An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography
Abstract
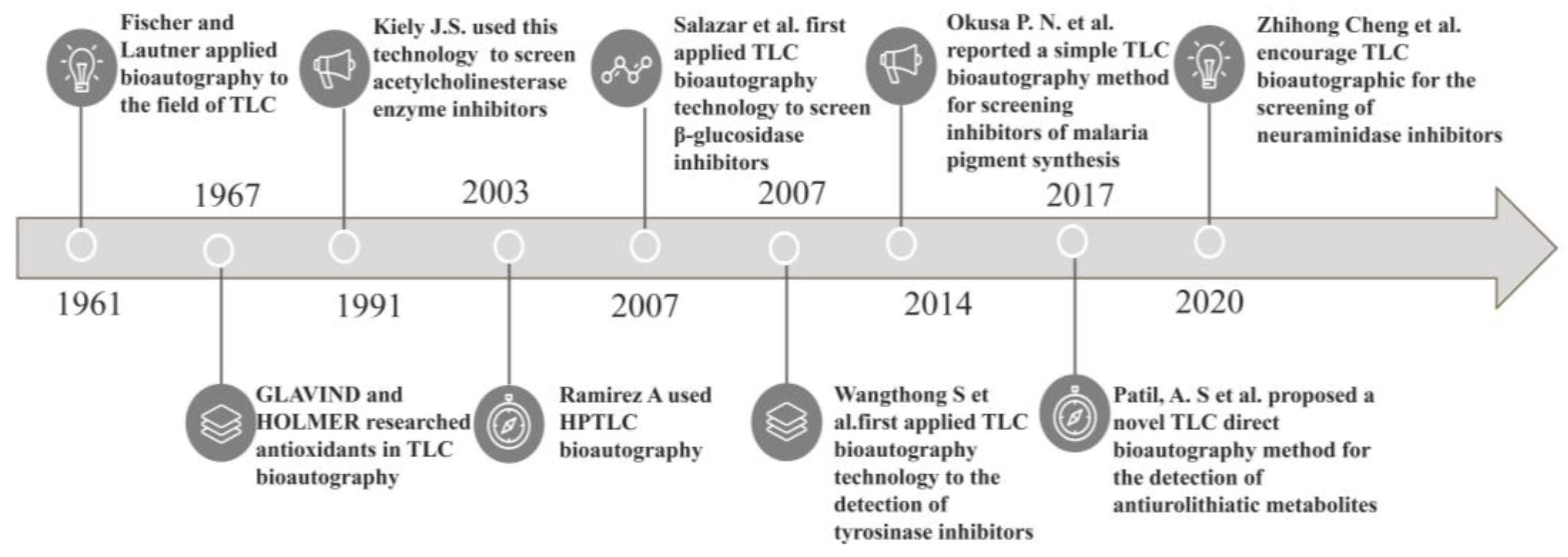
1. Introduction
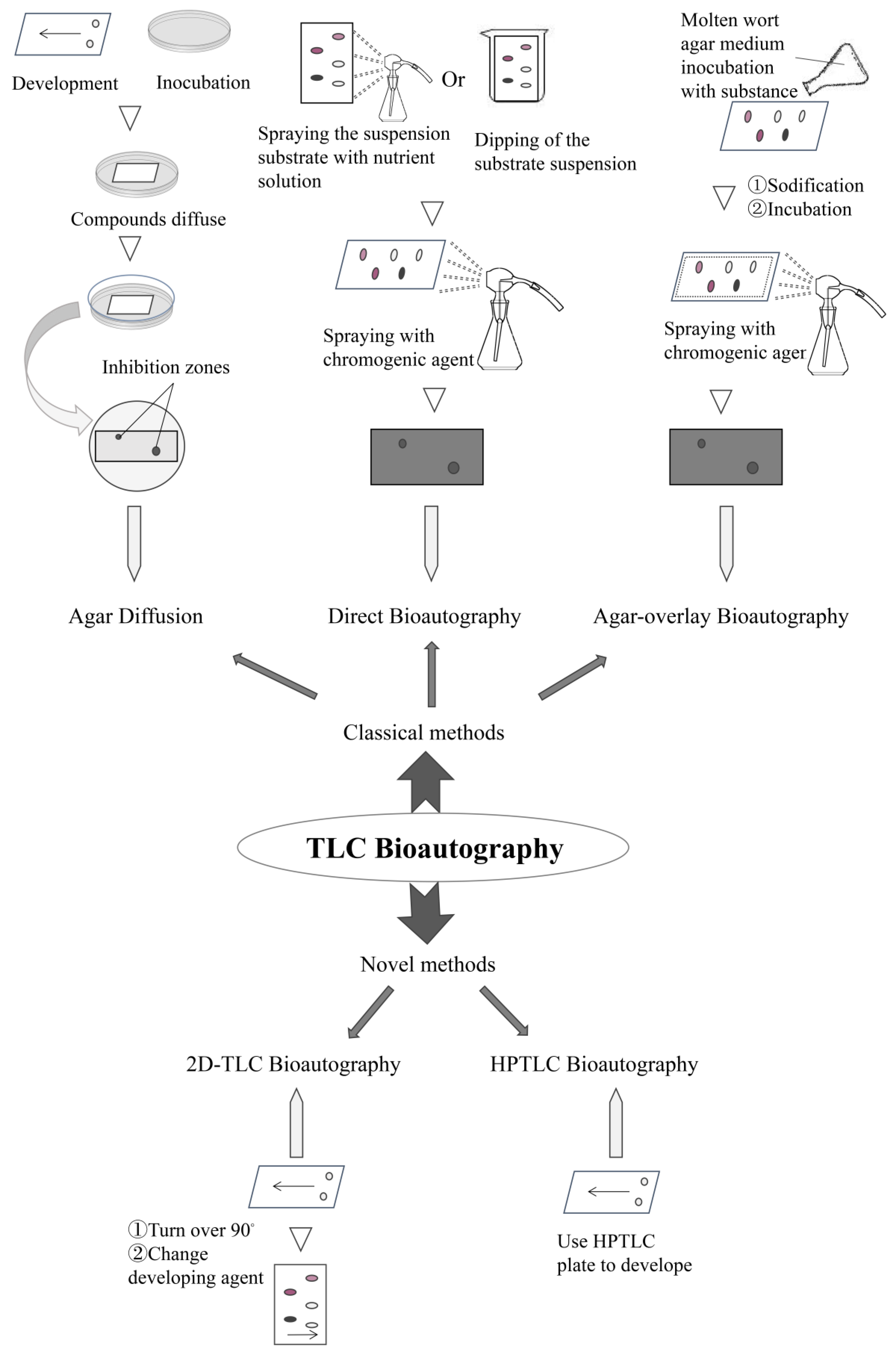
2. Classification
2.1. Classical Types
2.1.1. Agar Diffusion
2.1.2. Direct Bioautography
2.1.3. Agar Overlay Bioautography
2.2. Novel Types
2.2.1. High-Performance Thin-Layer Chromatography Bioautography
2.2.2. D-TLC Bioautography
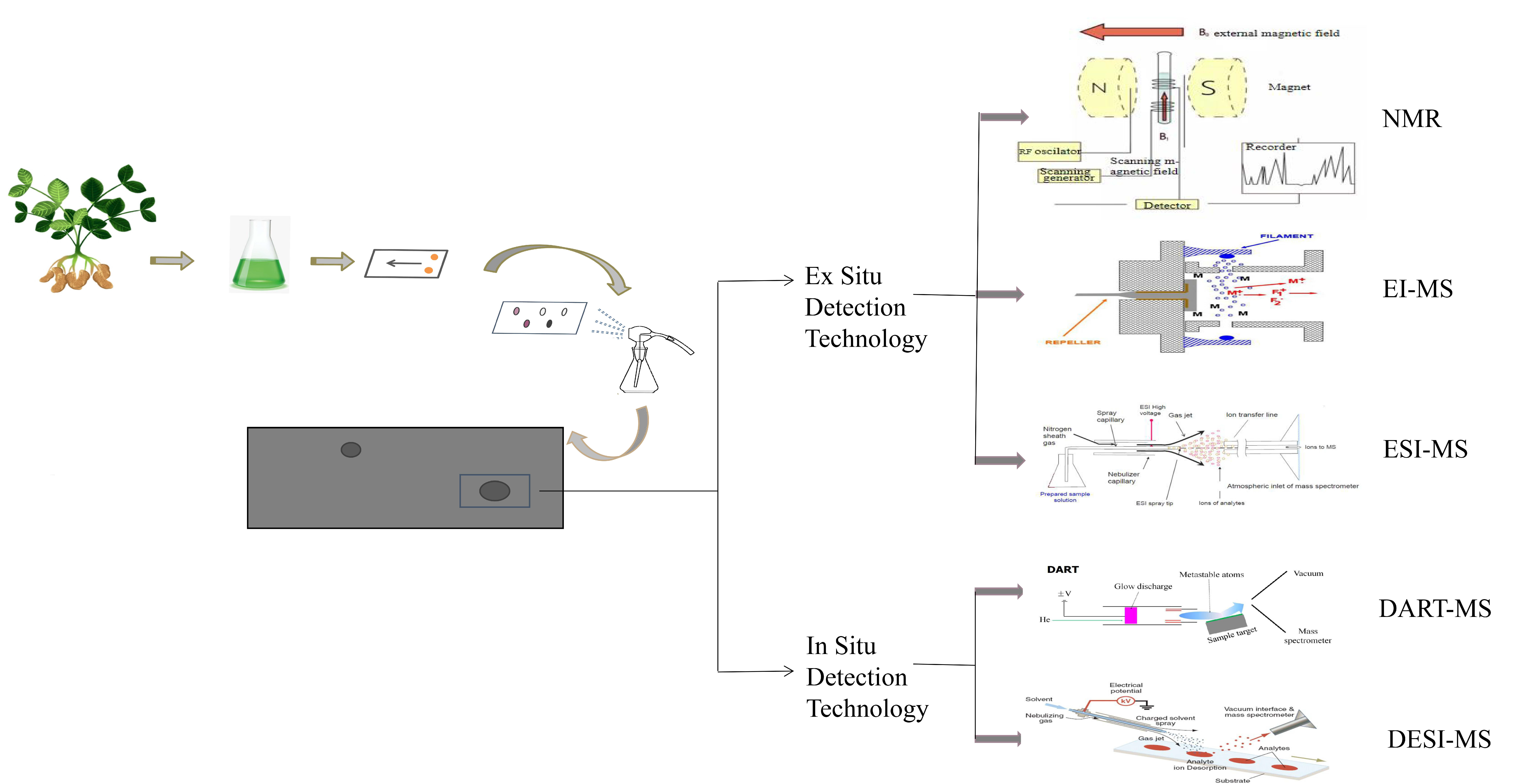
3. Detection Technique
3.1. Ex Situ Detection Technology
3.1.1. Nuclear Magnetic Resonance
3.1.2. Electron Ionization Mass Spectrometry
3.1.3. Electrospray Ionization Mass Spectrometry
3.2. In Situ Detection Technology
3.2.1. Direct Analysis in Real-Time Mass Spectrometry
3.2.2. Desorption Electrospray Ionization Mass Spectrometry
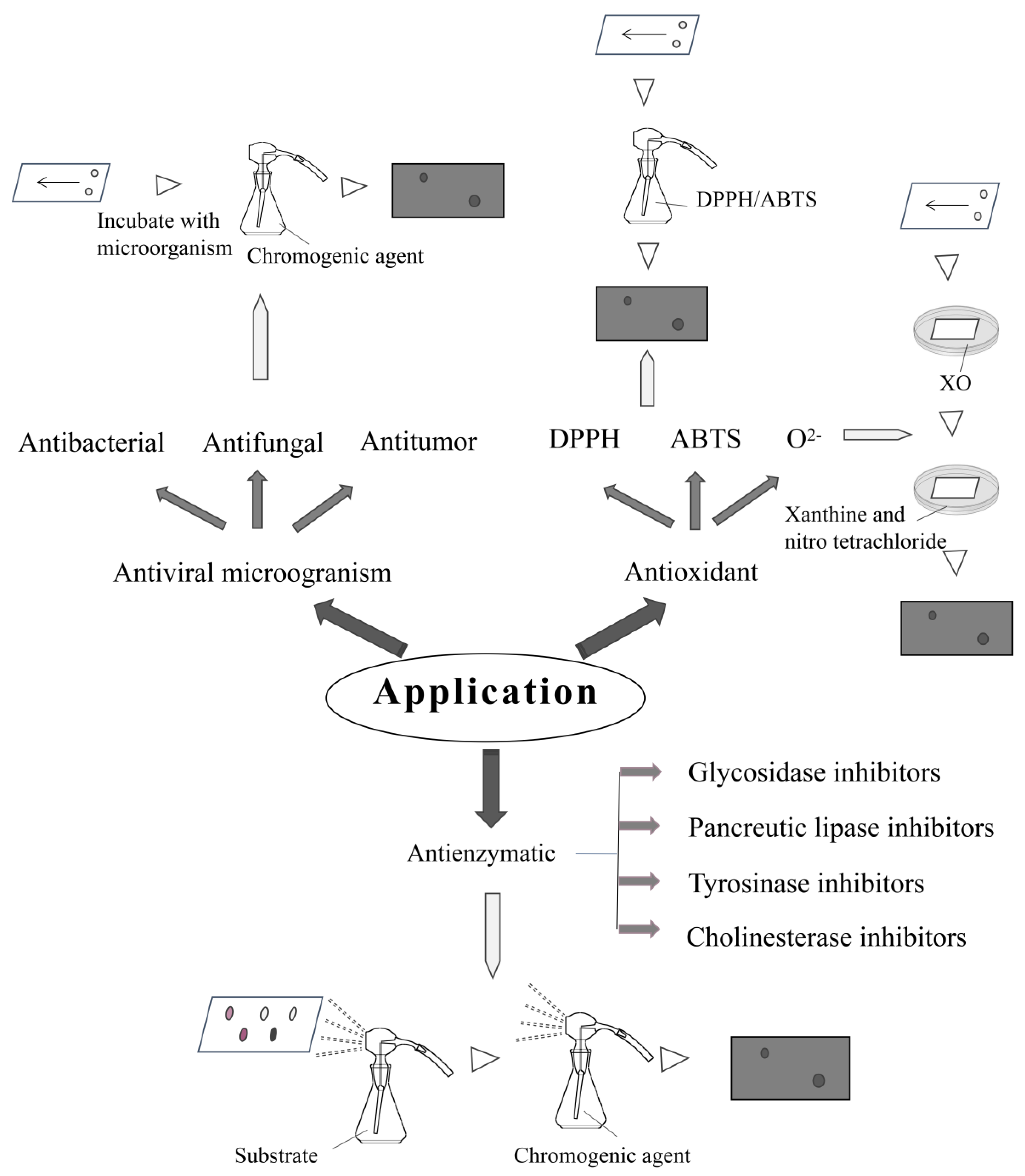
4. Biological Applications
4.1. Screening of Antimicrobial Compounds
4.1.1. Screening of Antibacterial Compounds
4.1.2. Screening of Antifungal Compounds
4.1.3. Screening of Antitumor Components
4.2. Screening of Antioxidant Compounds
4.3. Screening of Enzyme Inhibitors
4.3.1. Screening of Glucosidase Inhibitors
4.3.2. Screening of Pancreatic Lipase Inhibitors
4.3.3. Screening of Tyrosinase Inhibitors
4.3.4. Screening of Cholinesterase Inhibitors
4.4. Other Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bräm, S.; Wolfram, E. Recent Advances in Effect-directed Enzyme Assays based on Thin-layer Chromatography. Phytochem. Anal. 2017, 28, 74–86. [Google Scholar] [CrossRef]
- Deranieh, R.M.; Joshi, A.S.; Greenberg, M.L. Thin-Layer Chromatography of Phospholipids. Methods Mol. Biol. 2013, 1033, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Abramson, D.; Blecher, M. Quantitative Two-Dimensional Thin-Layer Chromatography of Natu-Rally Occurring Phospholipids. J. Lipid Res. 1964, 5, 628–631. [Google Scholar] [CrossRef]
- Sytar, O.; Bośko, P.; Živčák, M.; Brestic, M.; Smetanska, I. Bioactive Phytochemicals and Antioxidant Properties of the Grains and Sprouts of Colored Wheat Genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef]
- Dinakaran, S.K.; Sujiya, B.; Avasarala, H. Profiling and determination of phenolic compounds in Indian marketed hepatoprotective polyherbal formulations and their comparative evaluation. J. Ayurveda Integr. Med. 2018, 9, 3–12. [Google Scholar] [CrossRef]
- Ramallo, I.A.; Salazar, M.O.; Furlan, R.L.E. Enzymatic Bioautographic Methods. Methods Mol. Biol. 2019, 2089, 179–189. [Google Scholar] [CrossRef]
- Ciesla, L.; Waksmundzka-Hajnos, M.; Wojtunik-Kulesza, K.; Hajnos, M. Thin-layer chromatography coupled with biological detection to screen natural mixtures for potential drug leads. Phytochem. Lett. 2015, 11, 445–454. [Google Scholar] [CrossRef]
- Fischer, R.; Lautner, H. Zum papierchromatographischen Nachweis von Penicillinpräparaten. Arch. Pharm. 1961, 294, 1–7. [Google Scholar] [CrossRef]
- Glavind, J.; Holmer, G. Thin-layer chromatographic determination of antioxidants by the stable free radical α,α’-diphenyl-β-picrylhydrazyl. J. Am. Oil Chem. Soc. 1967, 44, 539–542. [Google Scholar] [CrossRef]
- Kiely, J.S.; Moos, W.H.; Pavia, M.R.; Schwarz, R.D.; Woodard, G.L. A silica gel plate-based qualitative assay for acetylcholinesterase activity: A mass method to screen for potential inhibitors. Anal. Biochem. 1991, 196, 439–442. [Google Scholar] [CrossRef]
- Raschetti, R.; Vivanti, A.J.; Vauloup-Fellous, C.; Loi, B.; Benachi, A.; De Luca, D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Sidorenko, Y.; Reichl, U. Structured model of influenza virus replication in MDCK cells. Biotechnol. Bioeng. 2004, 88, 1–14. [Google Scholar] [CrossRef]
- Abiri, R.; Abdul-Hamid, H.; Sytar, O.; Abiri, R.; de Almeida, E.B.; Sharma, S.; Bulgakov, V.; Arroo, R.; Malik, S. A Brief Overview of Potential Treatments for Viral Diseases Using Natural Plant Compounds: The Case of SARS-CoV. Molecules 2021, 26, 3868. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kumar, A.; Misra, N. On the Inhibition of COVID-19 Protease by Indian Herbal Plants: An in Silico Inves-tigation. arXiv 2020, arXiv:2004.03411. Available online: https://arxiv.org/abs/2004.03411 (accessed on 5 April 2020).
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Miao, Y.; Wu, T.; Cheng, Z. Development of a thin-layer chromatography bioautographic assay for neuraminidase inhibitors hyphenated with electrostatic field induced spray ionisation-mass spectrometry for identification of active Isatis indigotica root compounds. J. Chromatogr. A 2021, 1638, 461597. [Google Scholar] [CrossRef] [PubMed]
- Okusa, P.N.; Haddadi, Z.; Duez, P. Development of a simple TLC-bioautographic assay based on the inhibition of hemozoin synthesis for screening of natural sources antimalarial compounds. Eur. J. Integr. Med. 2014, 6, 126–127. [Google Scholar] [CrossRef]
- Patil, A.S.; Paikrao, H.; Kale, A.S.; Manik, S.R. A TLC-Direct Bioautography Method for Detection of Antiurolithiatic Metabolites. J. Chromatogr. Sci. 2017, 55, 578–585. [Google Scholar] [CrossRef][Green Version]
- Fan, H.; Ru, J.; Zhang, Y.; Wang, Q.; Li, Y. Fengycin produced by Bacillus subtilis 9407 plays a major role in the biocontrol of apple ring rot disease. Microbiol. Res. 2017, 199, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Komape, N.P.M.; Bagla, V.; Kabongo-Kayoka, P.; Masoko, P. Anti-mycobacteria potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province, South Africa. BMC Complement. Altern. Med. 2017, 17, 128. [Google Scholar] [CrossRef]
- Suleiman, M.; McGaw, L.; Naidoo, V.; Eloff, J. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 64–78. [Google Scholar] [CrossRef]
- Wedge, D.; Galindo, J.C.G.; Macías, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef]
- Mackeen, M.M.; Ali, A.M.; Lajis, N.H.; Kawazu, K.; Kikuzaki, H.; Nakatani, N. Antifungal Garcinia Acid Esters from The Fruits Of Garcinia Atroviridis. Z. Nat. C 2002, 57, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bisi-Johnson, M.A.; Obi, C.L.; Samuel, B.B.; Eloff, J.N.; Okoh, A.I. Antibacterial activity of crude extracts of some South African medicinal plants against multidrug resistant etiological agents of diarrhoea. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Balázs, V.L.; Horváth, B.; Kerekes, E.; Ács, K.; Kocsis, B.; Varga, A.; Böszörményi, A.; Nagy, D.U.; Krisch, J.; Széchenyi, A.; et al. Anti-Haemophilus Activity of Selected Essential Oils Detected by TLC-Direct Bioautography and Biofilm Inhibition. Molecules 2019, 24, 3301. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.M.; Grzelak, E.M. Bioautography detection in thin-layer chromatography. J. Chromatogr. A 2011, 1218, 2684–2691. [Google Scholar] [CrossRef]
- Nuthan, B.R.; Rakshith, D.; Marulasiddaswamy, K.M.; Rao, H.C.Y.; Ramesha, K.P.; Mohana, N.C.; Siddappa, S.; Darshan, D.; Kumara, K.K.S.; Satish, S. Application of Optimized and Validated Agar Overlay TLC–Bioautography Assay for Detecting the Antimicrobial Metabolites of Pharmaceutical Interest. J. Chromatogr. Sci. 2020, 58, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Ramirez, A.; Gutiérrez, R.; Diaz, G.; González, C.; Pérez, N.; Vega, S.; Noa, M. High-performance thin-layer chromatography–bioautography for multiple antibiotic residues in cow’s milk. J. Chromatogr. B 2003, 784, 315–322. [Google Scholar] [CrossRef]
- Tanvir, R.; Sajid, I.; Hasnain, S.; Kulik, A.; Grond, S. Rare actinomycetes Nocardia caishijiensis and Pseudonocardia carboxydivorans as endophytes, their bioactivity and metabolites evaluation. Microbiol. Res. 2016, 185, 22–35. [Google Scholar] [CrossRef]
- Betina, V. Bioautography in paper and thin-layer chromatography and its scope in the antibiotic field. J. Chromatogr. A 1973, 78, 41–51. [Google Scholar] [CrossRef]
- Marston, A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatogr. A 2011, 1218, 2676–2683. [Google Scholar] [CrossRef]
- Rasoamiaranjanahary, L.; Marston, A.; Guilet, D.; Schenk, K.; Randimbivololona, F.; Hostettmann, K. Antifungal diterpenes from Hypoestes serpens (Acanthaceae). Phytochemistry 2003, 62, 333–337. [Google Scholar] [CrossRef]
- Chaaib, F.; Queiroz, E.F.; Ndjoko, K.; Diallo, D.; Hostettmann, K. Antifungal and Antioxidant Compounds from the Root Bark ofFagara zanthoxyloides. Planta Med. 2003, 69, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wedge, D.E.; Nagle, D.G. A New 2D-TLC Bioautography Method for the Discovery of Novel Antifungal Agents to Control Plant Pathogens. J. Nat. Prod. 2000, 63, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- HE, J.; ZHAO, Q.-M.; CHEN, J. Study on Fast Detecting and Tracking of Antibacterial Active Components for Endophytic Fungi in Pseudolarix kaempferi Gord with 2D-TLC Bioautography. Food Sci. 2008, 29, 252–256. [Google Scholar]
- Feider, C.L.; Krieger, A.; DeHoog, R.J.; Eberlin, L.S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91, 4266–4290. [Google Scholar] [CrossRef]
- Dewanjee, S.; Gangopadhyay, M.; Bhattacharya, N.; Khanra, R.; Dua, T.K. Bioautography and its scope in the field of natural product chemistry. J. Pharm. Anal. 2015, 5, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Roy, R. The application of absolute quantitative1H NMR spectroscopy in drug discovery and development. Expert Opin. Drug Discov. 2016, 11, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Gouda, S.; Sahoo, S.K.; Thatoi, H.N. Chromatography separation, 1H NMR analysis and bioautography screening of methanol extract of Excoecaria agallocha L. from Bhitarkanika, Orissa, India. Asian Pac. J. Trop. Biomed. 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Adhami, H.-R.; Scherer, U.; Kaehlig, H.; Hettich, T.; Schlotterbeck, G.; Reich, E.; Krenn, L. Combination of Bioautography with HPTLC-MS/NMR: A Fast Identification of Acetylcholinesterase Inhibitors from Galbanum†. Phytochem. Anal. 2013, 24, 395–400. [Google Scholar] [CrossRef]
- Prasansuklab, A.; Theerasri, A.; Payne, M.; Ung, A.T.; Tencomnao, T. Acid-base fractions separated from Streblus asper leaf ethanolic extract exhibited antibacterial, antioxidant, anti-acetylcholinesterase, and neuroprotective activities. BMC Complement. Altern. Med. 2018, 18, 223. [Google Scholar] [CrossRef] [PubMed]
- Czernicka, L.; Grzegorczyk, A.; Marzec, Z.; Antosiewicz, B.; Malm, A.; Kukula-Koch, W. Antimicrobial Potential of Single Metabolites of Curcuma longa Assessed in the Total Extract by Thin-Layer Chromatography-Based Bioautography and Image Analysis. Int. J. Mol. Sci. 2019, 20, 898. [Google Scholar] [CrossRef]
- Kubec, R.; Cody, R.; Dane, A.J.; Musah, R.A.; Schraml, J.; Vattekkatte, A.; Block, E. Applications of Direct Analysis in Real Time−Mass Spectrometry (DART-MS) inAlliumChemistry. (Z)-ButanethialS-Oxide and 1-Butenyl Thiosulfinates and TheirS-(E)-1-ButenylcysteineS-Oxide Precursor from Allium siculum. J. Agric. Food Chem. 2010, 58, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Häbe, T.T.; Jamshidi-Aidji, M.; Macho, J.; Morlock, G.E. Direct bioautography hyphenated to direct analysis in real time mass spectrometry: Chromatographic separation, bioassay and mass spectra, all in the same sample run. J. Chromatogr. A 2018, 1568, 188–196. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Häbe, T.T.; Böszörményi, A.; Ott, P.G.; Morlock, G. Tracking and identification of antibacterial components in the essential oil of Tanacetum vulgare L. by the combination of high-performance thin-layer chromatography with direct bioautography and mass spectrometry. J. Chromatogr. A 2015, 1422, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pasilis, S.P.; Kertesz, V.; Van Berkel, G.J. Unexpected Analyte Oxidation during Desorption Electrospray Ionization-Mass Spectrometry. Anal. Chem. 2008, 80, 1208–1214. [Google Scholar] [CrossRef]
- Van Berkel, G.J.; Ford, M.J.; Deibel, M.A. Thin-Layer Chromatography and Mass Spectrometry Coupled Using Desorption Electrospray Ionization. Anal. Chem. 2005, 77, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, G.J.; Tomkins, B.; Kertesz, V. Thin-Layer Chromatography/Desorption Electrospray Ionization Mass Spectrometry: Investigation of Goldenseal Alkaloids. Anal. Chem. 2007, 79, 2778–2789. [Google Scholar] [CrossRef]
- Takáts, Z.; Wiseman, J.; Cooks, R.G. Ambient mass spectrometry using desorption electrospray ionization (DESI): Instrumentation, mechanisms and applications in forensics, chemistry, and biology. J. Mass Spectrom. 2005, 40, 1261–1275. [Google Scholar] [CrossRef]
- Pasilis, S.P.; Kertesz, V.; Van Berkel, G.J.; Schulz, M.; Schorcht, S. Using HPTLC/DESI-MS for peptide identification in 1D separations of tryptic protein digests. Anal. Bioanal. Chem. 2008, 391, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Takáts, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.; Wiseman, J.M.; Valentine, S.J.; Takats, Z.; Cooks, R.G.; Clemmer, D.E. Coupling Desorption Electrospray Ionization with Ion Mobility/Mass Spectrometry for Analysis of Protein Structure: Evidence for Desorption of Folded and Denatured States. J. Phys. Chem. B 2006, 110, 5045–5051. [Google Scholar] [CrossRef] [PubMed]
- Kauppila, T.; Wiseman, J.; Ketola, R.A.; Kotiaho, T.; Cooks, R.G.; Kostiainen, R. Desorption electrospray ionization mass spectrometry for the analysis of pharmaceuticals and metabolites. Rapid Commun. Mass Spectrom. 2005, 20, 387–392. [Google Scholar] [CrossRef]
- Nefliu, M.; Cooks, R.G.; Moore, C. Enhanced desorption ionization using oxidizing electrosprays. J. Am. Soc. Mass Spectrom. 2006, 17, 1091–1095. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 2299–2301. [Google Scholar] [CrossRef]
- Grzelak, E.M.; Hwang, C.; Cai, G.; Nam, J.-W.; Choules, M.; Gao, W.; Lankin, D.C.; McAlpine, J.B.; Mulugeta, S.G.; Napolitano, J.G.; et al. Bioautography with TLC-MS/NMR for Rapid Discovery of Anti-tuberculosis Lead Compounds from Natural Sources. ACS Infect. Dis. 2016, 2, 294–301. [Google Scholar] [CrossRef]
- Nicolaus, B.J.R.; Coronelli, C.; Binaghi, A. Mikrobiologischer Nachweis von Antibiotika auf Dünnschichtchromatogrammen. Cell. Mol. Life Sci. 1961, 17, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Meyers, E.; Smith, D.A. Bioautography of antibiotic spread-layer chromatograms. J. Chromatogr. A 1964, 14, 129–132. [Google Scholar] [CrossRef]
- Hamburger, M.O.; Cordell, G.A. A Direct Bioautographic Tlc Assay for Compounds Possessing Antibacterial Activity. J. Nat. Prod. 1987, 50, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Móricz, Á.M.; Ott, P.G.; Alberti, Á.; Böszörményi, A.; Lemberkovics, É.; Szőke, É.; Kéry, Á.; Mincsovics, E. Applicability of Preparative Overpressured Layer Chromatography and Direct Bioautography in Search of Antibacterial Chamomile Compounds. J. AOAC Int. 2013, 96, 1214–1221. [Google Scholar] [CrossRef]
- Kumar, R.R.; Jadeja, V.J. Characterization and partial purification of an antibacterial agent from halophilic actinomycetes Kocuria sp. strain rsk4. BioImpacts BI 2018, 8, 253–261. [Google Scholar] [CrossRef]
- Favre-Godal, Q.; Queiroz, E.F.; Wolfender, J.-L. Latest Developments in Assessing Antifungal Activity Using TLC-Bioautography: A Review. J. AOAC Int. 2013, 96, 1175–1188. [Google Scholar] [CrossRef]
- Sobhani, M.; Abbas-Mohammadi, M.; Ebrahimi, S.N.; Aliahmadi, A. Tracking leading anti-Candida compounds in plant samples; Plumbago europaea. Iran. J. Microbiol. 2018, 10, 187–193. [Google Scholar] [PubMed]
- Rahalison, L.; Hamburger, M.O.; Hostettmann, K.A.; Monod, M.; Frenk, E.A. A bioautographic agar overlay method for the detection of antifungal compounds from higher plants. Phytochem. Anal. 1991, 2, 199–203. [Google Scholar] [CrossRef]
- Tian, D.-M.; Cheng, H.-Y.; Jiang, M.-M.; Shen, W.-Z.; Tang, J.-S.; Yao, X.-S. Cardiac Glycosides from the Seeds of Thevetia peruviana. J. Nat. Prod. 2015, 79, 38–50. [Google Scholar] [CrossRef]
- Hanka, L.J.; Barnett, M.S. Microbiological Assays and Bioautography of Maytansine and Its Homologues. Antimicrob. Agents Chemother. 1974, 6, 651–652. [Google Scholar] [CrossRef][Green Version]
- Pourramezan, Z.; Oloomi, M.; Kasra-Kermanshahi, R. Antioxidant and Anticancer Activities of Lactobacillus Hilgardii Strain AG12a. Int. J. Prev. Med. 2020, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Adeli, A.; Nickavar, A. TLC-Bioautography and GC-MS Analyses for Detection and Identification of Antioxidant Constituents of Trachyspermum copticum Essential Oil. Iran. J. Pharm. Res. IJPR 2014, 13, 127–133. [Google Scholar] [PubMed]
- Corsino, J.; Silva, D.; Zanoni, M.V.B.; Bolzani, V.; Franca, S.; Pereira, A.M.S.; Furlan, M. Antioxidant flavan-3-ols and flavonol glycosides fromMaytenus aquifolium. Phytother. Res. 2003, 17, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, C.L.; Uchoa, A.; Salazar, J.R.; Perich, F.; Pardo, F. Plant Growth Inhibitory Activity ofp-Hydroxyacetophenones and Tremetones from Chilean EndemicBaccharisSpecies and Some Analogous: A Comparative Study. J. Agric. Food Chem. 2002, 50, 2283–2292. [Google Scholar] [CrossRef]
- Torres, P.; Avila, J.G.; De Vivar, A.R.; García, A.M.; Marín, J.C.; Aranda, E.; Cespedes, C. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry 2003, 64, 463–473. [Google Scholar] [CrossRef]
- Salazar, M.O.; Furlan, R.L.E. A rapid TLC autographic method for the detection of glucosidase inhibitors. Phytochem. Anal. 2007, 18, 209–212. [Google Scholar] [CrossRef]
- Su, X.; Li, X.; Tao, H.; Zhou, J.; Wu, T.; Chou, G.; Cheng, Z. Simultaneous isolation of seven compounds fromGlehnia littoralisroots by off-line overpressured layer chromatography guided by a TLC antioxidant autographic assay. J. Sep. Sci. 2013, 36, 3644–3650. [Google Scholar] [CrossRef]
- Simoes-Pires, C.; Hmicha, B.; Marston, A.; Hostettmann, K. A TLC bioautographic method for the detection of α- and β-glucosidase inhibitors in plant extracts. Phytochem. Anal. 1990, 20, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gu, L.; Xiao, Y.; Liu, Q.; Hu, H.; Wang, Z.; Chen, K. Rapid Identification of α-Glucosidase Inhibitors from Phlomis tuberosa by Sepbox Chromatography and Thin-Layer Chromatography Bioautography. PLoS ONE 2015, 10, e0116922. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.M.S. TLC Bioautographic Method for Detecting Lipase Inhibitors. Phytochem. Anal. 2011, 23, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhou, J.; Tang, Q.; Wu, T.; Cheng, Z. A new TLC bioautographic assay for qualitative and quantitative estimation of lipase inhibitors. Phytochem. Anal. 2015, 27, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Tuczek, F. Tyrosinase/catecholoxidase activity of hemocyanins: Structural basis and molecular mechanism. Trends Biochem. Sci. 2000, 25, 392–397. [Google Scholar] [CrossRef]
- Selinheimo, E.; Autio, K.; Kruus, K.; Buchert, J. Elucidating the Mechanism of Laccase and Tyrosinase in Wheat Bread Making. J. Agric. Food Chem. 2007, 55, 6357–6365. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, A.; Rodríguez-López, J.N.; Garcia-Canovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin against UV Damage in Human Skin†. Photochem. Photobiol. 2007, 84, 539–549. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Saenger, Y.M.; Pan, M.; Hashimoto, D.; Glodny, B.; Shah, S.; Houghton, A.N.; Merghoub, T.; Wolchok, J.D.; Merad, M. Abstract 4742: Anti-tyrosinase related protein 1 (trp1) antibody combined with cyclophosphamide induces adaptive immunity against melanoma. Cancer Res. 2011, 71, 4742. [Google Scholar]
- Martinez, M.; Whitaker, J.R. The biochemistry and control of enzymatic browning. Trends Food Sci. Technol. 1995, 6, 195–200. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Wangthong, S.; Tonsiripakdee, I.; Monhaphol, T.; Nonthabenjawan, R.; Wanichwecharungruang, S.P. Post TLC developing technique for tyrosinase inhibitor detection. Biomed. Chromatogr. 2006, 21, 94–100. [Google Scholar] [CrossRef]
- Taibon, J.; Ankli, A.; Schwaiger, S.; Magnenat, C.; Boka, V.-I.; Simões-Pires, C.; Aligiannis, N.; Cuendet, M.; Skaltsounis, A.-L.; Reich, E.; et al. Prevention of False-Positive Results: Development of an HPTLC Autographic Assay for the Detection of Natural Tyrosinase Inhibitors. Planta Med. 2015, 81, 1198–1204. [Google Scholar] [CrossRef]
- Girard, E.; Bernard, V.; Minic, J.; Chatonnet, A.; Krejci, E.; Molgó, J. Butyrylcholinesterase and the control of synaptic responses in acetylcholinesterase knockout mice. Life Sci. 2007, 80, 2380–2385. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; Gómez, E.; Muñoz-Torrero, D.; Font-Bardia, M.; Solans, X. Synthesis of diastereomeric 13-amido-substituted huprines as po-tential high affinity acetylcholinesterase inhibitors. Tetrahedron 2003, 59, 4143–4151. [Google Scholar] [CrossRef]
- Cavin, A.-L.; Hay, A.-E.; Marston, A.; Stoeckli-Evans, H.; Scopelliti, R.; Diallo, D.; Hostettmann, K. Bioactive Diterpenes from the Fruits of Detariummicrocarpum. J. Nat. Prod. 2006, 69, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Rhee, I.K.; van de Meent, M.; Ingkaninan, K.; Verpoorte, R. Screening for acetylcholinesterase inhibitors from Amaryllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromatogr. A 2001, 915, 217–223. [Google Scholar] [CrossRef]
- Marston, A.; Kissling, J.; Hostettmann, K. A rapid TLC bioautographic method for the detection of acetylcholinesterase and butyrylcholinesterase inhibitors in plants. Phytochem. Anal. 2002, 13, 51–54. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Duan, D.; Song, Z.; Yang, M.; Li, S. Modified TLC bioautographic method for screening acetylcholinesterase inhibitors from plant extracts. J. Sep. Sci. 2009, 32, 3257–3259. [Google Scholar] [CrossRef] [PubMed]
- Ramallo, I.A.; Garcia, P.; Furlan, R.L.E. A reversed-phase compatible thin-layer chromatography autography for the detection of acetylcholinesterase inhibitors. J. Sep. Sci. 2015, 38, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Salazar, M.O.; Viarengo, G.; Sciara, M.I.; Kieffer, P.M.; Véscovi, E.G.; Furlan, R.L.E. A Thin-layer Chromatography Autographic Method for the Detection of Inhibitors of the Salmonella PhoP-PhoQ Regulatory System. Phytochem. Anal. 2013, 25, 155–160. [Google Scholar] [CrossRef]
- Ložienė, K.; Švedienė, J.; Paškevičius, A.; Raudonienė, V.; Sytar, O.; Kosyan, A. Influence of plant origin natural α-pinene with different enantiomeric composition on bacteria, yeasts and fungi. Fitoterapia 2018, 127, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.R.; Savitske, B.J.; Credille, B.C. Concordance of disk diffusion, broth microdilution, and whole-genome sequencing for determination of in vitro antimicrobial susceptibility of Mannheimia haemolytica. J. Vet. Intern. Med. 2020, 34, 2158–2168. [Google Scholar] [CrossRef]
- Na, N.; Zhao, M.; Zhang, S.; Yang, C.; Zhang, X. Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 1859–1862. [Google Scholar] [CrossRef]
- Na, N.; Zhang, C.; Zhao, M.; Zhang, S.; Yang, C.; Fang, X.; Zhang, X. Direct detection of explosives on solid surfaces by mass spectrometry with an ambient ion source based on dielectric barrier discharge. J. Mass Spectrom. 2007, 42, 1079–1085. [Google Scholar] [CrossRef]
- Harper, J.D.; Charipar, N.A.; Mulligan, C.C.; Zhang, X.; Cooks, R.G.; Ouyang, Z. Low-Temperature Plasma Probe for Ambient Desorption Ionization. Anal. Chem. 2008, 80, 9097–9104. [Google Scholar] [CrossRef] [PubMed]
- McCullough, B.J.; Patel, K.; Francis, R.; Cain, P.; Douce, D.; Whyatt, K.; Bajic, S.; Lumley, N.; Hopley, C. Atmospheric Solids Analysis Probe Coupled to a Portable Mass Spectrometer for Rapid Identification of Bulk Drug Seizures. J. Am. Soc. Mass Spectrom. 2020, 31, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Lund, B.W.; Borggaard, C.; Birkler, R.I.D.; Jensen, K.; Støier, S. High throughput method for quantifying androstenone and skatole in adipose tissue from uncastrated male pigs by laser diode thermal desorption-tandem mass spectrometry. Food Chem. X 2021, 9, 100113. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ray, A.; Nasim, N.; Nayak, S.; Mohanty, S. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Teanpaisan, R.; Kawsud, P.; Pahumunto, N.; Puripattanavong, J. Screening for antibacterial and antibiofilm activity in Thai medicinal plant extracts against oral microorganisms. J. Tradit. Complement. Med. 2017, 7, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Rezaee, J.; Nickavar, A. Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran. J. Pharm. Res. IJPR 2016, 15, 241–246. [Google Scholar]
- Belaqziz, M.; Tan, S.P.; EL Abbassi, A.; Kiai, H.; Hafidi, A.; Donovan, O.O.; McLoughlin, P. Assessment of the antioxidant and antibacterial activities of different olive processing wastewaters. PLoS ONE 2017, 12, e0182622. [Google Scholar] [CrossRef]
- Legerská, B.; Chmelová, D.; Ondrejovič, M.; Miertuš, S. The TLC-Bioautography as a Tool for Rapid Enzyme Inhibitors detection—A Review. Crit. Rev. Anal. Chem. 2020, 41, 1–19. [Google Scholar] [CrossRef] [PubMed]
| Classification | Carrier | Culture Condition | Range | Sensitivity | Specificity |
|---|---|---|---|---|---|
| Agar diffusion | Culture medium | Diffusion requires incubation for several hours at 0~4 °C, and culture medium for a certain time at about 37 °C after diffusion | Suitable for broad-spectrum microorganisms | Low | Weak |
| Direct bioautography | Thin-layer plate | The incubation temperature is generally slightly higher than room temperature, and the incubation time is 2~3 days. It can also be cultured overnight at about 30 °C, sometimes with light | Fungal spores and certain bacteria that grow directly on thin-layer plates | High | Strong |
| Agar overlay bioautography | Thin-layer plate | After agar solidification, the laminates were cultured overnight at about 30 °C | Suitable for broad-spectrum microorganisms, especially for yeast, bacteria, etc. | Low | Weak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Y.; Wang, R.; Wang, Z.; Yang, B.; Kuang, H. An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules 2021, 26, 4647. https://doi.org/10.3390/molecules26154647
Wang M, Zhang Y, Wang R, Wang Z, Yang B, Kuang H. An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules. 2021; 26(15):4647. https://doi.org/10.3390/molecules26154647
Chicago/Turabian StyleWang, Meng, Yirong Zhang, Ruijie Wang, Zhibin Wang, Bingyou Yang, and Haixue Kuang. 2021. "An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography" Molecules 26, no. 15: 4647. https://doi.org/10.3390/molecules26154647
APA StyleWang, M., Zhang, Y., Wang, R., Wang, Z., Yang, B., & Kuang, H. (2021). An Evolving Technology That Integrates Classical Methods with Continuous Technological Developments: Thin-Layer Chromatography Bioautography. Molecules, 26(15), 4647. https://doi.org/10.3390/molecules26154647






