The Ultrastructure of Tissue Damage by Amyloid Fibrils
Abstract
1. Introduction
2. What Are ATTR Amyloidosis and AL Amyloidosis?
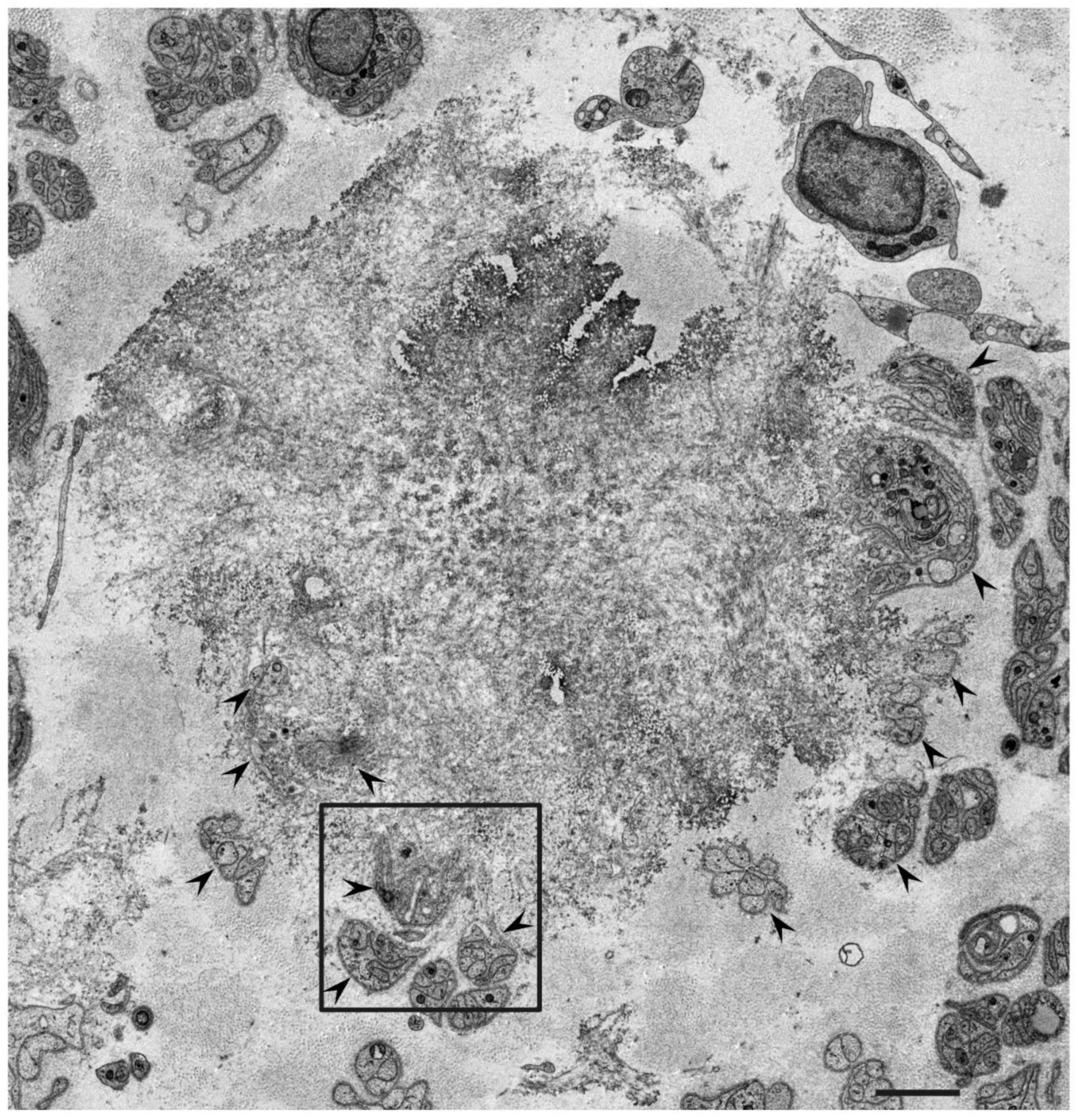
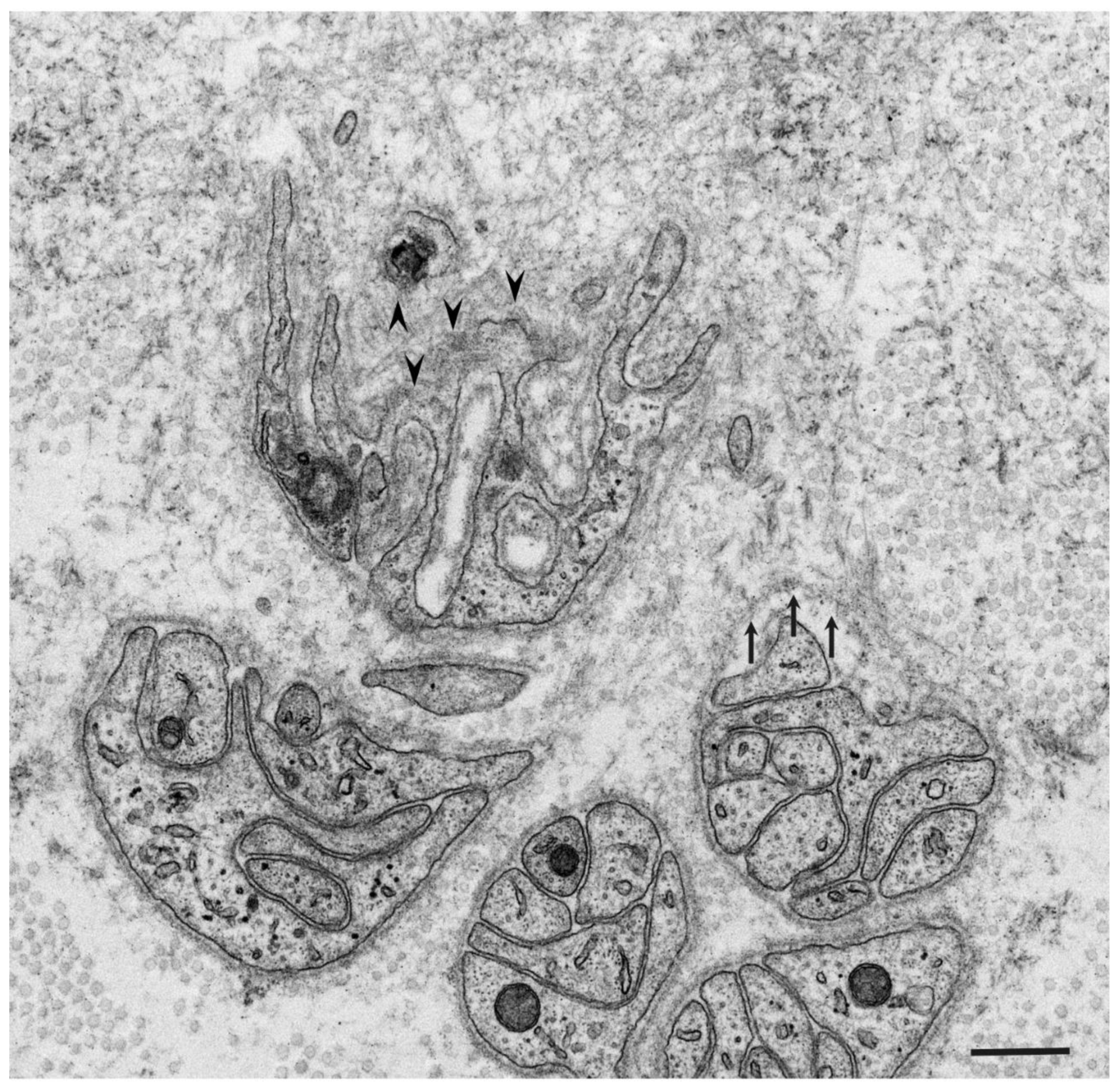
3. Ultrastructure of Tissue Damage
3.1. Atrophy and Degeneration Induced by Amyloid Fibrils
3.2. Obscuration of Basement and Cytoplasmic Membranes
4. Insights into Therapeutic Strategies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benson, M.D.; Buxbaum, J.N.; Eisenberg, D.S.; Merlini, G.; Saraiva, M.J.M.; Sekijima, Y.; Sipe, J.D.; Westermark, P. Amyloid nomencla-ture 2020: Update and recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid 2020, 27, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hamidi Asl, K.; Liepnieks, J.J.; Nakamura, M.; Benson, M.D. Organ-specific (localized) synthesis of Ig light chain amyloid. J. Immunol. 1999, 162, 5556–5560. [Google Scholar]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef]
- Koike, H.; Misu, K.; Sugiura, M.; Iijima, M.; Mori, K.; Yamamoto, M.; Hattori, N.; Mukai, E.; Ando, Y.; Ikeda, S.; et al. Pathology of early- vs late-onset TTR Met30 familial amyloid polyneuropathy. Neurology 2004, 63, 129–138. [Google Scholar] [CrossRef]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Katsuno, M. Transthyretin Amyloidosis: Update on the Clinical Spectrum, Pathogenesis, and Disease-Modifying Therapies. Neurol. Ther. 2020, 9, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Rosenblum, H.; Maurer, M.S. Pathophysiology and Therapeutic Approaches to Cardiac Amyloidosis. Circ. Res. 2021, 128, 1554–1575. [Google Scholar] [CrossRef]
- Koike, H.; Okumura, T.; Murohara, T.; Katsuno, M. Multidisciplinary Approaches for Transthyretin Amyloidosis. Cardiol. Ther. 2021, in press. [Google Scholar] [CrossRef]
- Koike, H.; Katsuno, M. Ultrastructure in Transthyretin Amyloidosis: From Pathophysiology to Therapeutic Insights. Biomedicines 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Immunoglobulin light chain amyloidosis: 2020 update on diagnosis, prognosis, and treatment. Am. J. Hematol. 2020, 95, 848–860. [Google Scholar] [CrossRef]
- Koike, H.; Tanaka, F.; Hashimoto, R.; Tomita, M.; Kawagashira, Y.; Iijima, M.; Fujitake, J.; Kawanami, T.; Kato, T.; Yamamoto, M.; et al. Natural history of transthyretin Val30Met familial amyloid polyneuropathy: Analysis of late-onset cases from non-endemic areas. J. Neurol. Neurosurg. Psychiatry 2012, 83, 152–158. [Google Scholar] [CrossRef]
- Andrade, C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain 1952, 75, 408–427. [Google Scholar] [CrossRef] [PubMed]
- Araki, S.; Mawatari, S.; Ohta, M.; Nakajima, A.; Kuroiwa, Y. Polyneuritic Amyloidosis in a Japanese Family. Arch. Neurol. 1968, 18, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Andersson, R. Familial amyloidosis with polyneuropathy. A clinical study based on patients living in northern Sweden. Acta Medica Scand. Suppl. 1976, 590, 1–64. [Google Scholar]
- Cornwell, G.G., III; Murdoch, W.L.; Kyle, R.A.; Westermark, P.; Pitkänen, P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am. J. Med. 1983, 75, 618–623. [Google Scholar] [CrossRef]
- Ueda, M.; Horibata, Y.; Shono, M.; Misumi, Y.; Oshima, T.; Su, Y.; Tasaki, M.; Shinriki, S.; Kawahara, S.; Jono, H.; et al. Clinicopathological features of senile systemic amyloidosis: An ante- and post-mortem study. Mod. Pathol. 2011, 24, 1533–1544. [Google Scholar] [CrossRef] [PubMed]
- Sekijima, Y.; Yazaki, M.; Ueda, M.; Koike, H.; Yamada, M.; Ando, Y. First nationwide survey on systemic wild-type ATTR amy-loidosis in Japan. Amyloid 2018, 25, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Koike, H.; Slama, M.; Coelho, T. Hereditary transthyretin amyloidosis: A model of medical progress for a fatal disease. Nat. Rev. Neurol. 2019, 15, 387–404. [Google Scholar] [CrossRef]
- Kyle, R.A.; Greipp, P.R. Amyloidosis (AL). Clinical and laboratory features in 229 cases. Mayo. Clin. Proc. 1983, 58, 665–683. [Google Scholar]
- Koike, H.; Misu, K.; Ikeda, S.; Ando, Y.; Nakazato, M.; Ando, E.; Yamamoto, M.; Hattori, N.; Sobue, G. Study Group for Hereditary Neuropathy in Japan. Type I (transthyretin Met30) familial amyloid polyneuropathy in Japan: Early- vs late-onset form. Arch. Neurol. 2002, 59, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Mouri, N.; Fukami, Y.; Iijima, M.; Matsuo, K.; Yagi, N.; Saito, A.; Nakamura, H.; Takahashi, K.; Nakae, Y.; et al. Two distinct mechanisms of neuropathy in immunoglobulin light chain (AL) amyloidosis. J. Neurol. Sci. 2021, 421, 117305. [Google Scholar] [CrossRef]
- Koike, H.; Hashimoto, R.; Tomita, M.; Kawagashira, Y.; Iijima, M.; Tanaka, F.; Sobue, G. Diagnosis of sporadic transthyretin Val30Met familial amyloid polyneuropathy: A practical analysis. Amyloid 2011, 18, 53–62. [Google Scholar] [CrossRef]
- González-López, E.; Gallego-Delgado, M.; Guzzo-Merello, G.; Moral, F.J.D.H.-D.; Cobo-Marcos, M.; Robles, C.; Bornstein, B.; Salas, C.; Lara-Pezzi, E.; Alonso-Pulpon, L.; et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 2015, 36, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Maurer, M.S. Transthyretin cardiac amyloidosis: A treatable form of heart failure with a preserved ejection frac-tion. Trends Cardiovasc. Med. 2021, 31, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sobue, G.; Nakao, N.; Murakami, K.; Yasuda, T.; Sahashi, K.; Mitsuma, T.; Sasaki, H.; Sakaki, Y.; Takahashi, A. Type I familial amyloid polyneuropathy. A pathological study of the peripheral nervous system. Brain 1990, 113, 903–919. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, A.; Andrade, C. Familial amyloid polyneuropathy: An electron microscope study of the peripheral nerve in five cases. I. interstitial changes. Brain 1971, 94, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.K.; King, R.H. Peripheral nerve changes in amyloid neuropathy. Brain 1974, 97, 395–406. [Google Scholar] [CrossRef][Green Version]
- Koike, H.; Ikeda, S.; Takahashi, M.; Kawagashira, Y.; Iijima, M.; Misumi, Y.; Ando, Y.; Ikeda, S.-I.; Katsuno, M.; Sobue, G. Schwann cell and endothelial cell damage in transthyretin familial amyloid polyneuropathy. Neurology 2016, 87, 2220–2229. [Google Scholar] [CrossRef]
- Koike, H.; Nishi, R.; Ikeda, S.; Kawagashira, Y.; Iijima, M.; Sakurai, T.; Shimohata, T.; Katsuno, M.; Sobue, G. The morphology of am-yloid fibrils and their impact on tissue damage in hereditary transthyretin amyloidosis: An ultrastructural study. J. Neurol. Sci. 2018, 394, 99–106. [Google Scholar] [CrossRef]
- Vital, C.; Vallat, J.M.; Deminiere, C.; Loubet, A.; Leboutet, M.J. Peripheral nerve damage during multiple myeloma and Walden-strom’s macroglobulinemia: An ultrastructural and immunopathologic study. Cancer 1982, 50, 1491–1497. [Google Scholar] [CrossRef]
- Sommer, C.; Schröder, J.M. Amyloid neuropathy: Immunocytochemical localization of intra- and extracellular immuno-globulin light chains. Acta. Neuropathol. 1989, 79, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Ando, Y.; Ueda, M.; Kawagashira, Y.; Iijima, M.; Fujitake, J.; Hayashi, M.; Yamamoto, M.; Mukai, E.; Nakamura, T.; et al. Distinct characteristics of amyloid deposits in early- and late-onset transthyretin Val30Met familial amyloid polyneuropathy. J. Neurol. Sci. 2009, 287, 178–184. [Google Scholar] [CrossRef]
- Koike, H.; Fukami, Y.; Nishi, R.; Kawagashira, Y.; Iijima, M.; Sobue, G.; Katsuno, M. Clinicopathological spectrum and recent ad-vances in the treatment of hereditary transthyretin amyloidosis. Neurol. Clin. Neurosci. 2019, 7, 166–173. [Google Scholar] [CrossRef]
- Aguzzi, A.; O’Connor, T. Protein aggregation diseases: Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 2010, 9, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Knowles, T.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Obici, L.; Adams, D. Acquired and inherited amyloidosis: Knowledge driving patients’ care. J. Peripher. Nerv. Syst. 2020, 25, 85–101. [Google Scholar] [CrossRef]
- Sousa, M.M.; Cardoso, I.; Fernandes, R.; Guimarães, A.; Saraiva, M.J. Deposition of transthyretin in early stages of familial amy-loidotic polyneuropathy: Evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 2001, 159, 1993–2000. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Sousa, M.M.; Cardoso, I.; do Amaral, J.B.; Guimarães, A.; Saraiva, M.J. Activation of ERK1/2 MAP kinases in familial amyloidotic polyneuropathy. J. Neurochem. 2006, 97, 151–161. [Google Scholar] [CrossRef]
- Fong, V.H.; Vieira, A. Pro-oxidative effects of aggregated transthyretin in human Schwannoma cells. NeuroToxicology 2013, 39, 109–113. [Google Scholar] [CrossRef]
- Ibrahim, R.B.; Yeh, S.Y.; Lin, K.P.; Ricardo, F.; Yu, T.Y.; Chan, C.C.; Tsai, J.W.; Liu, Y.T. Cellular Secretion and Cytotoxicity of Transthy-retin Mutant Proteins Underlie Late-Onset Amyloidosis and Neurodegeneration. Cell Mol. Life Sci. 2020, 77, 1421–1434. [Google Scholar] [CrossRef] [PubMed]
- Madhivanan, K.; Greiner, E.R.; Alves-Ferreira, M.; Soriano-Castell, D.; Rouzbeh, N.; Aguirre, C.A.; Paulsson, J.F.; Chapman, J.; Jiang, X.; Ooi, F.K.; et al. Cellular clearance of circulating transthyretin decreases cell-nonautonomous proteotoxicity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2018, 115, E7710–E7719. [Google Scholar] [CrossRef] [PubMed]
- Said, G.; Ropert, A.; Faux, N. Length-dependent degeneration of fibers in Portuguese amyloid polyneuropathy: A clinico-pathologic study. Neurology 1984, 34, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Misumi, Y.; Ando, Y.; Ueda, M.; Obayashi, K.; Jono, H.; Su, Y.; Yamashita, T.; Uchino, M. Chain reaction of amyloid fibril formation with induction of basement membrane in familial amyloidotic polyneuropathy. J. Pathol. 2009, 219, 481–490. [Google Scholar] [CrossRef]
- Hou, X.; Mechler, A.; Martin, L.L.; Aguilar, M.I.; Small, D.H. Cholesterol and anionic phospholipids increase the binding of amy-loidogenic transthyretin to lipid membranes. Biochim. Biophys. Acta 2008, 1778, 198–205. [Google Scholar] [CrossRef]
- Holmgren, G.; Steen, L.; Ekstedt, J.; Groth, C.-G.; Ericzon, B.-G.; Eriksson, S.; Andersen, O.; Karlberg, I.; Nordén, G.; Nakazato, M.; et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30). Clin. Genet. 1991, 40, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthy-retin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef]
- Emdin, M.; Aimo, A.; Rapezzi, C.; Fontana, M.; Perfetto, F.; Seferović, P.M.; Barison, A.; Castiglione, V.; Vergaro, G.; Giannoni, A.; et al. Treatment of cardiac transthyretin amyloidosis: An update. Eur. Hear. J. 2019, 40, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, N.R.; Rissing, S.M.; Smith, J.; Jung, J.; Benson, M.D. Inotersen therapy of transthyretin amyloid cardiomyopathy. Amyloid 2019, 27, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Coelho, T.; Maia, L.; da Silva, A.M.; Cruz, M.W.; Planté-Bordeneuve, V.; Lozeron, P.; Suhr, O.; Campistol, J.M.; Conceicao, I.; Schmidt, H.H.-J.; et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology 2012, 79, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Berk, J.L.; Suhr, O.B.; Obici, L.; Sekijima, Y.; Zeldenrust, S.R.; Yamashita, T.; Heneghan, M.A.; Gorevic, P.D.; Litchy, W.J.; Wiesman, J.F.; et al. Diflunisal Trial Consortium. Repurposing diflunisal for familial amyloid poly-neuropathy: A randomized clinical trial. JAMA 2013, 310, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Car-diomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Amaral, J.; Guimarães, A.; Saraiva, M.J. Up-regulation of the extracellular matrix remodeling genes, biglycan, neutrophil gelatinase-associated lipocalin and matrix metalloproteinase-9 in familial amyloid polyneuropathy. FASEB J. 2004, 19, 124–126. [Google Scholar] [CrossRef]
- Biolo, A.; Ramamurthy, S.; Connors, L.H.; O’Hara, C.J.; Meier-Ewert, H.K.; Soo Hoo, P.T.; Sawyer, D.B.; Seldin, D.C.; Sam, F. Matrix met-alloproteinases and their tissue inhibitors in cardiac amyloidosis: Relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ. Heart Fail. 2008, 1, 249–257. [Google Scholar] [CrossRef]
- Obici, L.; Cortese, A.; Lozza, A.; Lucchetti, J.; Gobbi, M.; Palladini, G.; Perlini, S.; Saraiva, M.J.; Merlini, G. Doxycycline plus taurour-sodeoxycholic acid for transthyretin amyloidosis: A phase II study. Amyloid 2012, 19 (Suppl. 1), 34–36. [Google Scholar] [CrossRef] [PubMed]
- Karlstedt, E.; Jimenez-Zepeda, V.; Howlett, J.G.; White, J.A.; Fine, N.M. Clinical Experience with the Use of Doxycycline and Ur-sodeoxycholic Acid for the Treatment of Transthyretin Cardiac Amyloidosis. J. Card Fail. 2019, 25, 147–153. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.; Szabo, A.; Flynn, K.E.; Dhakal, B.; Chhabra, S.; Pasquini, M.C.; Weihrauch, D.; Hari, P.N. Adjuvant doxycycline to en-hance anti-amyloid effects: Results from the dual phase 2 trial. EClinicalMedicine 2020, 23, 100361. [Google Scholar] [CrossRef]
- Bodin, K.; Ellmerich, S.; Kahan, M.C.; Tennent, G.A.; Loesch, A.; Gilbertson, J.A.; Hutchinson, W.L.; Mangione, P.P.; Gallimore, J.R.; Millar, D.J.; et al. Antibodies to human serum amyloid P component eliminate visceral amyloid deposits. Nature 2010, 468, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.; Cookson, L.M.; Berges, A.C.; Barton, S.V.; Lane, T.; Ritter, J.M.; Fontana, M.; Moon, J.; Pinzani, M.; Gillmore, J.D.; et al. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N. Engl. J. Med. 2015, 373, 1106–1114. [Google Scholar] [CrossRef]
- Wall, J.S.; Kennel, S.J.; Stuckey, A.C.; Long, M.J.; Townsend, D.W.; Smith, G.T.; Wells, K.J.; Fu, Y.; Stabin, M.G.; Weiss, D.T.; et al. Ra-dioimmunodetection of amyloid deposits in patients with AL amyloidosis. Blood 2010, 116, 2241–2244. [Google Scholar] [CrossRef]
- Gertz, M.A.; Landau, H.; Comenzo, R.L.; Seldin, D.; Weiss, B.; Zonder, J.; Merlini, G.; Schönland, S.; Walling, J.; Kinney, G.G.; et al. First-in-Human Phase I/II Study of NEOD001 in Patients with Light Chain Amyloidosis and Persistent Organ Dysfunction. J. Clin. Oncol. 2016, 34, 1097–1103. [Google Scholar] [CrossRef]
- Hosoi, A.; Su, Y.; Torikai, M.; Jono, H.; Ishikawa, D.; Soejima, K.; Higuchi, H.; Guo, J.; Ueda, M.; Suenaga, G.; et al. Novel Antibody for the Treatment of Transthyretin Amyloidosis. J. Biol. Chem. 2016, 291, 25096–25105. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Rappaport, M.; Shimoni, S.; Goland, S.; Voldarsky, I.; Fabricant, Y.; Edri, O.; Cuciuc, V.; Lifshitz, S.; Tshori, S.; et al. novel monoclonal antibody targeting aggregated transthyretin facilitates its removal and functional recovery in an exper-imental model. Eur. Heart J. 2020, 41, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Michalon, A.; Hagenbuch, A.; Huy, C.; Varela, E.; Combaluzier, B.; Damy, T.; Suhr, O.B.; Saraiva, M.J.; Hock, C.; Nitsch, R.M.; et al. A human antibody selective for transthyretin amyloid removes cardiac amyloid through phagocytic immune cells. Nat. Commun. 2021, 12, 3142. [Google Scholar] [CrossRef] [PubMed]

| ATTR Amyloidosis | AL Amyloidosis | ||
|---|---|---|---|
| ATTRwt Amyloidosis | ATTRv Amyloidosis | ||
| Precursor protein | Transthyretin | Transthyretin | Immunoglobulin light chain |
| Acquired or hereditary | Acquired | Hereditary | Acquired |
| Underlying condition | Aging | Mutation in the TTR gene | Plasma cell dyscrasia |
| Major organ involvement | Heart | Heart | Heart |
| Tendon/ligament | Peripheral nervous system | Peripheral nervous system | |
| Gastrointestinal tract | Gastrointestinal tract | ||
| Eye | Kidney | ||
| Liver | |||
| Soft tissue | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, H.; Katsuno, M. The Ultrastructure of Tissue Damage by Amyloid Fibrils. Molecules 2021, 26, 4611. https://doi.org/10.3390/molecules26154611
Koike H, Katsuno M. The Ultrastructure of Tissue Damage by Amyloid Fibrils. Molecules. 2021; 26(15):4611. https://doi.org/10.3390/molecules26154611
Chicago/Turabian StyleKoike, Haruki, and Masahisa Katsuno. 2021. "The Ultrastructure of Tissue Damage by Amyloid Fibrils" Molecules 26, no. 15: 4611. https://doi.org/10.3390/molecules26154611
APA StyleKoike, H., & Katsuno, M. (2021). The Ultrastructure of Tissue Damage by Amyloid Fibrils. Molecules, 26(15), 4611. https://doi.org/10.3390/molecules26154611







