine Compounds (D10C–D500C)
4.3. Synthesis of Mannosylated Dextran-S-Cysteine Compounds (D10CM–D500CM)
4.4. Synthesis of 99mTc Complexes
4.5. In Vitro Stability Studies of 99mTc Complexes
4.6. Animal Distribution Studies
4.7. Imaging Studies
4.7.1. Imaging Systems
4.7.2. Animal Imaging Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eckelman, W.C.; Reba, R.C.; Gibson, R.E.; Rzeszotarski, W.J.; Vieras, F.; Mazaitis, J.K.; Francis, B. Receptor-Binding Radiotracers: A Class of Potential Radiopharmaceuticals. J. Nucl. Med. 1979, 20, 350. [Google Scholar]
- Castronovo, F.P., Jr. Technetium-99m: Basic nuclear physics and chemical properties. Am. J. Hosp. Pharm. 1975, 32, 480–488. [Google Scholar] [CrossRef]
- Keshtgar, M.; Zaknun, J.J.; Sabih, D.; Lago, G.; Cox, C.E.; Leong, S.P.L.; Mariani, G. Implementing Sentinel Lymph Node Biopsy Programs in Developing Countries: Challenges and Opportunities. World J. Surg. 2011, 35, 1159–1168. [Google Scholar] [CrossRef]
- Morton, D.L.; Chan, A.D. The concept of sentinel node localization: How it started. Semin. Nucl. Med. 2000, 30, 4–10. [Google Scholar] [CrossRef]
- Morais, M.; Campello, M.P.C.; Xavier, C.; Heemskerk, J.; Correia, J.D.G.; Lahoutte, T.; Caveliers, V.; Hernot, S.; Santos, I. Radiolabeled Mannosylated Dextran Derivatives Bearing an NIR-Fluorophore for Sentinel Lymph Node Imaging. Bioconjug. Chem. 2014, 25, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Shayan, R.; Achen, M.G.; Stacker, S.A. Lymphatic vessels in cancer metastasis: Bridging the gaps. Carcinogenesis 2006, 27, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Galimberti, V.; Zurrida, S.; Pigatto, F.; Veronesi, P.; Robertson, C.; Paganelli, G.; Sciascia, V.; Viale, G. Sentinel lymph node biopsy as an indicator for axillary dissection in early breast cancer. Eur. J. Cancer 2001, 37, 454–458. [Google Scholar] [CrossRef]
- Wilhelm, A.J.; Mijnhout, G.S.; Franssen, E.J. Radiopharmaceuticals in sentinel lymph-node detection—An overview. Eur. J. Nucl. Med. 1999, 26, S36–S42. [Google Scholar] [CrossRef]
- Fritzberg, A.R.; Kasina, S.; Eshima, D.; Johnson, D.L. Synthesis and biological evaluation of technetium-99m MAG3 as a hippuran replacement. J. Nucl. Med. 1986, 27, 111–116. [Google Scholar]
- Ocampo-García, B.E.; Ramírez, F.d.M.; Ferro-Flores, G.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; Morales-Avila, E.; de Murphy, C.A.; Pedraza-López, M.; Medina, L.A.; Camacho-López, M.A. 99mTc-labelled gold nanoparticles capped with HYNIC-peptide/mannose for sentinel lymph node detection. Nucl. Med. Biol. 2011, 38, 1–11. [Google Scholar] [CrossRef]
- Estudiante-Mariquez, O.J.; Rodríguez-Galván, A.; Ramírez-Hernández, D.; Contreras-Torres, F.F.; Medina, L.A. Technetium-Radiolabeled Mannose-Functionalized Gold Nanoparticles as Nanoprobes for Sentinel Lymph Node Detection. Molecules 2020, 25, 1982. [Google Scholar] [CrossRef]
- Vera, D.R.; Wisner, E.R.; Stadalnik, R.C. Sentinel node imaging via a nonparticulate receptor-binding radiotracer. J. Nucl. Med. 1997, 38, 530–535. [Google Scholar] [PubMed]
- Jeong, J.M.; Hong, M.K.; Kim, Y.J.; Lee, J.; Kang, J.H.; Lee, D.S.; Chung, J.K.; Lee, M.C. Development of 99mTc-neomannosyl human serum albumin (99mTc-MSA) as a novel receptor binding agent for sentinel lymph node imaging. Nucl. Med. Commun. 2004, 25, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Uehara, T.; Kaneko, E.; Nakayama, M.; Koizumi, M.; Endo, K.; Arano, Y. 99mTc-labeled mannosyl-neoglycoalbumin for sentinel lymph node identification. Nucl. Med. Biol. 2004, 31, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Vera, D.R.; Wallace, A.M.; Hoh, C.K.; Mattrey, R.F. A synthetic macromolecule for sentinel node detection: 99mTc-DTPA-mannosyl-dextran. J. Nucl. Med. 2001, 42, 951–959. [Google Scholar]
- Hoh, C.K.; Wallace, A.M.; Vera, D.R. Preclinical studies of [99mTc] DTPA-mannosyl-dextran. Nucl. Med. Biol. 2003, 30, 457–464. [Google Scholar] [CrossRef]
- Unkart, J.T.; Wallace, A.M. Use of 99mTc-Tilmanocept as a Single Agent for Sentinel Lymph Node Identification in Breast Cancer: A Retrospective Pilot Study. J. Nucl. Med. Technol. 2017, 45, 181. [Google Scholar] [CrossRef][Green Version]
- Wallace, A.M.; Hoh, C.K.; Darrah, D.D.; Schulteis, G.; Vera, D.R. Sentinel lymph node mapping of breast cancer via intradermal administration of Lymphoseek. Nucl. Med. Biol. 2007, 34, 849–853. [Google Scholar] [CrossRef]
- Vera, D.R.; Wallace, A.M.; Hoh, C.K. [99mTc] MAG3-mannosyl-dextran: A receptor-binding radiopharmaceutical for sentinel node detection. Nucl. Med. Biol. 2001, 28, 493–498. [Google Scholar] [CrossRef]
- Morais, M.; Subramanian, S.; Pandey, U.; Samuel, G.; Venkatesh, M.; Martins, M.; Pereira, S.; Correia, J.D.G.; Santos, I. Mannosylated Dextran Derivatives Labeled with fac-[M(CO)3]+ (M = 99mTc, Re) for Specific Targeting of Sentinel Lymph Node. Mol. Pharm. 2011, 8, 609–620. [Google Scholar] [CrossRef]
- Wallace, A.M.; Hoh, C.K.; Ellner, S.J.; Darrah, D.D.; Schulteis, G.; Vera, D.R. Lymphoseek: A molecular imaging agent for melanoma sentinel lymph node mapping. Ann. Surg. Oncol. 2007, 14, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Fernández, S.; Pietzsch, H.-J.; Dematteis, S.; Moreno, M.; Pacheco, J.P.; Cerecetto, H.; Rey, A. Synthesis, in vitro and in vivo characterization of novel 99mTc-‘4+1’-labeled 5-nitroimidazole derivatives as potential agents for imaging hypoxia. Nucl. Med. Biol. 2012, 39, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Pirmettis, I.; Arano, Y.; Tsotakos, T.; Okada, K.; Yamaguchi, A.; Uehara, T.; Morais, M.; Correia, J.D.G.; Santos, I.; Martins, M.; et al. New 99mTc(CO)3 Mannosylated Dextran Bearing S-Derivatized Cysteine Chelator for Sentinel Lymph Node Detection. Mol. Pharm. 2012, 9, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Pandey, U.; Papadopoulos, M.; Pirmettis, I.; Venkatesh, M.; Samuel, G. Studies toward the biological efficacy of 99mTc-labeled dextran-cysteine-mannose ([99mTc(CO)3] DCM20) for sentinel lymph node detection. Cancer Biother. Radiopharm. 2012, 27, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Rajaram, M.V.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef]
- Timmons, S.C.; Jakeman, D.L. Stereoselective Chemical Synthesis of Sugar Nucleotides via Direct Displacement of Acylated Glycosyl Bromides. Org. Lett. 2007, 9, 1227–1230. [Google Scholar] [CrossRef]
- Lee, Y.C.; Stowell, C.P.; Krantz, M.J. 2-Imino-2-methoxyethyl 1-thioglycosides: New reagents for attaching sugars to proteins. Biochemistry 1976, 15, 3956–3963. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P.; Abram, U.; Kaden, T.A. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]- in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Fysikopoulos, E.; Rouchota, E.M.; Eleftheriadis, V.; Gatsiou, C.A.; Pilatis, I.; Sarpaki, S.; Loudos, G.; Kostopoulos, S.; Glotsos, D. Photograph to X-ray image translation for ana-tomical mouse mapping in preclinical nuclear molecular imaging. In Proceedings of the 2nd International Conference on Medical Image and Computer-Aided Diagnosis, Birmingham, UK, 25–26 March 2021. [Google Scholar]
- Georgiou, M.; Loudos, G.; Fysikopoulos, E.; Lamprou, E.; Mikropoulos, K.; Shegani, A.; Georgoulias, P. λ-Eye: A high-sensitivity γ imaging probe for axillary sentinel lymph node mapping. Nucl. Med. Commun. 2016, 37, 1001–1009. [Google Scholar] [CrossRef]


| DCM Derivative | Cysteine Moieties | Mannose Moieties | MWcalculated |
|---|---|---|---|
| D10CM | 7 | 19 | 21,297 |
| D20CM | 17 | 23 | 31,097 |
| D40CM | 29 | 33 | 59,459 |
| D75CM | 23 | 74 | 111,238 |
| D150CM | 95 | 160 | 236,320 |
| D500CM | 142 | 645 | 805,567 |
| Organ | 99mTc-D75C | 99mTc-D75CM | ||||
|---|---|---|---|---|---|---|
| 15 min | 60 min | 180 min | 15 min | 60 min | 180 min | |
| Blood | 6.16 ± 1.23 | 10.10 ± 2.19 | 10.13 ± 1.36 | 1.93 ± 0.91 | 0.40 ± 0.19 | 0.18 ± 0.04 |
| Liver | 2.11 ± 0.23 | 3.22 ± 0.39 | 3.22 ± 0.61 | 3.62 ± 0.87 | 3.70 ± 1.44 | 2.22 ± 0.16 |
| Heart | 0.99 ± 0.24 | 1.65 ± 0.56 | 1.88 ± 0.46 | 0.44 ± 0.12 | 0.34 ± 0.11 | 0.35 ± 0.03 |
| Kidneys | 5.28 ± 1.00 | 5.61 ± 1.16 | 4.92 ± 0.86 | 2.22 ± 0.47 | 0.90 ± 0.09 | 0.76 ± 0.13 |
| Stomach | 0.46 ± 0.10 | 1.32 ± 0.92 | 1.38 ± 0.35 | 0.33 ± 0.17 | 0.29 ± 0.04 | 1.08 ± 0.66 |
| Intestines | 0.40 ± 0.08 | 0.54 ± 0.11 | 1.00 ± 0.13 | 0.36 ± 0.12 | 0.33 ± 0.08 | 0.81 ± 0.60 |
| Spleen | 0.58 ± 0.07 | 1.08 ± 0.29 | 1.99 ± 0.47 | 0.73 ± 0.22 | 1.43 ± 1.33 | 1.19 ± 0.33 |
| Muscle | 0.19 ± 0.01 | 0.27 ± 0.01 | 0.34 ± 0.07 | 0.17 ± 0.11 | 0.15 ± 0.03 | 0.34 ± 0.11 |
| Lungs | 1.44 ± 0.07 | 2.32 ± 0.65 | 2.43 ± 0.07 | 0.96 ± 0.50 | 0.31 ± 0.06 | 0.38 ± 0.06 |
| Urine * | 2.47 ± 1.32 | 6.54 ± 4.84 | 22.46 ± 3.55 | 2.06 ± 0.74 | 1.69 ± 2.39 | 4.45 ±3.23 |
| 1st node * | 1.04 ± 0.37 | 1.94 ± 0.80 | 2.43 ± 0.64 | 4.20 ± 0.84 | 15.00 ± 1.50 | 13.53 ± 0.45 |
| 2nd node * | 0.53 ± 0.38 | 0.80 ± 0.34 | 1.72 ± 1.13 | 0.99 ± 0.75 | 1.81 ± 0.77 | 1.49 ± 0.20 |
| Injection site * | 60.91 ± 1.52 | 42.63 ± 3.27 | 24.05 ± 1.61 | 66.84 ± 1.96 | 53.20 ± 2.96 | 51.04 ± 1.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papasavva, A.; Shegani, A.; Kiritsis, C.; Roupa, I.; Ischyropoulou, M.; Makrypidi, K.; Pilatis, I.; Loudos, G.; Pelecanou, M.; Papadopoulos, M.; et al. Comparative Study of a Series of 99mTc(CO)3 Mannosylated Dextran Derivatives for Sentinel Lymph Node Detection. Molecules 2021, 26, 4797. https://doi.org/10.3390/molecules26164797
Papasavva A, Shegani A, Kiritsis C, Roupa I, Ischyropoulou M, Makrypidi K, Pilatis I, Loudos G, Pelecanou M, Papadopoulos M, et al. Comparative Study of a Series of 99mTc(CO)3 Mannosylated Dextran Derivatives for Sentinel Lymph Node Detection. Molecules. 2021; 26(16):4797. https://doi.org/10.3390/molecules26164797
Chicago/Turabian StylePapasavva, Afroditi, Antonio Shegani, Christos Kiritsis, Ioanna Roupa, Myrto Ischyropoulou, Konstantina Makrypidi, Irineos Pilatis, George Loudos, Maria Pelecanou, Minas Papadopoulos, and et al. 2021. "Comparative Study of a Series of 99mTc(CO)3 Mannosylated Dextran Derivatives for Sentinel Lymph Node Detection" Molecules 26, no. 16: 4797. https://doi.org/10.3390/molecules26164797
APA StylePapasavva, A., Shegani, A., Kiritsis, C., Roupa, I., Ischyropoulou, M., Makrypidi, K., Pilatis, I., Loudos, G., Pelecanou, M., Papadopoulos, M., & Pirmettis, I. (2021). Comparative Study of a Series of 99mTc(CO)3 Mannosylated Dextran Derivatives for Sentinel Lymph Node Detection. Molecules, 26(16), 4797. https://doi.org/10.3390/molecules26164797