Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol
Abstract
:1. Introduction
2. Chemical Features and Anticancer Effect of Curcumin and Resveratrol
3. Hybrid Compounds Containing Curcumin and Resveratrol
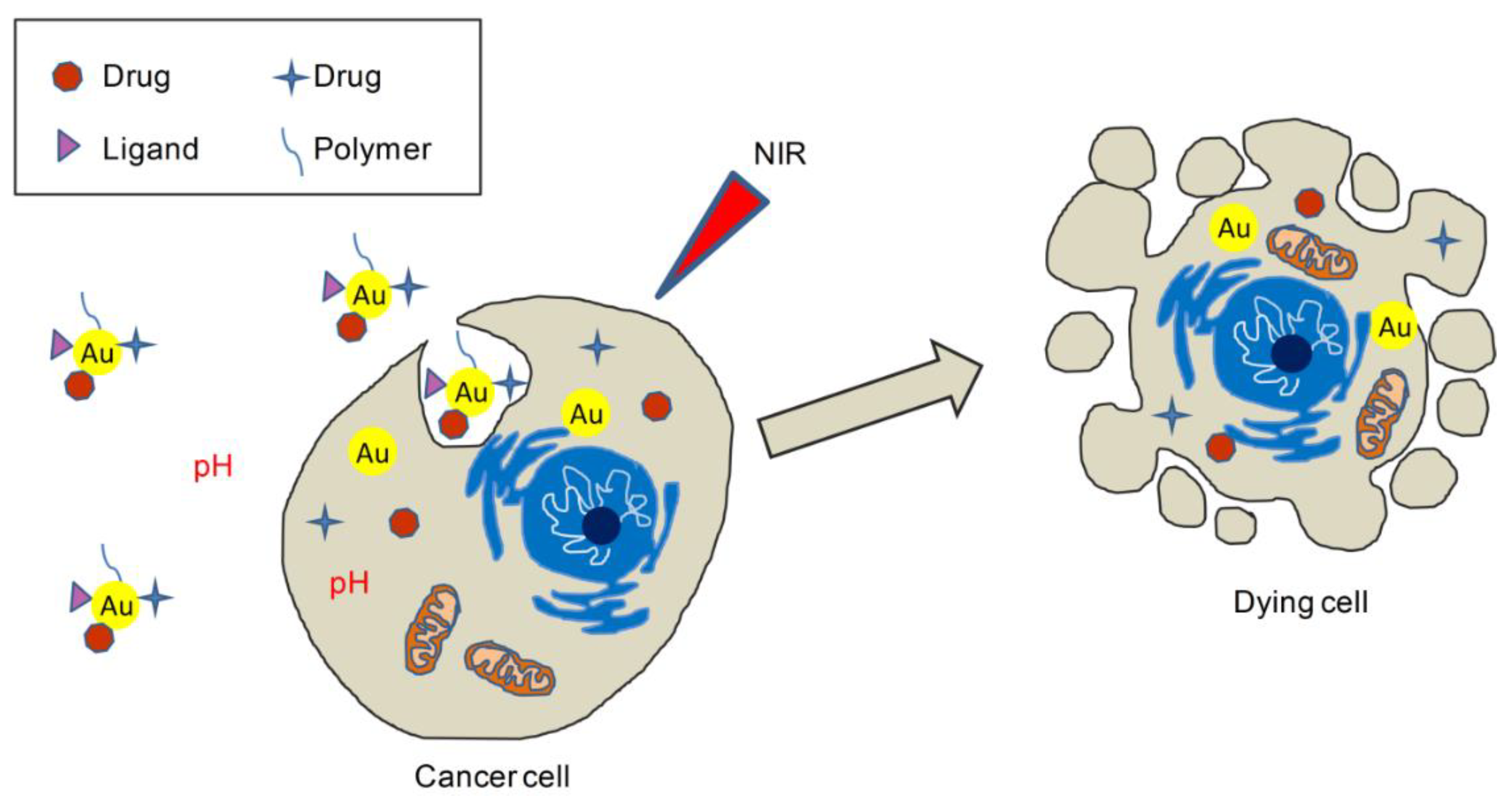
4. Gold-Based Hybrid Nanosystems
5. Perspectives and Conclusions
Funding
Conflicts of Interest
Abbreviations
| AuNP | gold nanoparticle |
| CTS | chitosan |
| Cur | curcumin |
| Cur@AuNP | Cur-containing AuNP |
| DDS | drug delivery system |
| DNMT | DNA methyltransferase |
| Dox | doxorubicin |
| DSS | dextran sodium sulfate |
| ECM | extracellular matrix |
| EGFR TK | epidermal growth factor receptor tyrosine kinase |
| ER | estrogen receptor |
| FA | folic acid |
| G | graphene |
| GO | graphene oxide |
| GSH | reduced glutathione |
| HSPC | hydrogenated soya phosphatidylcholine |
| LA | lipoic acid |
| Lip | liposome |
| MMP | matrix metalloproteinase |
| MRI | magnetic resonance imaging |
| MUC-1 | mucin-1 |
| NF-κB | nuclear factor-κB |
| NIR | near-infrared |
| NP | nanoparticle |
| PAMAM | poly(amidoamine) |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PS | polystyrene |
| PTT | photothermal therapy |
| PVP | polyvinylpyrrolidone |
| Res | resveratrol |
| rGO | reduced graphene oxide |
| ROS | reactive oxygen species |
| SAR | structure-activity relationship |
| SDT | sonodynamic treatment |
| STAT3 protein | signal transducer and activator of transcription 3 protein |
| TDD | targeted drug delivery |
| TPA | 12-O-tetradecanoylphorbol-13-acetate |
| TrxR | thioredoxin reductase |
| VEGF | vascular endothelial growth factor |
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 6 February 2021).
- Bredberg, A. Cancer: More of polygenic disease and less of multiple mutations? A quantitative viewpoint. Cancer 2011, 117, 440–445. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Piccolo, M.T.; Menale, C.; Crispi, S. Combined anticancer therapies: An overview of the latest applications. Anticancer Agents Med. Chem. 2015, 15, 408–422. [Google Scholar] [CrossRef]
- Choudhary, S.; Singh, P.K.; Verma, H.; Singh, H.; Silakari, O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018, 151, 62–97. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorg. Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Lu, J.; Lin, W. Hybrid nanoparticles for combination therapy of cancer. J. Control. Release 2015, 219, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2011, 12, 39–50. [Google Scholar] [CrossRef]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 14. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Zou, Y.; Hu, J.; Li, Y.; Cheng, Y. Natural polyphenols in drug delivery systems: Current status and future challenges. Giant 2020, 3, 100022. [Google Scholar] [CrossRef]
- Pavan, A.R.; Silva, G.D.; Jornada, D.H.; Chiba, D.E.; Fernandes, G.F.; Man Chin, C.; Dos Santos, J.L. Unraveling the Anticancer Effect of Curcumin and Resveratrol. Nutrients 2016, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The Basic Properties of Gold Nanoparticles and their Applications in Tumor Diagnosis and Treatment. Int. J. Mol. Sci. 2020, 21, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent Developments in the Facile Bio-Synthesis of Gold Nanoparticles (AuNPs) and Their Biomedical Applications. Int J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Cha, B.S.; Kim, S.; Park, K.S. Eco-friendly synthesis and biomedical applications of gold nanoparticles: A review. Microchem. J. 2020, 152, 104296. [Google Scholar] [CrossRef]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free Radicals as a Double-Edged Sword: The Cancer Preventive and Therapeutic Roles of Curcumin. Molecules 2020, 25, 22. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.H.; Sethi, G.; Um, J.Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulda, S.; Debatin, K.M. Resveratrol modulation of signal transduction in apoptosis and cell survival: A mini-review. Cancer Detect. Prev. 2006, 30, 217–223. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. A review of the chemopreventative and chemotherapeutic properties of the phytochemicals berberine, resveratrol and curcumin, and their influence on cell death via the pathways of apoptosis and autophagy. Cell Biol. Int. 2020, 44, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xia, J.; Gao, J.; Inagaki, Y.; Tang, W.; Kokudo, N. Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov. Ther. 2015, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Rana, P.; Shrama, A.; Mandal, C.C. Molecular insights into phytochemicals-driven break function in tumor microenvironment. J. Food Biochem. 2021, e13824. [Google Scholar]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, M.; Saravanan, C.; Singh, S.K. Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opin. Ther. Targets 2012, 16, 959–972. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, L.; Xie, J.; Chen, G.; Wang, F. Targeting miRNAs by natural products: A new way for cancer therapy. Biomed. Pharmacother. 2020, 130, 110546. [Google Scholar] [CrossRef]
- Avila-Carrasco, L.; Majano, P.; Sánchez-Toméro, J.A.; Selgas, R.; López-Cabrera, M.; Aguilera, A.; González Mateo, G. Natural Plants Compounds as Modulators of Epithelial-to-Mesenchymal Transition. Front. Pharmacol. 2019, 10, 715. [Google Scholar] [CrossRef] [Green Version]
- Benassi, R.; Ferrari, E.; Lazzari, S.; Spagnolo, F.; Saladini, M. Theoretical study on Curcumin: A comparison of calculated spectroscopic properties with NMR, UV–vis and IR experimental data. J. Mol. Struct. 2008, 892, 168–176. [Google Scholar] [CrossRef]
- Tonnesen, H.; Karlsen, J.; Mostad, A. Structural Studies of Curcuminoids. I. The Crystal Structure of Curcumin. Acta Chem. Scand. 1982, 36b, 475–479. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.; Rohani, S.; Gillies, E.R. Curcumin, a promising anti-cancer therapeutic: A review of its chemical properties, bioactivity and approaches to cancer cell delivery. RSC Adv. 2014, 4, 10815–10829. [Google Scholar] [CrossRef]
- Sultana, S.; Munir, N.; Mahmood, Z.; Riaz, M.; Akram, M.; Rebezov, M.; Kuderinova, N.; Moldabayeva, Z.; Shariati, M.A.; Rauf, A.; et al. Molecular targets for the management of cancer using Curcuma longa Linn. phytoconstituents: A Review. Biomed. Pharmacother. 2021, 135, 111078. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Grover, J.; Kania, M.; Jachak, S.M. Recent developments in chemistry and biology of curcumin analogues. RSC Adv. 2014, 4, 13946–13978. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.; Cao, J.; Li, J.; Wu, E.; Ma, Q.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Khalid, S.; Ahmad, A. Regulation of Cell Signaling Pathways and miRNAs by Resveratrol in Different Cancers. Int. J. Mol. Sci. 2018, 19, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Jo, H.; Cho, J.H.; Dhanasekaran, D.N.; Song, Y.S. Resveratrol as a Tumor-Suppressive Nutraceutical Modulating Tumor Microenvironment and Malignant Behaviors of Cancer. Int. J. Mol. Sci. 2019, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Zhu, W.; Feng, W.; Lee, S.S.; Leung, A.W.; Shen, J.; Gao, L.; Xu, C. A Review of Resveratrol as a Potent Chemoprotective and Synergistic Agent in Cancer Chemotherapy. Front. Pharmacol. 2018, 9, 1534. [Google Scholar] [CrossRef]
- He, S.; Yan, X. From resveratrol to its derivatives: New sources of natural antioxidant. Curr. Med. Chem. 2013, 20, 1005–1017. [Google Scholar] [PubMed]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic Versatility of Resveratrol Derivatives. Nutrients 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbo, B.D.; André-Miral, C.; Nasre-Nasser, R.G.; Schimith, L.E.; Santos, M.G.; Costa-Silva, D.; Muccillo-Baisch, A.L.; Hort, M.A. Resveratrol Derivatives as Potential Treatments for Alzheimer’s and Parkinson’s Disease. Front. Aging Neurosci. 2020, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef]
- Ivasiv, V.; Albertini, C.; Gonçalves, A.E.; Rossi, M.; Bolognesi, M.L. Molecular Hybridization as a Tool for Designing Multitarget Drug Candidates for Complex Diseases. Curr. Top. Med. Chem. 2019, 19, 1694–1711. [Google Scholar] [CrossRef]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs-A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 9. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Guo, Y.; Wang, Y.; Mao, F.; Huang, L.; Li, X. Design, synthesis, and biological evaluation of benzoselenazole-stilbene hybrids as multi-target-directed anti-cancer agents. Eur. J. Med. Chem. 2015, 95, 220–229. [Google Scholar] [CrossRef]
- Yin, Y.; Lian, B.P.; Xia, Y.Z.; Shao, Y.Y.; Kong, L.Y. Design, synthesis and biological evaluation of resveratrol-cinnamoyl derivates as tubulin polymerization inhibitors targeting the colchicine binding site. Bioorg. Chem. 2019, 93, 103319. [Google Scholar] [CrossRef]
- Aldawsari, F.S.; Aguayo-Ortiz, R.; Kapilashrami, K.; Yoo, J.; Luo, M.; Medina-Franco, J.L.; Velázquez-Martínez, C.A. Resveratrol-salicylate derivatives as selective DNMT3 inhibitors and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, W.; Yang, Y.; Ma, T.; Guo, J.; Wang, S.; Yu, W.; Kong, L. Discovery of oral-available resveratrol-caffeic acid based hybrids inhibiting acetylated and phosphorylated STAT3 protein. Eur. J. Med. Chem. 2016, 124, 1006–1018. [Google Scholar] [CrossRef]
- Ning, W.; Hu, Z.; Tang, C.; Yang, L.; Zhang, S.; Dong, C.; Huang, J.; Zhou, H.B. Novel Hybrid Conjugates with Dual Suppression of Estrogenic and Inflammatory Activities Display Significantly Improved Potency against Breast Cancer. J. Med. Chem. 2018, 61, 8155–8173. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Silva, M.; Coelho, L.F.; Guirelli, I.M.; Pereira, R.M.; Ferreira-Silva, G.; Graravelli, G.Y.; Horvath, R.O.; Caixeta, E.S.; Ionta, M.; Viegas, C. Synthetic resveratrol-curcumin hybrid derivative inhibits mitosis progression in estrogen positive MCF-7 breast cancer cells. Toxicol. In Vitro 2018, 50, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; Moreno, G.; Herrera-R, A.; Cardona-G, W. New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells. Molecules 2021, 26, 2661. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Pandya, V.; Bhullar, K.S.; Kerek, E.; Wong, Y.F.; Losch, R.; Ou, J.; Aldawsari, F.S.; Velazquez-Martinez, C.; Thiesen, A.; et al. Resveratrol and Resveratrol-Aspirin Hybrid Compounds as Potent Intestinal Anti-Inflammatory and Anti-Tumor Drugs. Molecules 2020, 25, 17. [Google Scholar] [CrossRef] [PubMed]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef]
- Ai, Y.; Zhu, B.; Ren, C.; Kang, F.; Li, J.; Huang, Z.; Lai, Y.; Peng, S.; Ding, K.; Tian, J.; et al. Discovery of New Monocarbonyl Ligustrazine-Curcumin Hybrids for Intervention of Drug-Sensitive and Drug-Resistant Lung Cancer. J. Med. Chem. 2016, 59, 1747–1760. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, M.; Nepali, K.; Gupta, M.K.; Saxena, A.K.; Sharma, S.; Bedi, P.M.S. Triazole tethered C5-curcuminoid-coumarin based molecular hybrids as novel antitubulin agents: Design, synthesis, biological investigation and docking studies. Eur. J. Med. Chem. 2016, 116, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gupta, M.K.; Saxena, A.K.; Bedi, P.M. Triazole linked mono carbonyl curcumin-isatin bifunctional hybrids as novel anti tubulin agents: Design, synthesis, biological evaluation and molecular modeling studies. Bioorg. Med. Chem. 2015, 23, 7165–7180. [Google Scholar] [CrossRef]
- Banuppriya, G.; Sribalan, R.; Padmini, V. Synthesis and characterization of curcumin-sulfonamide hybrids: Biological evaluation and molecular docking studies. J. Mol. Struct. 2018, 1155, 90–100. [Google Scholar] [CrossRef]
- Elmegeed, G.A.; Yahya, S.M.; Abd-Elhalim, M.M.; Mohamed, M.S.; Mohareb, R.M.; Elsayed, G.H. Evaluation of heterocyclic steroids and curcumin derivatives as anti-breast cancer agents: Studying the effect on apoptosis in MCF-7 breast cancer cells. Steroids 2016, 115, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.H.; Yu, K.; Zhang, X.; Chen, G.; Hoover, A.; Leon, F.; Wang, R.; Subrahmanyam, N.; Addo Mekuria, E.; Harinantenaina Rakotondraibe, L. A new class of hybrid anticancer agents inspired by the synergistic effects of curcumin and genistein: Design, synthesis, and anti-proliferative evaluation. Bioorg. Med. Chem. Lett. 2015, 25, 4553–4556. [Google Scholar] [CrossRef]
- Wang, J.Q.; Wang, X.; Wang, Y.; Tang, W.J.; Shi, J.B.; Liu, X.H. Novel curcumin analogue hybrids: Synthesis and anticancer activity. Eur. J. Med. Chem. 2018, 156, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Filippi, S.; Bizzarri, B.M.; Zippilli, C.; Meschini, R.; Pogni, R.; Baratto, M.C.; Villanova, L.; Saladino, R. Synthesis and Evaluation of Artemisinin-Based Hybrid and Dimer Derivatives as Antimelanoma Agents. ACS Omega 2020, 5, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zhang, D.; Chojnacki, J.; Du, Y.; Fu, H.; Grant, S.; Zhang, S. Design and biological characterization of hybrid compounds of curcumin and thalidomide for multiple myeloma. Org. Biomol. Chem. 2013, 11, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Song, B.A.; Zhao, H.J.; Qi, X.B.; Huang, Y.J.; Liu, X.H. Novel myricetin derivatives: Design, synthesis and anticancer activity. Eur. J. Med. Chem. 2015, 97, 155–163. [Google Scholar] [CrossRef]
- Dandriyal, J.; Singla, R.; Kumar, M.; Jaitak, V. Recent developments of C-4 substituted coumarin derivatives as anticancer agents. Eur. J. Med. Chem. 2016, 119, 141–168. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Emami, S. Recent advances of cytotoxic chalconoids targeting tubulin polymerization: Synthesis and biological activity. Eur. J. Med. Chem. 2016, 121, 610–639. [Google Scholar] [CrossRef]
- Bijian, K.; Zhang, Z.; Xu, B.; Jie, S.; Chen, B.; Wan, S.; Wu, J.; Jiang, T.; Alaoui-Jamali, M.A. Synthesis and biological activity of novel organoselenium derivatives targeting multiple kinases and capable of inhibiting cancer progression to metastases. Eur. J. Med. Chem. 2012, 48, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.K.; Chen, K.J.; Qian, Z.H.; Weng, W.L.; Qian, M.Y. Tetramethylpyrazine in the treatment of cardiovascular and cerebrovascular diseases. Planta Med. 1983, 47, 89. [Google Scholar]
- Fernandes, G.F.S.; Silva, G.D.B.; Pavan, A.R.; Chiba, D.E.; Chin, C.M.; Dos Santos, J.L. Epigenetic Regulatory Mechanisms Induced by Resveratrol. Nutrients 2017, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Pardoll, D.; Jove, R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer 2009, 9, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Cherubini, F.; Monteleone, G.; Stolfi, C. STAT3 Interactors as Potential Therapeutic Targets for Cancer Treatment. Int. J. Mol. Sci. 2018, 19, 6. [Google Scholar] [CrossRef] [Green Version]
- Mendelsohn, J.; Baselga, J. The EGF receptor family as targets for cancer therapy. Oncogene 2000, 19, 6550–6565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate 2010, 70, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Yu, J.; Lu, Y.; China, T.; Kumamoto, T.; Koseki, T.; Muto, S.; Horie, S. Testosterone augments polyphenol-induced DNA damage response in prostate cancer cell line, LNCaP. Cancer Sci. 2011, 102, 468–471. [Google Scholar] [CrossRef]
- Lai, H.C.; Singh, N.P.; Sasaki, T. Development of artemisinin compounds for cancer treatment. Investig. New Drugs 2013, 31, 230–246. [Google Scholar] [CrossRef]
- Biswas, D.K.; Singh, S.; Shi, Q.; Pardee, A.B.; Iglehart, J.D. Crossroads of estrogen receptor and NF-kappaB signaling. Sci. STKE 2005, 2005, pe27. [Google Scholar]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. Biofactors 2019, 45, 304–317. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin--synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef] [Green Version]
- Capek, I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. [Google Scholar] [CrossRef]
- Gupta, N.; Malviya, R. Understanding and advancement in gold nanoparticle targeted photothermal therapy of cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188532. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, J.Q.; Ashby, C.R., Jr.; Zeng, L.; Fan, Y.F.; Chen, Z.S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Mahato, K.; Nagpal, S.; Shah, M.A.; Srivastava, A.; Maurya, P.K.; Roy, S.; Jaiswal, A.; Singh, R.; Chandra, P. Gold nanoparticle surface engineering strategies and their applications in biomedicine and diagnostics. 3 Biotech. 2019, 9, 57. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Liu, R.; Liu, L.; Li, H.; Li, X.; Chen, Y.; Lu, C.; Liu, Y. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif Cells Nanomed. Biotechnol. 2019, 47, 4222–4233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashraf, R.; Amna, T.; Sheikh, F.A. Unique Properties of the Gold Nanoparticles: Synthesis, Functionalization and Applications. In Application of Nanotechnology in Biomedical Sciences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 75–98. [Google Scholar]
- Fadel, M.; Kassab, K.; Youssef, T.; El-Kholy, A.I. One-step synthesis of phyto-polymer coated gold nanospheres as a delivery system to enhance resveratrol cytotoxicity. Drug Dev. Ind. Pharm. 2019, 45, 937–945. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-Gold Loaded with Resveratrol Enhance the Anti-Hepatoma Effect of Resveratrol In Vitro and In Vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef]
- Park, S.Y.; Chae, S.Y.; Park, J.O.; Lee, K.J.; Park, G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol. Rep. 2016, 35, 3248–3256. [Google Scholar] [CrossRef]
- Thipe, V.C.; Panjtan Amiri, K.; Bloebaum, P.; Raphael Karikachery, A.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, R.K.; Thennarasu, S.; Mandal, A.B. Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surf. B. Biointerfaces 2014, 114, 138–143. [Google Scholar] [CrossRef]
- Tomoaia, G.; Horovitz, O.; Mocanu, A.; Nita, A.; Avram, A.; Racz, C.P.; Soritau, O.; Cenariu, M.; Tomoaia-Cotisel, M. Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids Surf. B. Biointerfaces 2015, 135, 726–734. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold nanoshell coated thermo-pH dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy. J. Mater. Chem B 2017, 5, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, Q.; Yu, T.; Li, X.; Gao, Y.; Li, J.; Liu, Y.; Rong, L.; Wang, Z.; Sun, H.; et al. Surfactant-Free Preparation of Au@Resveratrol Hollow Nanoparticles with Photothermal Performance and Antioxidant Activity. ACS Appl. Mater. Interfaces 2017, 9, 3376–3387. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Rengaraj, A.; Arivazhagan, R.; Huh, Y.S.; Yun, K. Curcumin-Conjugated Gold Clusters for Bioimaging and Anticancer Applications. Bioconjug. Chem. 2018, 29, 363–370. [Google Scholar] [CrossRef]
- Al-Ani, L.A.; Yehye, W.A.; Kadir, F.A.; Hashim, N.M.; AlSaadi, M.A.; Julkapli, N.M.; Hsiao, V.K.S. Hybrid nanocomposite curcumin-capped gold nanoparticle-reduced graphene oxide: Anti-oxidant potency and selective cancer cytotoxicity. PLoS ONE 2019, 14, e0216725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75. [Google Scholar] [CrossRef]
- Ghorbani, M.; Bigdeli, B.; Jalili-Baleh, L.; Baharifar, H.; Akrami, M.; Dehghani, S.; Goliaei, B.; Amani, A.; Lotfabadi, A.; Rashedi, H.; et al. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold-iron oxide nanocomposites: A pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur. J. Pharm. Sci. 2018, 114, 175–188. [Google Scholar] [CrossRef]
- Mathew, M.S.; Vinod, K.; Jayaram, P.S.; Jayasree, R.S.; Joseph, K. Improved Bioavailability of Curcumin in Gliadin-Protected Gold Quantum Cluster for Targeted Delivery. ACS Omega 2019, 4, 14169–14178. [Google Scholar] [CrossRef]
- Dai, M.; Frezzo, J.A.; Sharma, E.; Chen, R.; Singh, N.; Yuvienco, C.; Caglar, E.; Xiao, S.; Saxena, A.; Montclare, J.K. Engineered Protein Polymer-Gold Nanoparticle Hybrid Materials for Small Molecule Delivery. J. Nanomed Nanotechnol 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekmohammadi, S.; Hadadzadeh, H.; Farrokhpour, H.; Amirghofran, Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: A new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter 2018, 14, 2400–2410. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int. J. Biol. Macromol. 2018, 112, 119–125. [Google Scholar] [CrossRef]
- Alvi, S.B.; Appidi, T.; Deepak, B.P.; Rajalakshmi, P.S.; Minhas, G.; Singh, S.P.; Begum, A.; Bantal, V.; Srivastava, R.; Khan, N.; et al. The “nano to micro” transition of hydrophobic curcumin crystals leading to in situ adjuvant depots for Au-liposome nanoparticle mediated enhanced photothermal therapy. Biomater. Sci. 2019, 7, 3866–3875. [Google Scholar] [CrossRef]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR triggered liposome gold nanoparticles entrapping curcumin as in situ adjuvant for photothermal treatment of skin cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef]
- Kayani, Z.; Dehdari Vais, R.; Soratijahromi, E.; Mohammadi, S.; Sattarahmady, N. Curcumin-gold-polyethylene glycol nanoparticles as a nanosensitizer for photothermal and sonodynamic therapies: In vitro and animal model studies. Photodiagn. Photodyn. Ther. 2021, 33, 102139. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, F.; Azarpira, N.; Sattarahmady, N. Evaluation of a nanocomposite of PEG-curcumin-gold nanoparticles as a near-infrared photothermal agent: An in vitro and animal model investigation. Lasers Med. Sci. 2018, 33, 1769–1779. [Google Scholar] [CrossRef]
- Rahimi-Moghaddam, F.; Sattarahmady, N.; Azarpira, N. Gold-Curcumin Nanostructure in Photo-thermal Therapy on Breast Cancer Cell Line: 650 and 808 nm Diode Lasers as Light Sources. J. Biomed. Phys. Eng. 2019, 9, 473–482. [Google Scholar]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int J. Nanomedicine 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, G.; Chen, J.; Fang, G. Green Synthesis and Characterization of Gold Nanoparticles Using Lignin Nanoparticles. Nanomaterials 2020, 10, 1869. [Google Scholar] [CrossRef]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 2017, 3, 516–527. [Google Scholar]
- Teimuri-mofrad, R.; Hadi, R.; Tahmasebi, B.; Farhoudian, S.; Mehravar, M.; Nasiri, R. Green synthesis of gold nanoparticles using plant extract: Mini-review. Nanochem. Res. 2017, 2, 8–19. [Google Scholar]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, K.; Rajaram, A.; Sreeram, K.J.; Rajaram, R. Curcumin conjugated gold nanoparticle synthesis and its biocompatibility. RSC Adv. 2014, 4, 1808–1818. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, C.; Bonacchi, D.; Lorenzi, G.; Hermanns, M.I.; Pohl, C.; Baldi, G.; Unger, R.E.; Kirkpatrick, C.J. Gold nanoparticles induce cytotoxicity in the alveolar type-II cell lines A549 and NCIH441. Part. Fibre Toxicol. 2009, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Freese, C.; Uboldi, C.; Gibson, M.I.; Unger, R.E.; Weksler, B.B.; Romero, I.A.; Couraud, P.O.; Kirkpatrick, C.J. Uptake and cytotoxicity of citrate-coated gold nanospheres: Comparative studies on human endothelial and epithelial cells. Part. Fibre Toxicol. 2012, 9, 23. [Google Scholar] [CrossRef]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, C.J.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
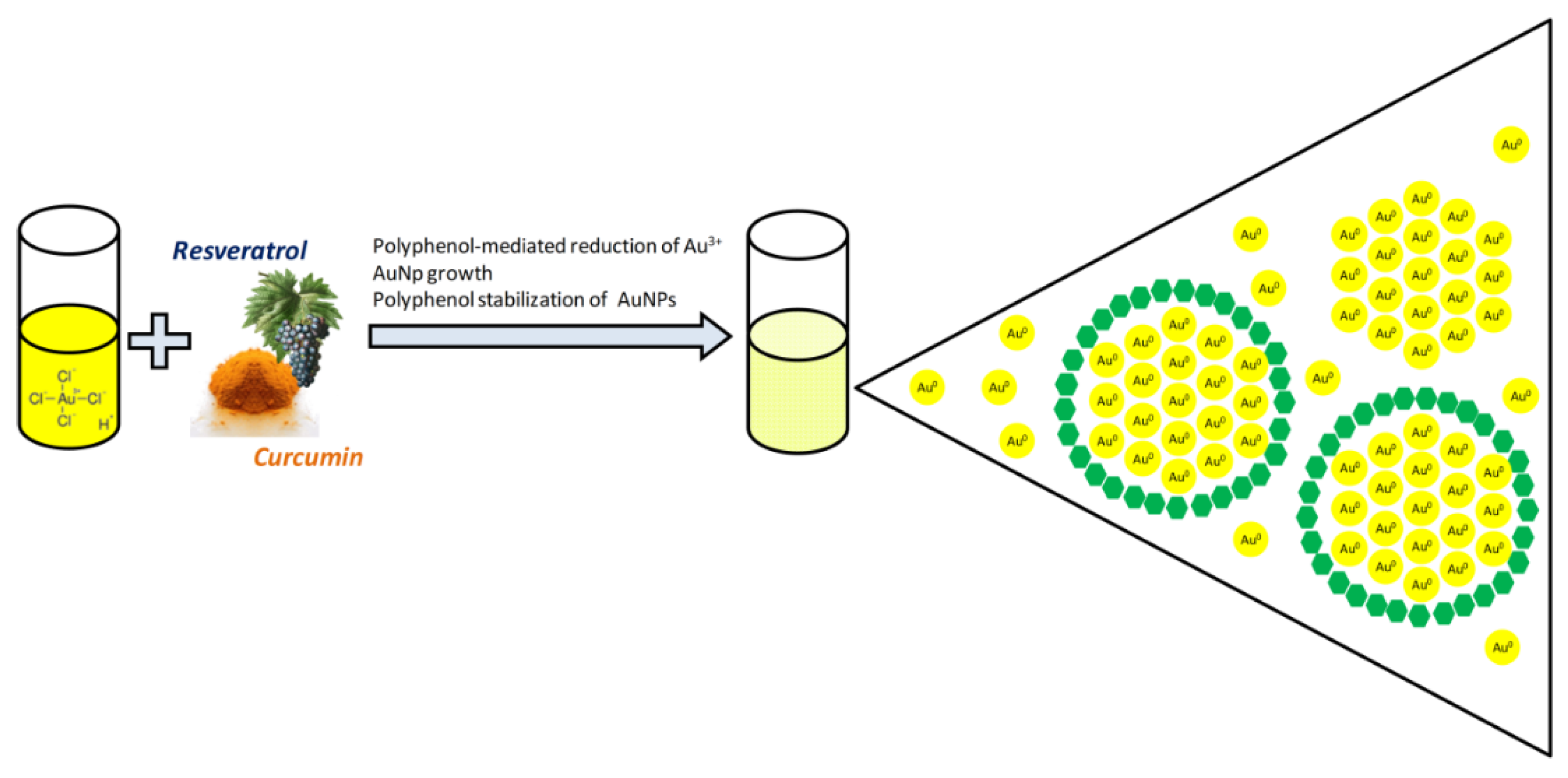
- Sanna, V.; Pala, N.; Dessì, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. (Hoboken) 2021, e1353. [Google Scholar]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic photothermal therapy (PPTT) using gold nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Tamura, A.; Murotani, H.; Oishi, M.; Jinji, Y.; Matsuishi, K.; Nagasaki, Y. Large payloads of gold nanoparticles into the polyamine network core of stimuli-responsive PEGylated nanogels for selective and noninvasive cancer photothermal therapy. Nanoscale 2010, 2, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.M.; Kim, E.B.; Hyun, M.S.; Kim, B.B.; Park, T.J. Self-assembly of biogenic gold nanoparticles and their use to enhance drug delivery into cells. Colloids Surf. B. Biointerfaces 2015, 135, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, X.N.; Chen, J.; Wang, W.X.; Lu, Z.F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Zhang, D.D. Resveratrol, a polyphenol phytoalexin, protects against doxorubicin-induced cardiotoxicity. J. Cell. Mol. Med. 2015, 19, 2324–2328. [Google Scholar] [CrossRef]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, P.; Duan, H.; Chen, X. Plasmonic Vesicles of Amphiphilic Nanocrystals: Optically Active Multifunctional Platform for Cancer Diagnosis and Therapy. Acc. Chem. Res. 2015, 48, 2506–2515. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Malinick, A.S.; Yang, T.; Cheng, W.; Cheng, Q. Gold nanoparticle-coupled liposomes for enhanced plasmonic biosensing. Sens. Actuators Rep. 2020, 2, 100023. [Google Scholar] [CrossRef]
- Huo, M.; Chen, Y.; Shi, J. Triggered-release drug delivery nanosystems for cancer therapy by intravenous injection: Where are we now? Expert Opin. Drug Deliv. 2016, 13, 1195–1198. [Google Scholar] [CrossRef]
- Moussa, H.G.; Martins, A.M.; Husseini, G.A. Review on triggered liposomal drug delivery with a focus on ultrasound. Curr. Cancer Drug Targets 2015, 15, 282–313. [Google Scholar] [CrossRef]
- Butt, A.M.; Amin, M.C.; Katas, H.; Abdul Murad, N.A.; Jamal, R.; Kesharwani, P. Doxorubicin and siRNA Codelivery via Chitosan-Coated pH-Responsive Mixed Micellar Polyplexes for Enhanced Cancer Therapy in Multidrug-Resistant Tumors. Mol. Pharm. 2016, 13, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ye, F.; Hu, M.; Yin, P.; Zhang, W.; Li, Y.; Yu, X.; Deng, Y. Influence of phospholipid types and animal models on the accelerated blood clearance phenomenon of PEGylated liposomes upon repeated injection. Drug Deliv. 2015, 22, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Soucy, J.R.; Hebert, J.; Auguste, D.T. Advances in Receptor-Mediated, Tumor-Targeted Drug Delivery. Adv. Ther. 2019, 2, 1800091. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Mohanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304. [Google Scholar] [CrossRef]
- Joye, I.J.; Davidov-Pardo, G.; Ludescher, R.D.; McClements, D.J. Fluorescence quenching study of resveratrol binding to zein and gliadin: Towards a more rational approach to resveratrol encapsulation using water-insoluble proteins. Food Chem. 2015, 185, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sharma, A.K.; Kumar, P. Nanoscale Self-Assembly for Therapeutic Delivery. Front. Bioeng. Biotechnol. 2020, 8, 127. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wei, P.; Zhou, Z.; Wei, T. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights. Adv. Drug Deliv Rev. 2016, 105 Pt B, 145–162. [Google Scholar] [CrossRef] [Green Version]
- Neri, G.; Micale, N.; Scala, A.; Fazio, E.; Mazzaglia, A.; Mineo, P.G.; Montesi, M.; Panseri, S.; Tampieri, A.; Grassi, G.; et al. Silibinin-conjugated graphene nanoplatform: Synthesis, characterization and biological evaluation. FlatChem 2017, 1, 34–41. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Gurunathan, S.; Kim, J.-H. Graphene Oxide–Silver Nanocomposite Enhances Cytotoxic and Apoptotic Potential of Salinomycin in Human Ovarian Cancer Stem Cells (OvCSCs): A Novel Approach for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 710. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramazani, A.; Abrvash, M.; Sadighian, S.; Rostamizadeh, K.; Fathi, M. Preparation and characterization of curcumin loaded gold/graphene oxide nanocomposite for potential breast cancer therapy. Res. Chem. Intermed. 2018, 44, 7891–7904. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hadadzadeh, H.; Farrokhpour, H.; Haghighi, F.H.; Abyar, F.; Mirahmadi-Zare, S.Z. In situ generation of the gold nanoparticles–bovine serum albumin (AuNPs–BSA) bioconjugated system using pulsed-laser ablation (PLA). Mater. Chem. Phys. 2016, 177, 360–370. [Google Scholar] [CrossRef]
- Leung, K.C.; Xuan, S.; Zhu, X.; Wang, D.; Chak, C.P.; Lee, S.F.; Ho, W.K.; Chung, B.C. Gold and iron oxide hybrid nanocomposite materials. Chem. Soc. Rev. 2012, 41, 1911–1928. [Google Scholar] [CrossRef]
- Maczurek, A.; Hager, K.; Kenklies, M.; Sharman, M.; Martins, R.; Engel, J.; Carlson, D.A.; Münch, G. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 6. [Google Scholar] [CrossRef] [Green Version]
- Donelli, D.; Antonelli, M.; Firenzuoli, F. Considerations about turmeric-associated hepatotoxicity following a series of cases occurred in Italy: Is turmeric really a new hepatotoxic substance? Intern. Emerg. Med. 2020, 15, 725–726. [Google Scholar] [CrossRef] [PubMed]
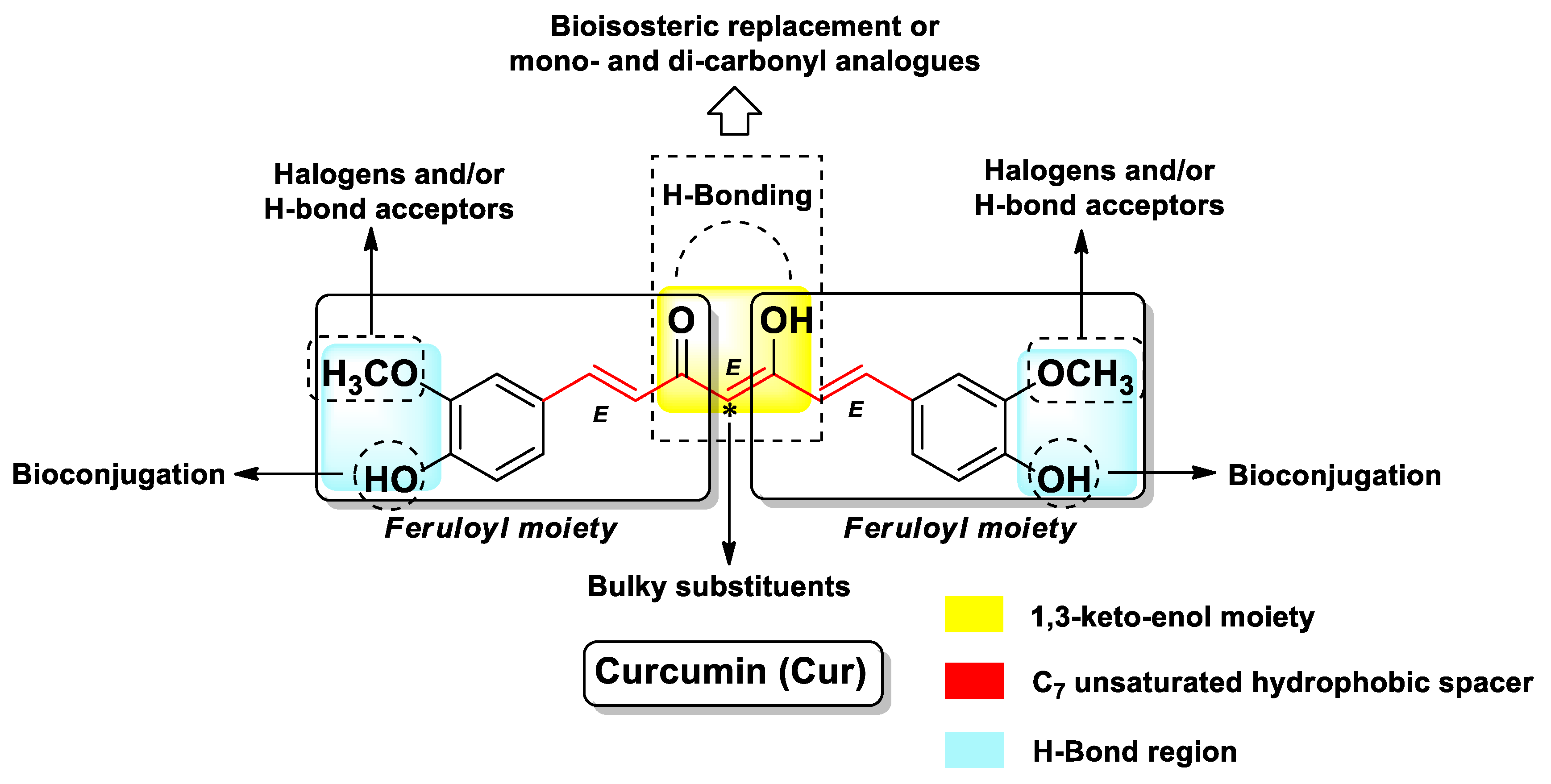
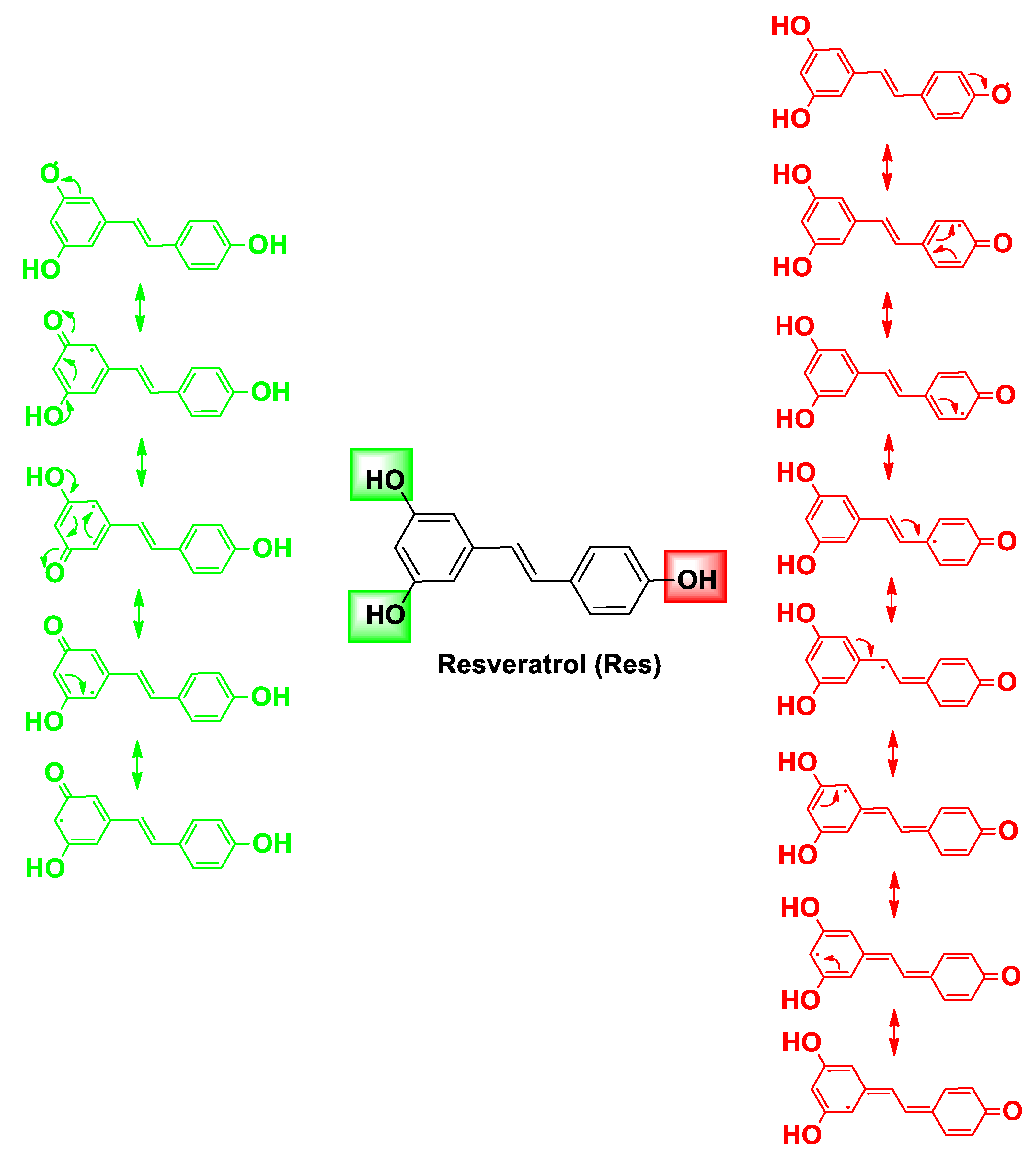
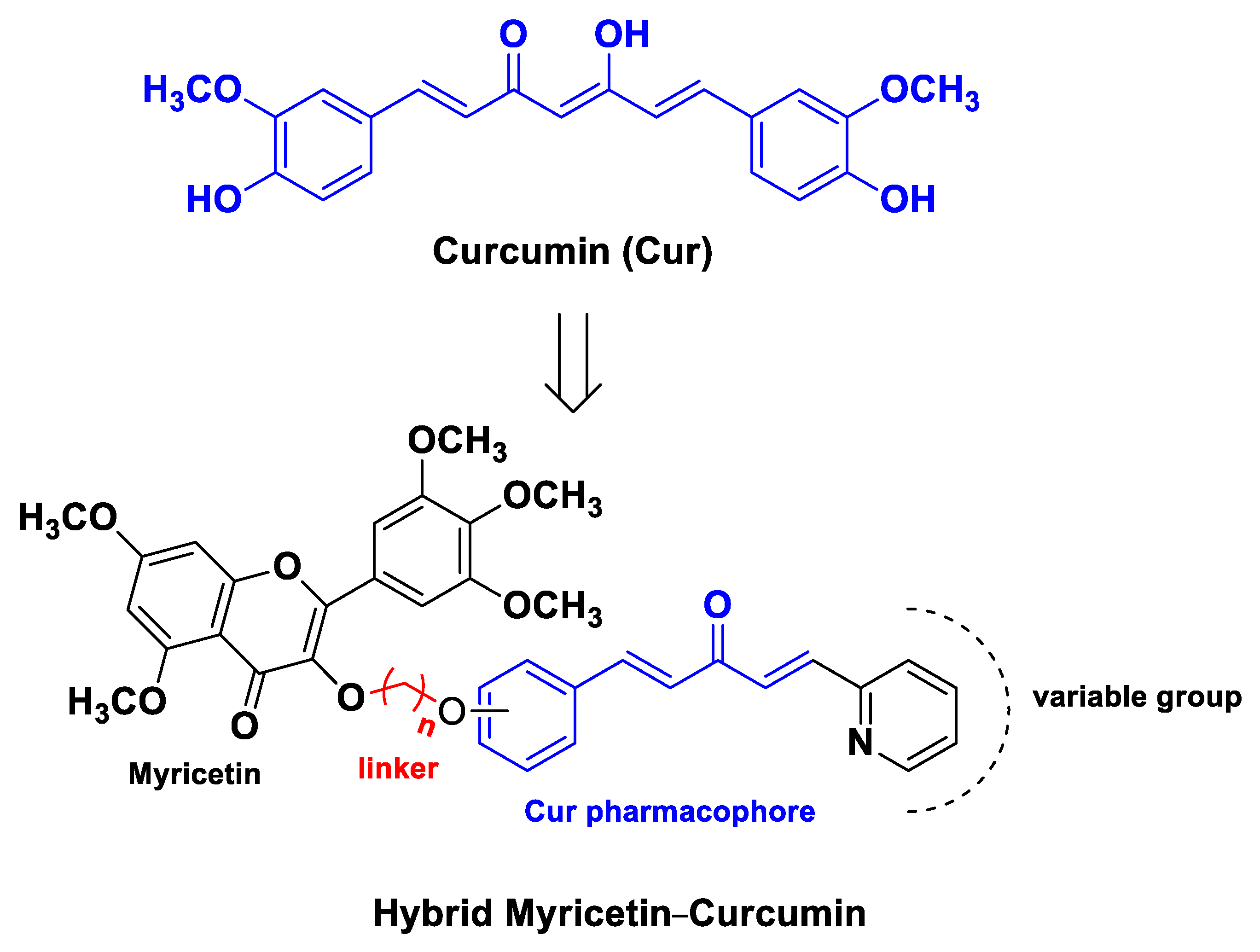
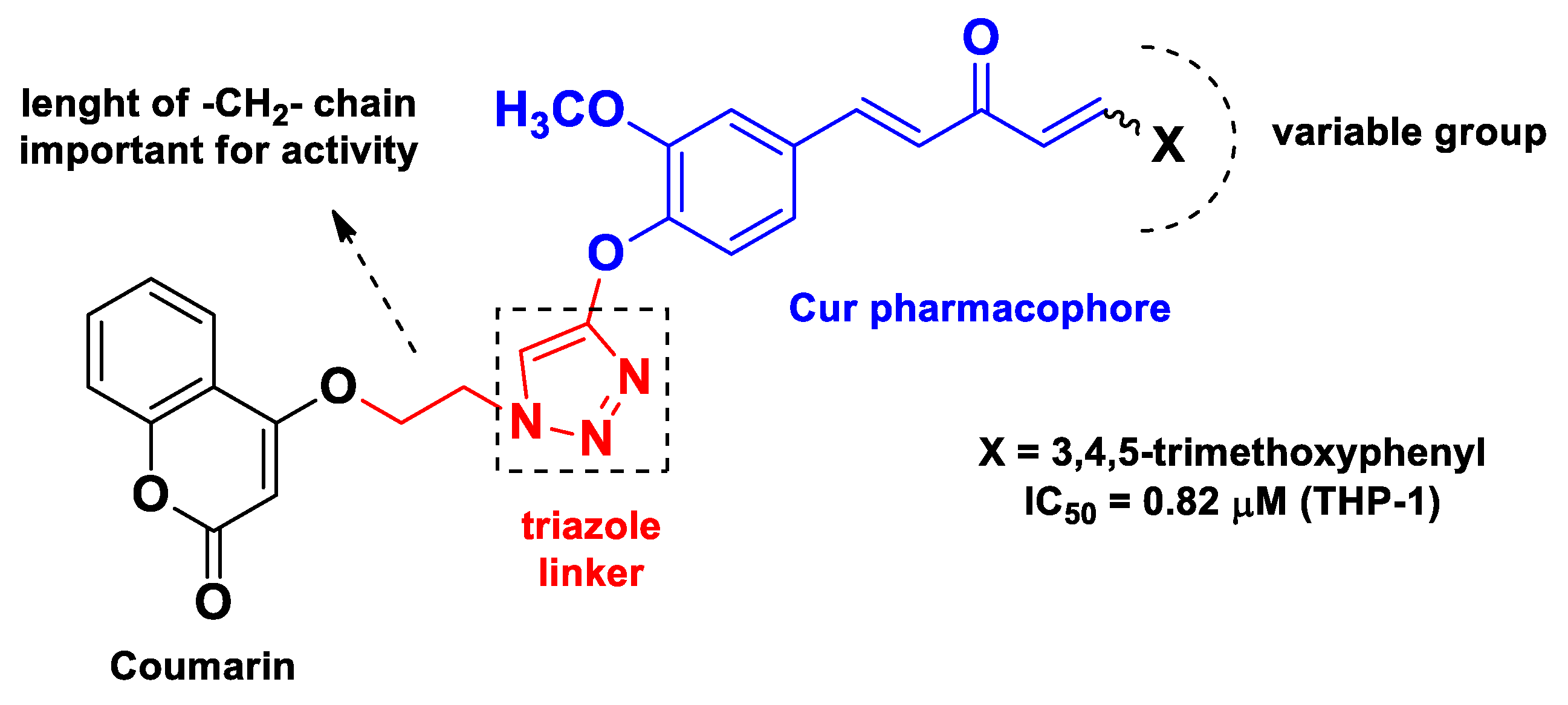
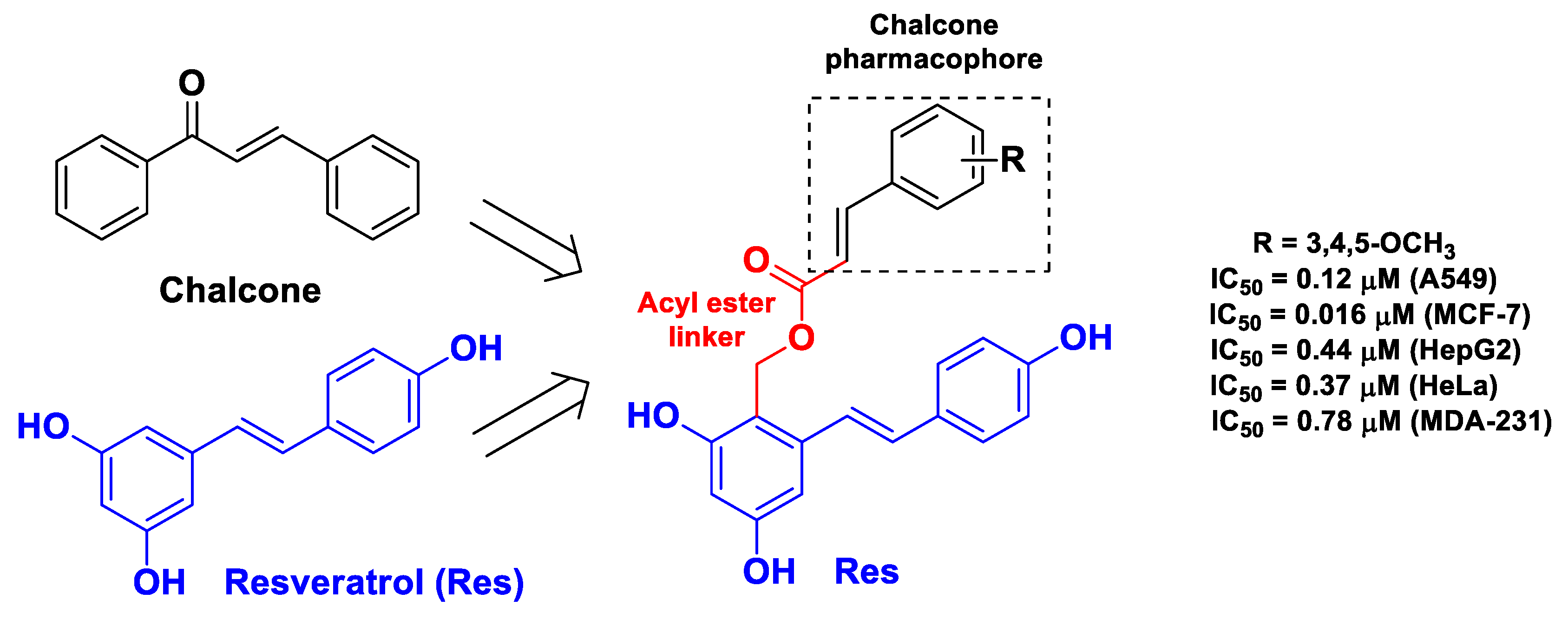


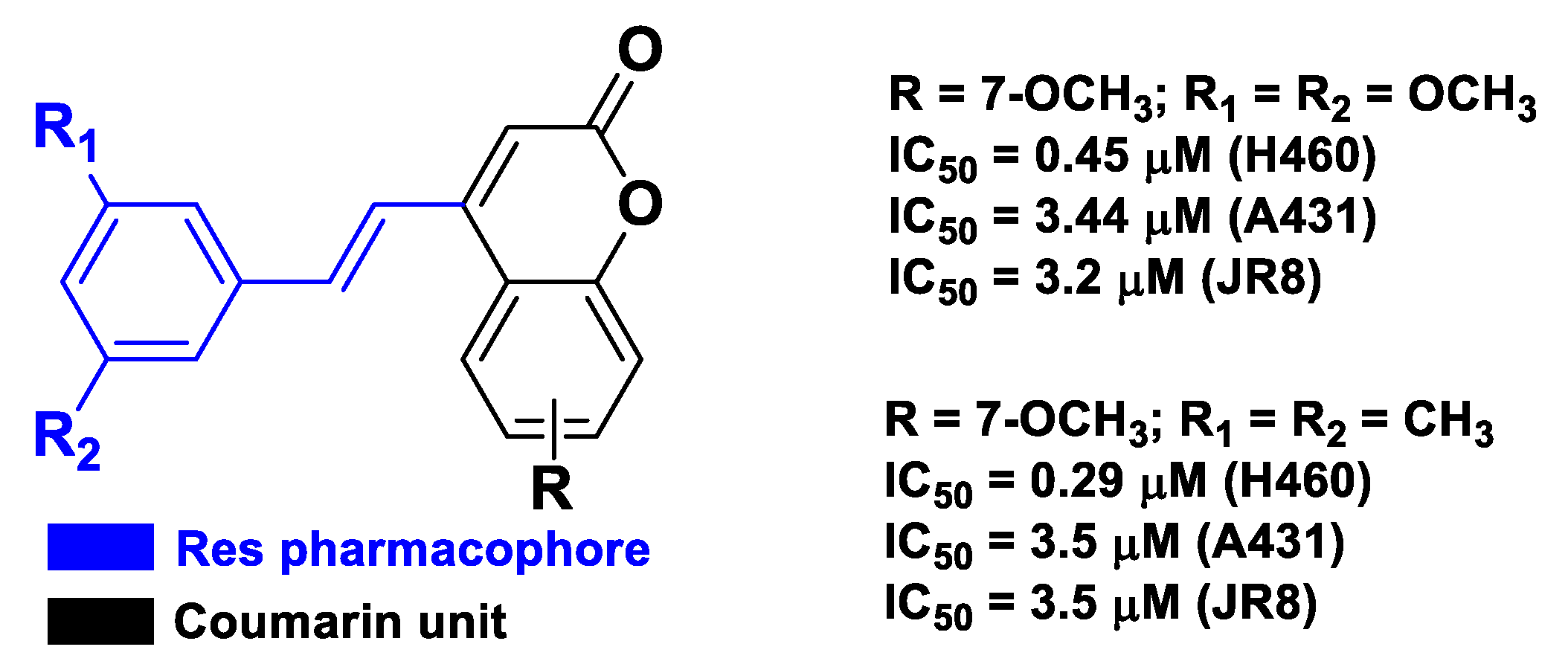
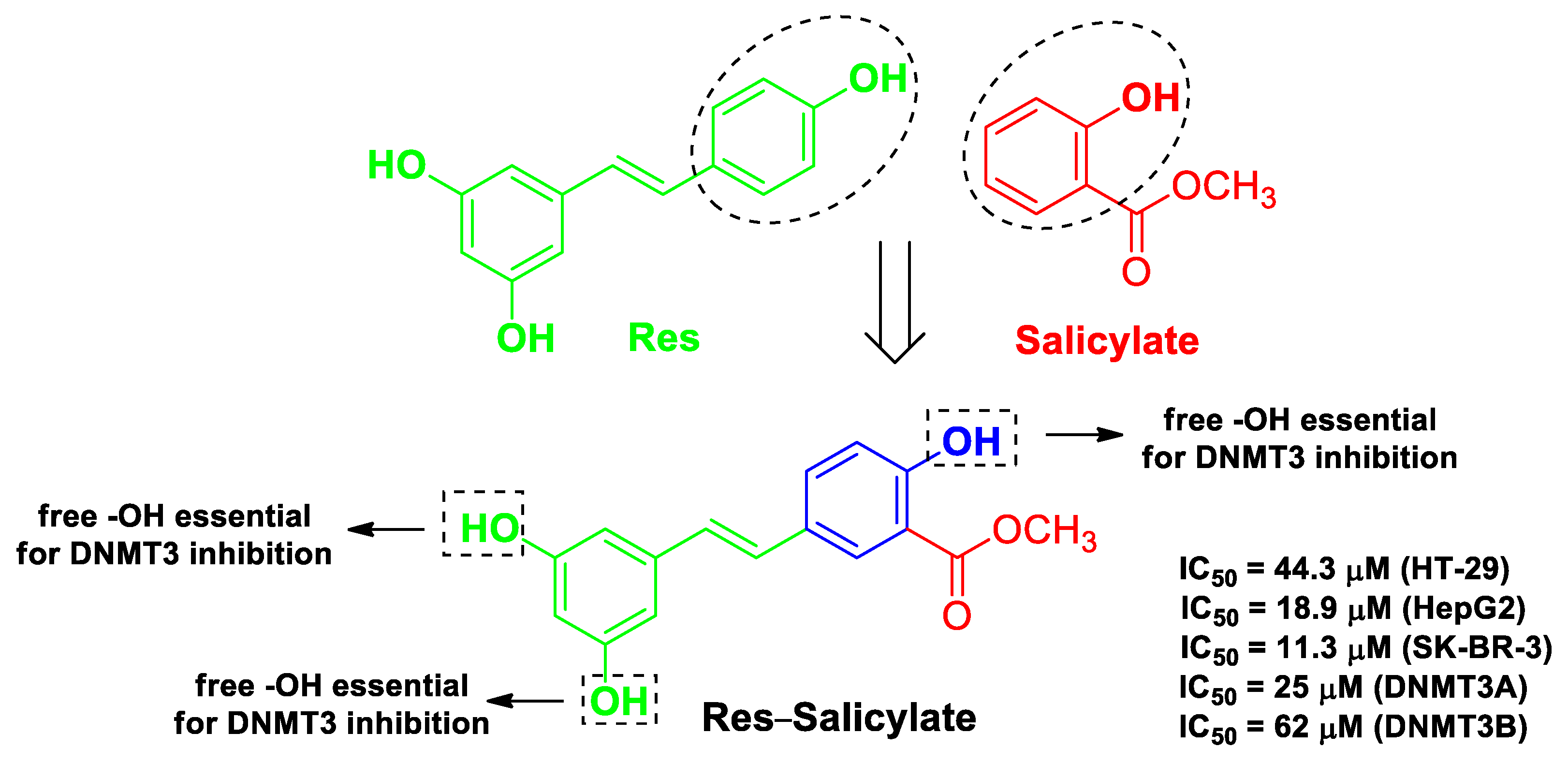
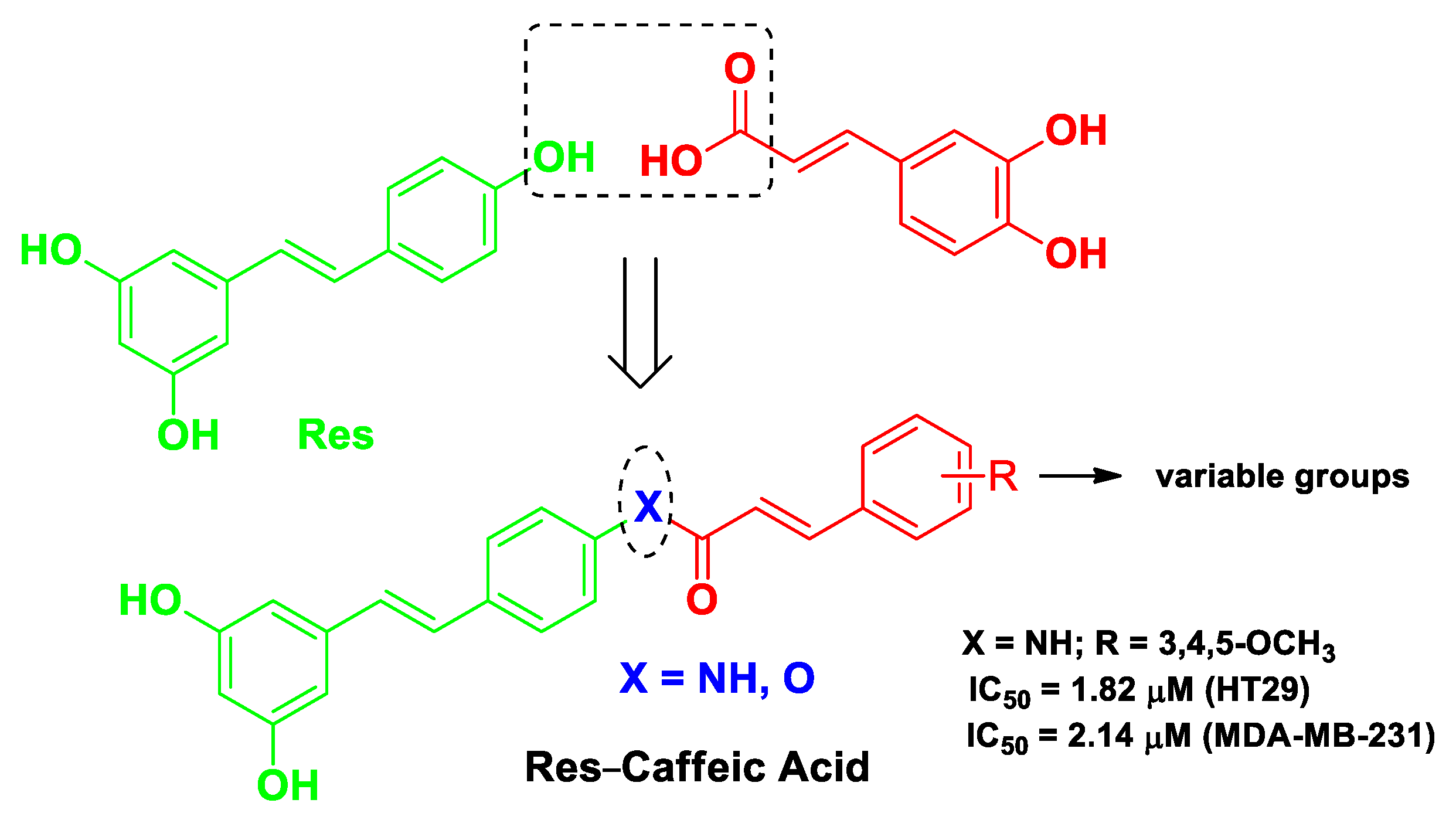

| Pharmacophore 1 | Pharmacophore 2 | Hybrids | Pathology | In Vitro Cell-Free or In Silico Model | In Vitro Cell-Based Model | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|---|
| Res | ebselen | Benzoselenazole–stilbene hybrids | cancer | inhibition of TrxR | human liver carcinoma Bel-7402 | [48] | |
| Res | cinnamic acid | Res linked to cinnamic acid through an acyl ester group | cancer | tubulin polymerization assay; molecular docking with tubulin | lung cancer A549, MCF-7, hepatoma HepG2, cervical cancer HeLa, and breast cancer MDA-231 cells | [49] | |
| Res | salicylate | addition of a carboxylic acid or its methyl ester attached ortho to one of the phenol groups present in hydroxystilbene | cancer | molecular docking with human DNMT | human colorectal adenocarcinoma HT-29, hepatoma HepG2 cells, and mammary gland/breast SK-BR-3 cells | [50] | |
| Res | caffeic acid | Res-caffeic acid hybrids possessing an amide linker or an ester linkage | breast cancer | molecular docking with STAT3 protein | human breast cancer MDA-MB-231 cells and colonic carcinoma HT29 cells | female Kunming mice bearing breast cancer 4T1 cells | [51] |
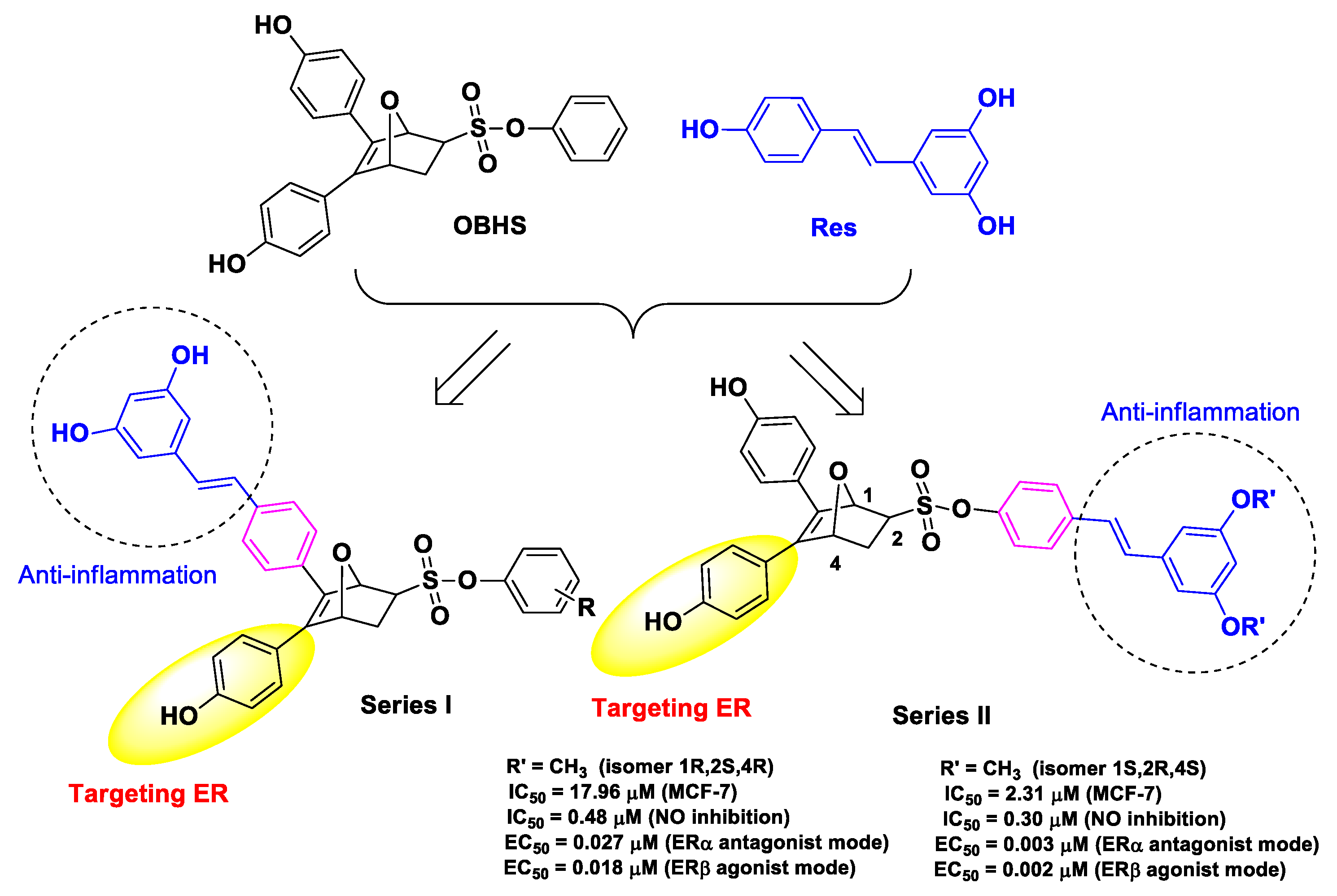
| Res | obhs | conjugation of Res with OBHS | breast cancer | estrogen receptor ERα antagonistic activity | human breast cancer MCF-7 cells | female Balb/c nude mice inoculated with MCF-7 breast cancer cells | [52] |
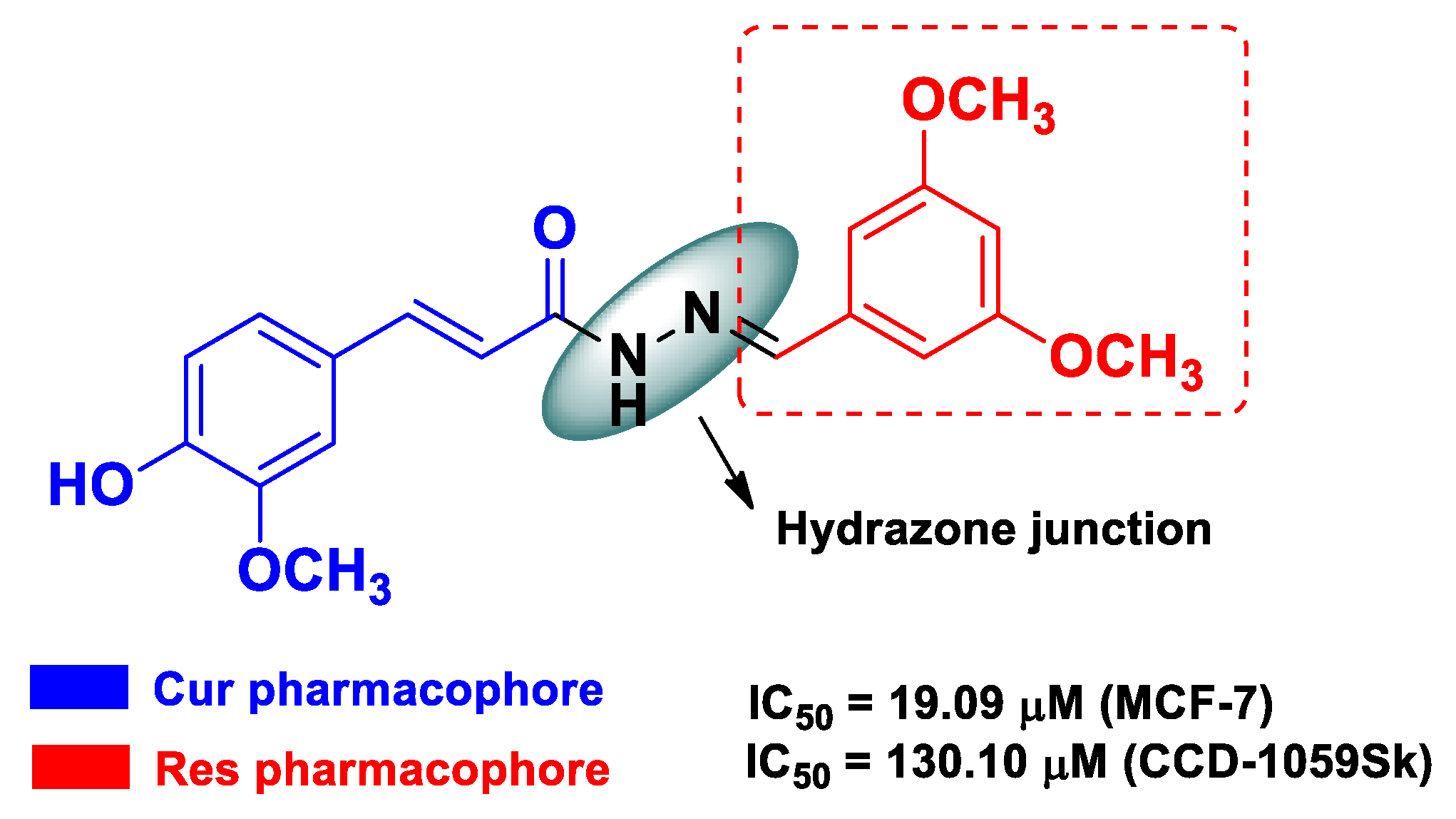
| Res | Cur | an o-substituted conjugated-phenyl system from Res linked to a 3-methoxy-4-hydroxycynamoil subunit, with a hydrazone functionality as a spacer | breast cancer | estrogen-positive human breast cancer MCF-7 cells | [53] | ||
| Res | Cur | Res linked to a 3-methoxy-4-hydroxycynamoil subunit from Cur | colonic cancer | human colon adenocarcinoma SW480 and SW620 cells | [54] | ||
| Res | Aspirin | addition of a carboxylic acid group adjacently to one of the phenols in the Res structure | colonic cancer and intestinal inflammation | normal mouse intestinal ModeK cells and human colon cancer HCT116 cells | C57BL/6 mice bearing HCT116 colon cancer cells; DSS-induced colitis in male C57BL/6 mice | [55] | |
| Res | coumarin | a substituted trans-vinylbenzene moiety on a coumarin backbone | cancer | human lung carcinoma H460, squamous carcinoma A431, and melanoma JR8 cells | [56] |
| Pharmacophore 1 | Pharmacophore 2 | Hybrids | Pathology | In Vitro Cell-Free or In Silico Model | In Vitro Cell-Based Model | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|---|
| Cur | ligustrazine | substituting one of the two aromatic rings of Cur analogs with ligustrazine | lung cancer | inhibition of TrxR | human lung cancer A549, drug-resistant human lung cancer A549/DDP cells | athymic BALB/c nude mice inoculated with A549/DDP cells | [57] |
| Cur | coumarin | monocarbonyl Cur linked to coumarin with a trizole as spacer | cancer | molecular docking with tubulin | human leukemia THP-1, colon adenocarcinoma COLO-205, colorectal cancer HCT-116 cells | [58] | |
| Cur | Isatin | monocarbonyl Cur linked to isatin with a trizole as a spacer | cancer | tubulin polymerization assay; molecular docking with tubulin | human leukemia THP-1, colon adenocarcinoma COLO-205, colorectal cancer HCT-116, prostate cancer PC-3 cells | [59] | |
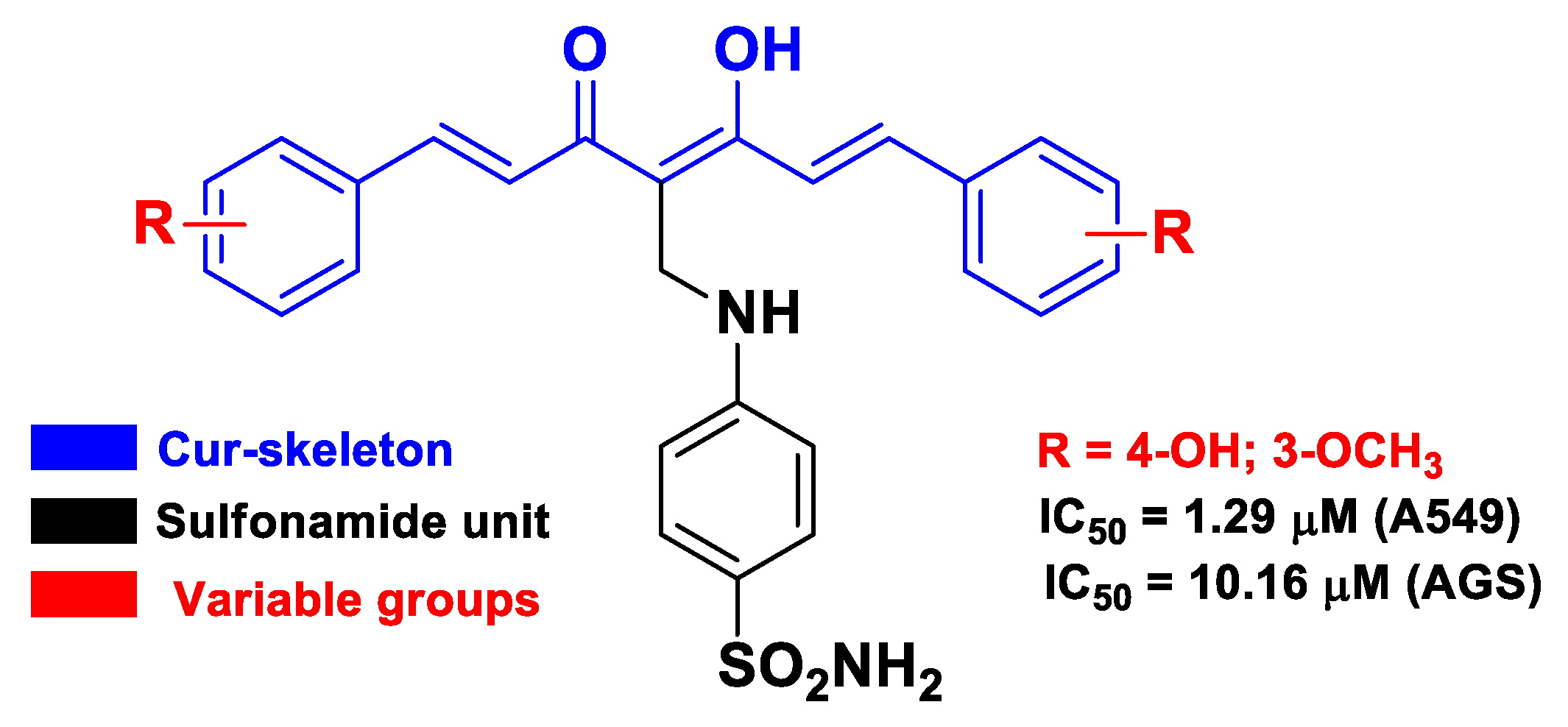
| Cur | sulfonamide | introduction of sulfanilamide unit into the methylene part of Cur | cancer | molecular docking with EGFR TK | human gastric adenocarcinoma AGS and lung cancer A549 cells | [60] | |
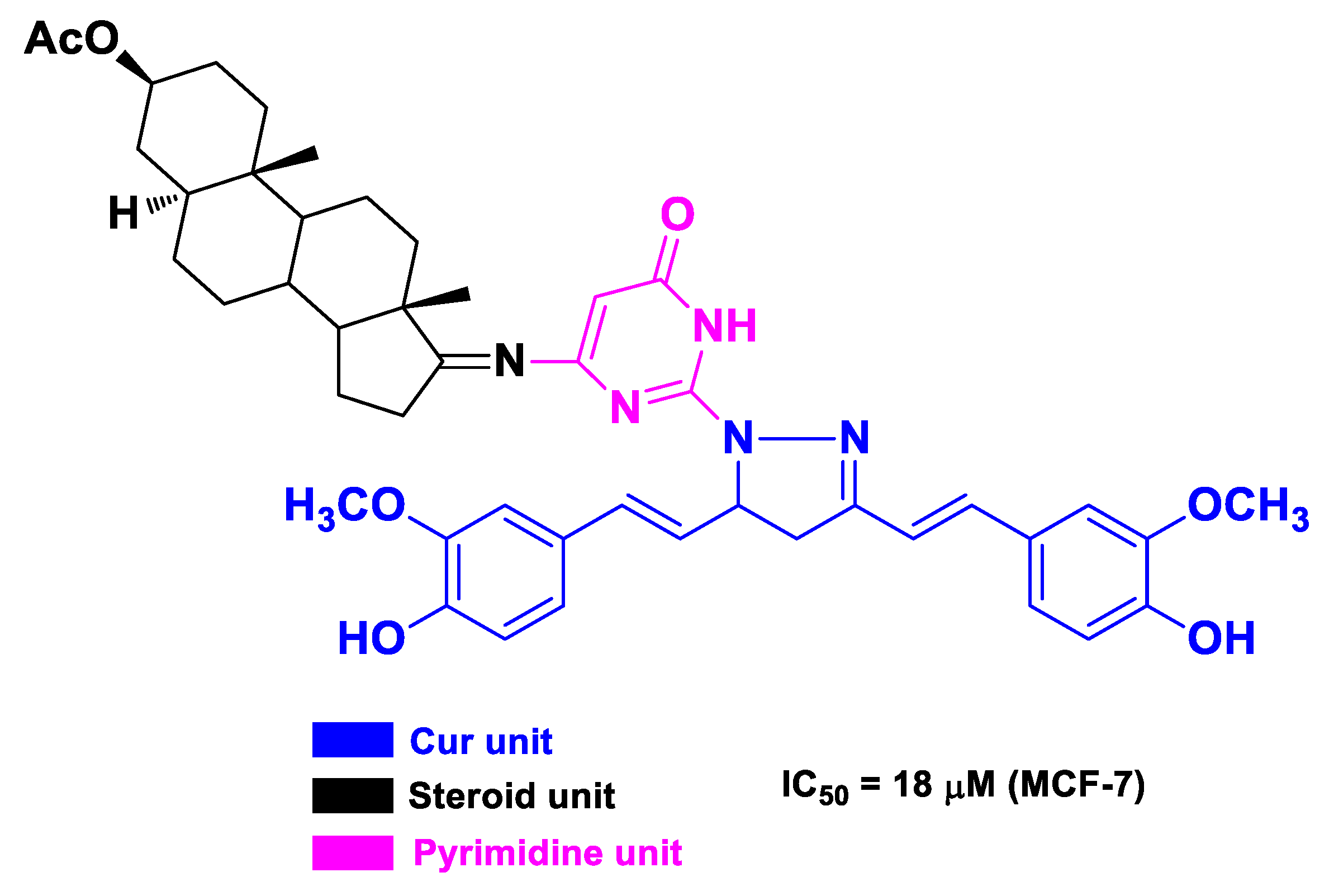
| Cur | steroids | pyrazolocurcumin-pyrimidinyl androstane derivative | breast cancer | human breast cancer MCF-7 cells | [61] | ||
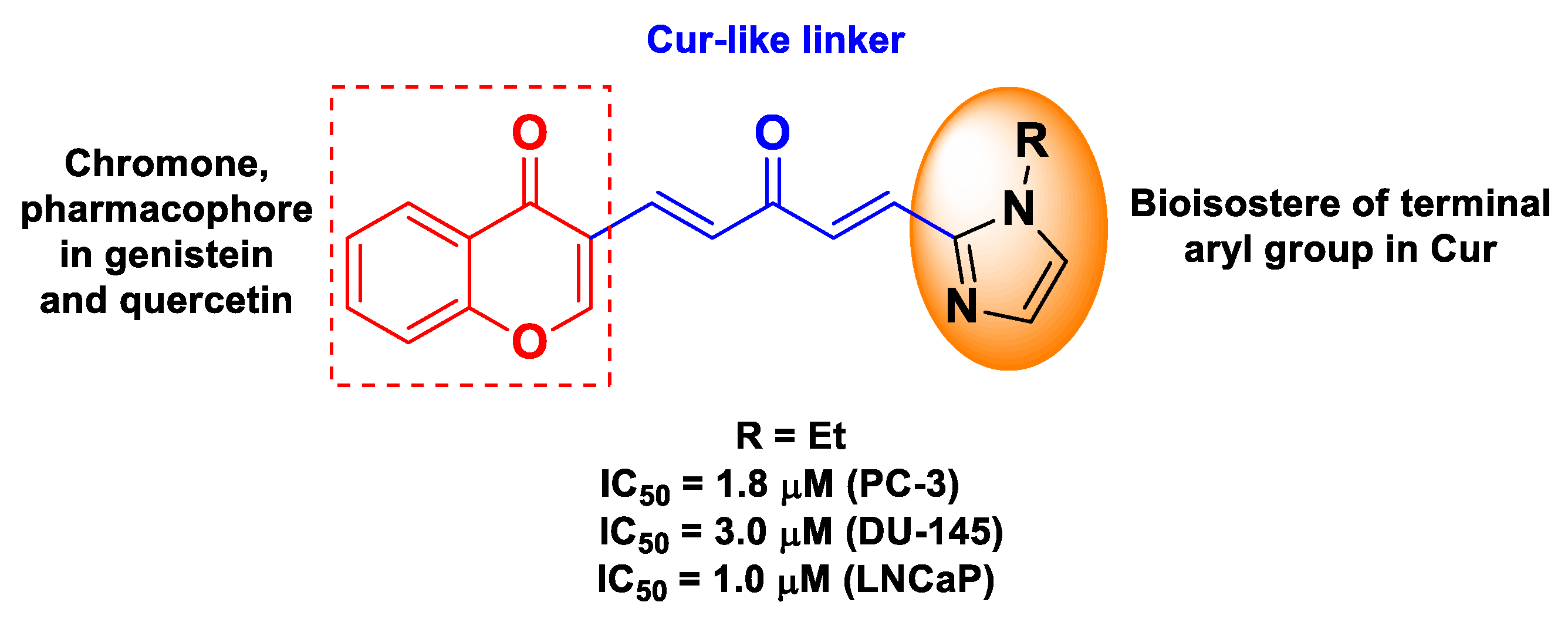
| Cur | quercetin or genistein | ester of (1E,4E)-1,4-penta-dien-3-one (from Cur) and chromone (from quercetin or genistein) | prostate cancer | human prostate cancer androgen-independent PC-3b and DU-145 cells, and androgen-dependent LNCaP cells | [62] | ||
| Cur | myricetin | monocarbonyl analogs of curcumin linked to myricetin | gastric cancer | human gastric cancer SGC-7901 cells | [63] | ||
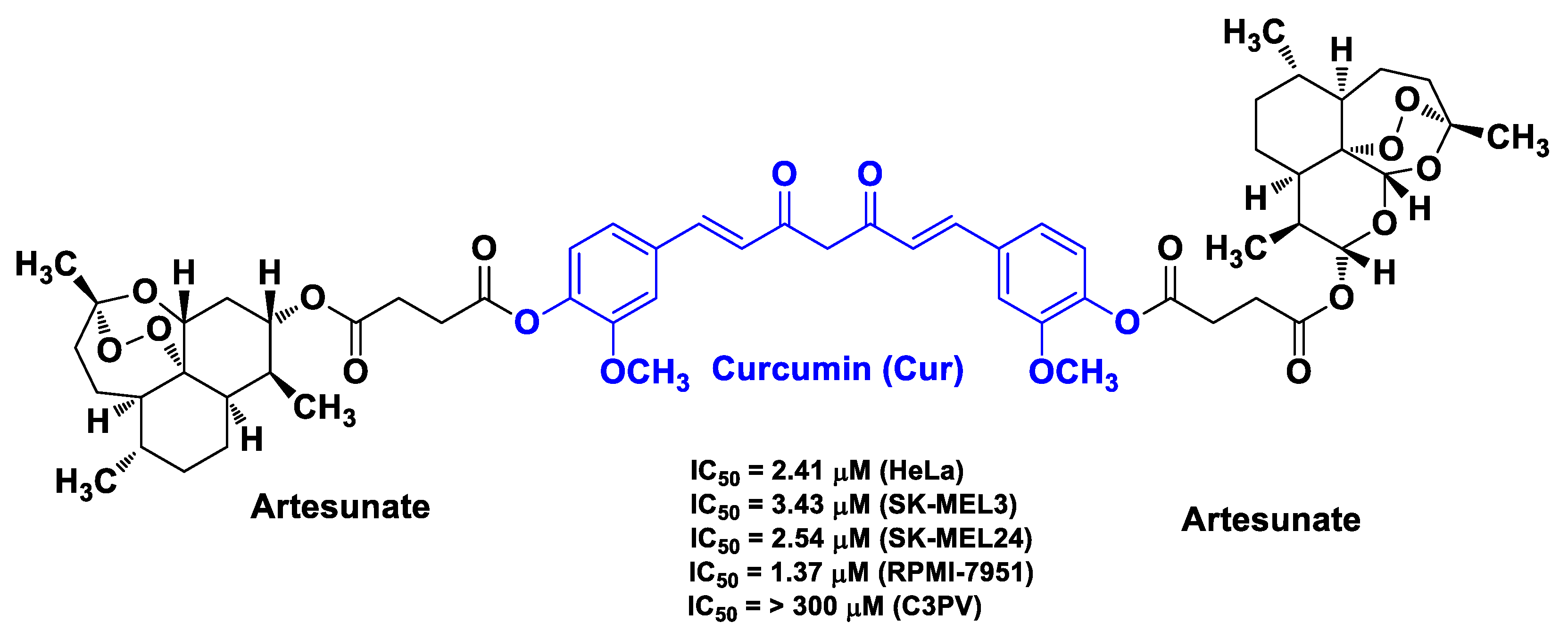
| Cur | artesunate | linkage of Cur with two artesunate molecules | melanoma | melanoma SK-MEL3, SK-MEL24, and RPMI-7951 cells | [64] | ||
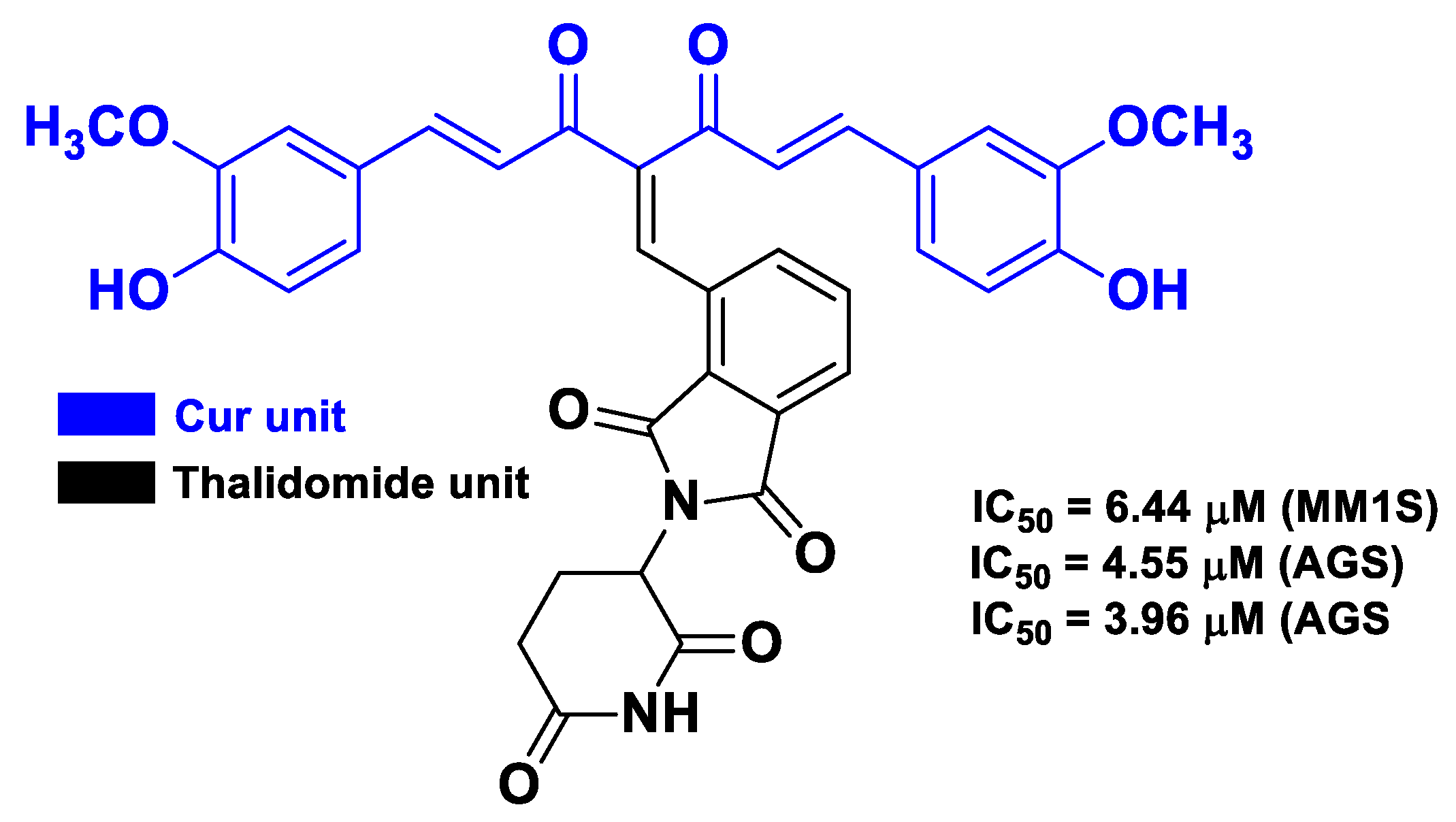
| Cur | thalidomide | thalidomide linked at the methylene position between the two carbonyls of Cur; monoketone Cur linked to thalidomide | multiple myeloma | human multiple myeloma MM1S, RPMI8226, U266 cells | [65] |
| Cytotoxic Drug | Hybrid Materials | Aim | Functionalization | Pathology | In Vitro Model | In Vivo Model | Ref. |
|---|---|---|---|---|---|---|---|
| Res | Res-loaded Au nanospheres | DD | liver cancer | Human liver HepG2 cells | [91] | ||
| Res | Res-loaded AuNPs | DD | liver cancer | Human liver HepG2 cells | 15 BALC/c nude mice bearing HepG2 cells | [92] | |
| Res | Res-conjugated AuNPs | DD | breast cancer | TPA-induced migration and invasion in breast cancer MCF-7 cells | [93] | ||
| Res | Res-conjugated AuNPs stabilized by gum arabic | DD | cancer | breast cancer MDAMB-231, pancreatic cancer PANC-1, and prostate cancer PC-3 cells | [94] | ||
| Res Dox | Res-stabilized AuNPs | DD | brain cancer | human glioma LN 229 cells | [95] | ||
| Res Dox | AuNPs capped with Res | DD | cervical cancer | human cervical cancer (HeLa, HPV-18 positive, and CaSki, HPV-16 positive) cells | [96] | ||
| Res | Res-loaded in chitosan modified liposomes coated by gold nanoshells | NIR- and pH-responsive system | AuNPs Chitosan | cervical cancer | human epithelioid cervix carcinoma HeLa cells | [97] | |
| Res | Hollow NPs based on Au-Res complexes | NIR responsive system | AuNPs | melanoma | malignant melanoma A375 cells | [98] |
| Cytotoxic Drug | Hybrid Materials | Aim | Functionalization | Pathology | In Vitro Model | In Vivo Model | References |
|---|---|---|---|---|---|---|---|
| Cur | Cur-conjugated AuNPs | DD | cervical cancer | cervical cancer HeLa cells | [99] | ||
| Cur | Cur-capped AuNP-reduced graphene oxide nanocomposite | DD | colon and liver cancer | human colon cancer HT-29 and SW-948 cells | [100] | ||
| Cur | MUC-1 aptamer conjugated and Cur-loaded PEGylated amine-terminated generation 5 poly(amidoamine) dendrimers/gold hybrid structures | active TDD | MUC-1 aptamer | colon adenocarcinoma | colon cancer HT29 and C26 cells | C26 tumor-bearing BALB/c female mice | [101] |
| Cur Lipoic acid | Lipoic acid-Cur and GSH attached to gold-iron oxide nanocomposites | active targeted and pH-responsive DD MRI | GSH Lipoic acid Iron NPs | Brain cancer | fetal human astrocyte and U87MG cell lines | [102] | |
| Cur | Cur-loaded gliadin-stabilized folic acid-functionalized Au quantum clusters | active targeted and pH-responsive DD | Folic acid Gliadin | Cancer | brain cancer C6 glioma cells and breast cancer MDA-MB231 cells | [103] | |
| Cur | Cur-loaded in protein polymer-Au NPs (protein polymer based on elastin-like peptide and the coiled-coil region of Cartilage Oligomeric Matrix protein, both bearing an N-terminal hexahistidine group) | Breast cancer | human breast cancer MCF7 cells | [104] | |||
| Cur | Folate-Cur-loaded Au-polyvinylpyrrolidone NPs | active targeted DD | Folic acid | breast cancer | human breast adenocarcinoma MDA-MB-231 and MCF-7, epithelial MCF 10A cells; mouse mammary carcinoma 4T1 cells | female Balb/c mice bearing 4T1 cancer | [105] |
| Cur | AuNPs immobilized on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets loaded with Cur | active targeted DD NIR- and pH-responsive system | AuNPs Folic acid | breast cancer | human breast adenocarcinoma MCF-7 cells | [106] | |
| Cur | chitosan-coated halloysite nanotubes loaded with Cur-Au NPs | NIR- and pH-responsive systems | AuNPs Chitosan | breast cancer | human breast adenocarcinoma MCF-7 cells | [107] | |
| Cur | Cur-loaded in gold-coated liposome NPs | NIR-responsive system | AuNPs | melanoma | mouse melanoma B16 cells | C57BL/6 female mice bearing B16 cells | [108] |
| Cur | Cur-loaded HSPC liposomes coated with gold | NIR-responsive system | AuNPs | melanoma | mouse melanoma B16 F10 cells | [109] | |
| Cur | Cur-Au-PEG-NPs | NIR-responsive and sonosensitive system | AuNPs Ultrasounds | melanoma | mouse melanoma C540 (B16/F10) cells | inbred male BALB/c mice bearing B16/F10 cells | [110] |
| Cur | PEG-Cur-AuNPs | NIR-responsive system | AuNPs | melanoma | mouse melanoma C540 (B16/F10) cells | [111] | |
| Cur | PEG-Cur-AuNPs | NIR-responsive system | AuNPs | melanoma | mouse melanoma C540 (B16/F10) cells | male C57/inbred mice implanted with B16/F10 cells | [112] |
| Cur | Cur-loaded Ag/Au bimetallic NPs coated with polystyrene- and PEG-based gel layers | NI- responsive system | AuNPs | melanoma | mouse melanoma B16F10 cells | [113] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristani, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. https://doi.org/10.3390/molecules26154665
Micale N, Molonia MS, Citarella A, Cimino F, Saija A, Cristani M, Speciale A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules. 2021; 26(15):4665. https://doi.org/10.3390/molecules26154665
Chicago/Turabian StyleMicale, Nicola, Maria Sofia Molonia, Andrea Citarella, Francesco Cimino, Antonina Saija, Mariateresa Cristani, and Antonio Speciale. 2021. "Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol" Molecules 26, no. 15: 4665. https://doi.org/10.3390/molecules26154665
APA StyleMicale, N., Molonia, M. S., Citarella, A., Cimino, F., Saija, A., Cristani, M., & Speciale, A. (2021). Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules, 26(15), 4665. https://doi.org/10.3390/molecules26154665











