Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sample Preparation
2.2.1. Bacterial Strains, Culture Conditions
2.2.2. Sample Preparation
2.3. Analytical Instrumentation
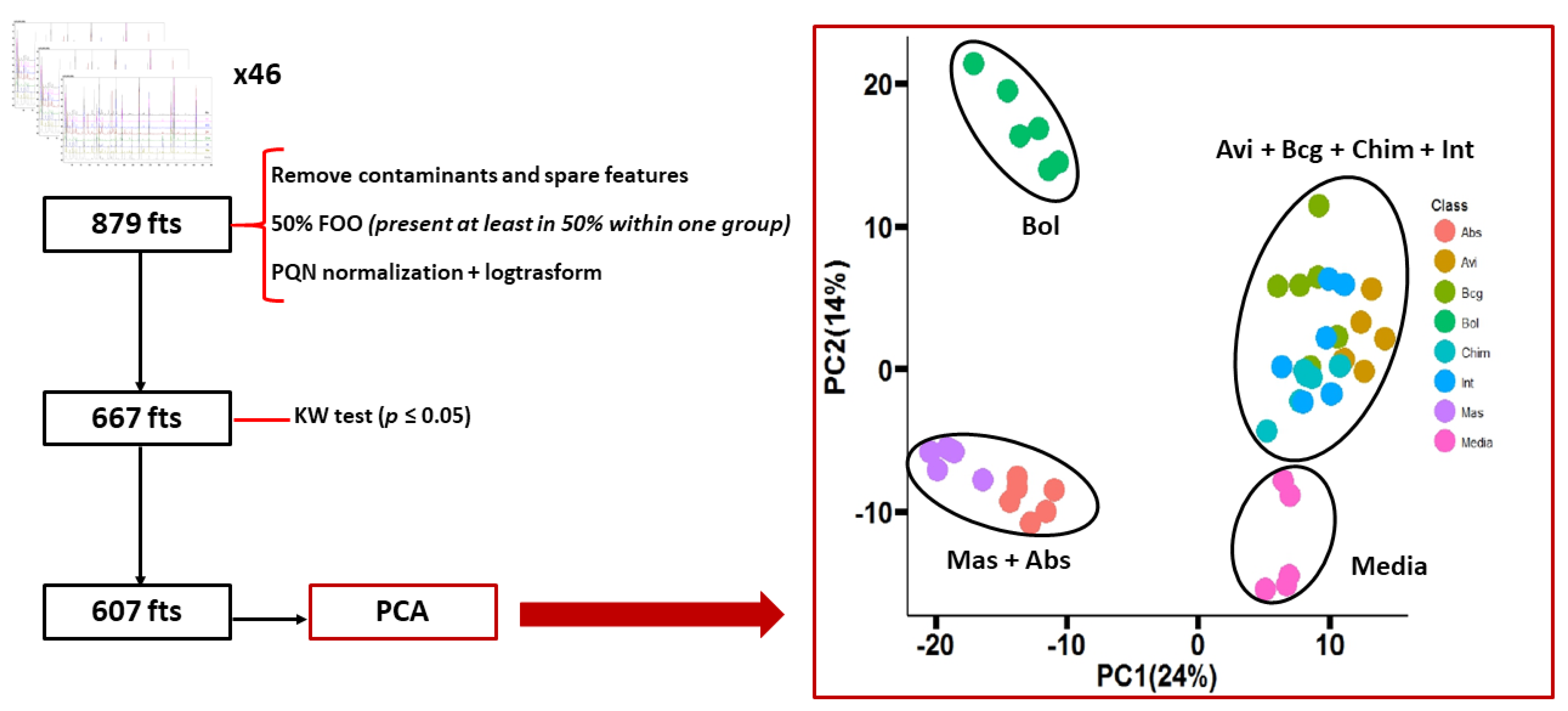
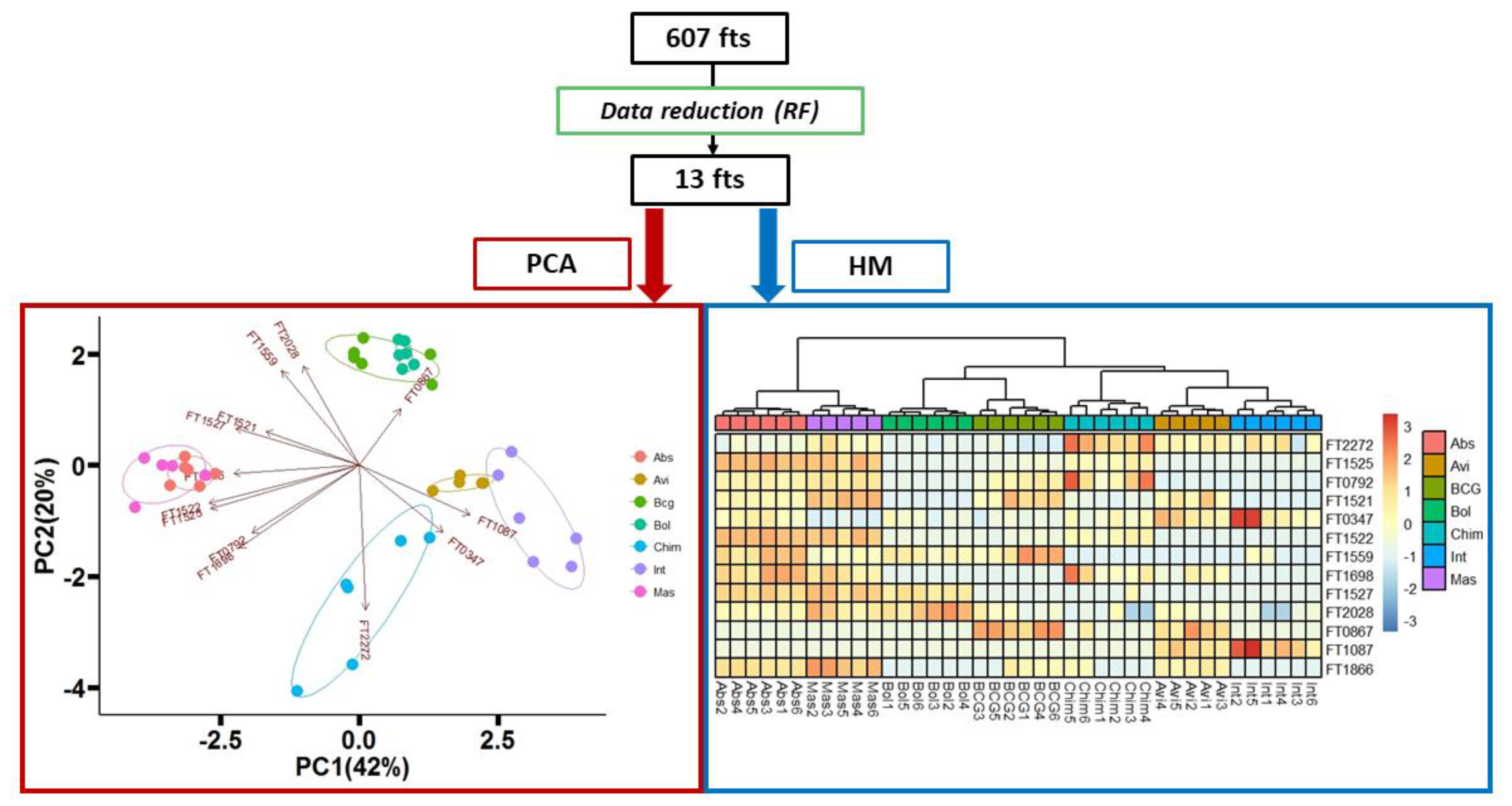
2.4. Statistical Analysis
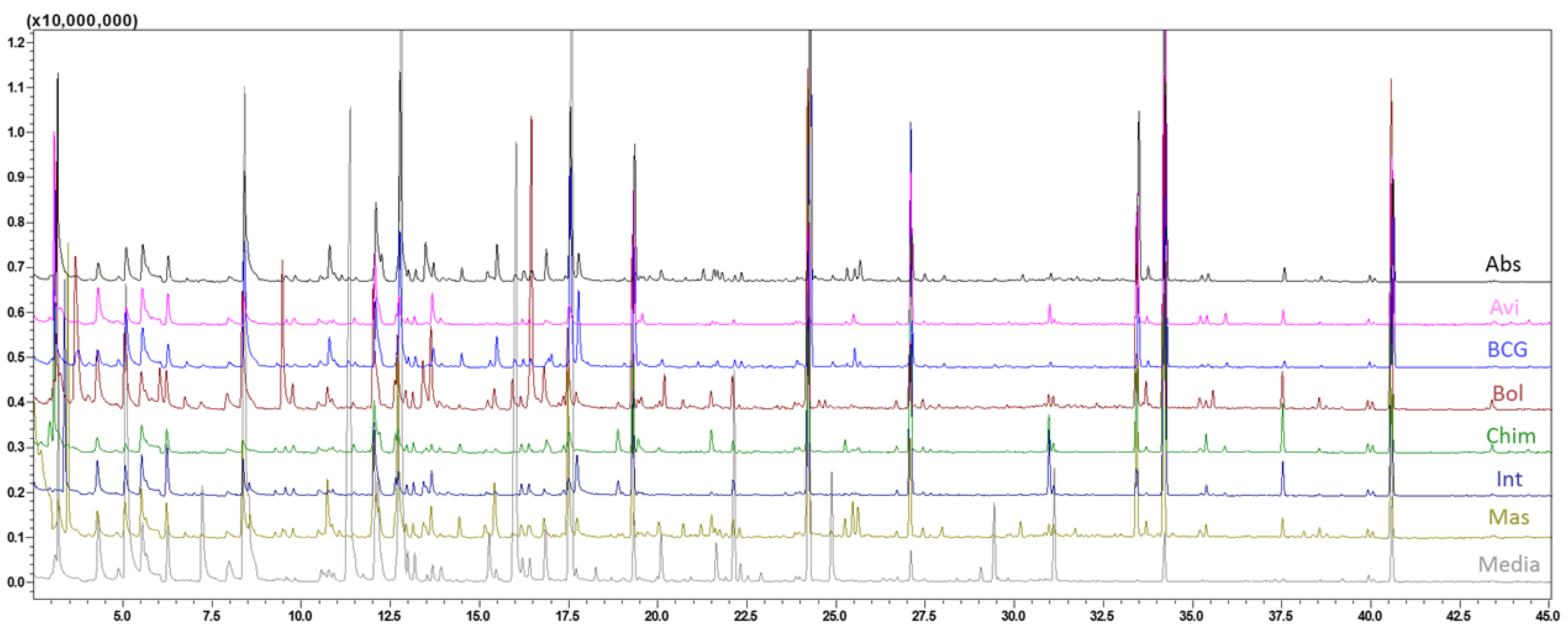
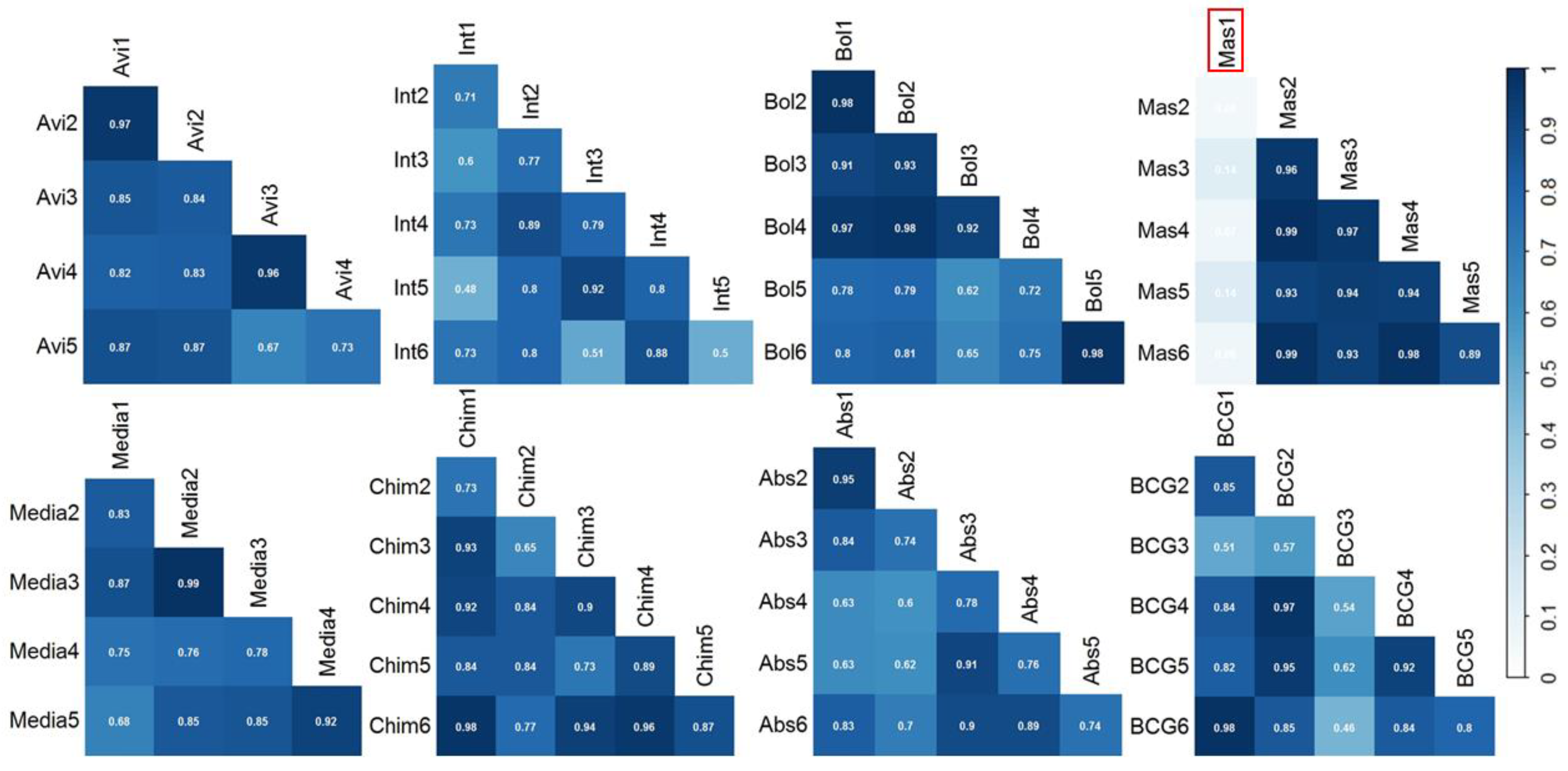
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rastogi, N.; Legrand, E.; Sola, C. The Mycobacteria: An introduction to nomenclature and pathogenesis. OIE Rev. Sci. Tech. 2001, 20, 21–54. [Google Scholar] [CrossRef]
- Johnson, M.M.; Odell, J.A. Nontuberculous mycobacterial pulmonary infections. J. Thorac. Dis. 2014, 6, 210–220. [Google Scholar] [CrossRef]
- Köhler, H.; Gierke, F.; Möbius, P. Paratuberculosis-current concepts and future of the diagnosis. Magy. Állatorvosok Lapja 2008, 130, 67–69. [Google Scholar]
- Beccaria, M.; Bobak, C.; Maitshotlo, B.; Mellors, T.R.; Purcaro, G.; Franchina, F.A.; Rees, C.A.; Nasir, M.; Black, A.; Hill, J.E. Exhaled human breath analysis in active pulmonary tuberculosis diagnostics by comprehensive gas chromatography-mass spectrometry and chemometric techniques. J. Breath Res. 2019, 13, 016005. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, M.; Mellors, T.R.; Petion, J.S.; Rees, C.A.; Nasir, M.; Systrom, H.K.; Sairistil, J.W.; Jean-Juste, M.A.; Rivera, V.; Lavoile, K.; et al. Preliminary investigation of human exhaled breath for tuberculosis diagnosis by multidimensional gas chromatography—Time of flight mass spectrometry and machine learning. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1074–1075, 46–50. [Google Scholar] [CrossRef]
- Küntzel, A.; Oertel, P.; Fischer, S.; Bergmann, A.; Trefz, P.; Schubert, J.; Miekisch, W.; Reinhold, P.; Köhler, H. Comparative analysis of volatile organic compounds for the classification and identification of mycobacterial species. PLoS ONE 2018, 13, e0194348. [Google Scholar] [CrossRef] [PubMed]
- Mellors, T.R.; Rees, C.A.; Wieland-Alter, W.F.; Von Reyn, C.F.; Hill, J.E. The volatile molecule signature of four mycobacteria species. J. Breath Res. 2017, 11, 031002. [Google Scholar] [CrossRef] [PubMed]
- van Mastrigt, E.; de Jongste, J.C.; Pijnenburg, M.W. The analysis of volatile organic compounds in exhaled breath and biomarkers in exhaled breath condensate in children-clinical tools or scientific toys? Clin. Exp. Allergy 2015, 45, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Fens, N.; van der Schee, M.P.; Brinkman, P.; Sterk, P.J. Exhaled breath analysis by electronic nose in airways disease. Established issues and key questions. Clin. Exp. Allergy 2013, 43, 705–715. [Google Scholar] [CrossRef]
- Oh, E.H.; Song, H.S.; Park, T.H. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb. Technol. 2011, 48, 427–437. [Google Scholar] [CrossRef]
- Burklund, A.; Saturley-Hall, H.K.; Franchina, F.A.; Hill, J.E.; Zhang, J.X.J. Printable QR code paper microfluidic colorimetric assay for screening volatile biomarkers. Biosens. Bioelectron. 2019, 128, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, K.; Chemie, F.; Rev, M.S.; Introduction, I.; Reference, C. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2011, 30, 236–267. [Google Scholar] [CrossRef]
- Lindinger, W.; Hansel, A.; Jordan, A. On-line monitoring of volatile organic compounds at pptv levels by means of Proton-Transfer-Reaction Mass Spectrometry (PTR-MS) Medical applications, food control and environmental research. Int. J. Mass Spectrom. Ion Process. 1998, 173, 191–241. [Google Scholar] [CrossRef]
- Baumbach, J.I. Ion mobility spectrometry coupled with multi-capillary columns for metabolic profiling of human breath. J. Breath Res. 2009, 3, 034001. [Google Scholar] [CrossRef]
- Purcaro, G.; Stefanuto, P.H.; Franchina, F.A.; Beccaria, M.; Wieland-Alter, W.F.; Wright, P.F.; Hill, J.E. SPME-GC×GC-TOF MS fingerprint of virally-infected cell culture: Sample preparation optimization and data processing evaluation. Anal. Chim. Acta 2018, 1027, 158–167. [Google Scholar] [CrossRef]
- Longo, V.; Forleo, A.; Provenzano, S.P.; Coppola, L.; Zara, V.; Ferramosca, A.; Siciliano, P.; Capone, S. HS-SPME-GC-MS metabolomics approach for sperm quality evaluation by semen volatile organic compounds (VOCs) analysis. Biomed. Phys. Eng. Express 2019, 5, 015006. [Google Scholar] [CrossRef]
- Maurer, D.L.; Ellis, C.K.; Thacker, T.C.; Rice, S.; Koziel, J.A.; Nol, P.; VerCauteren, K.C. Screening of Microbial Volatile Organic Compounds for Detection of Disease in Cattle: Development of Lab-scale Method. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Bojko, B.; Reyes-Garcés, N.; Bessonneau, V.; Goryński, K.; Mousavi, F.; Souza Silva, E.A.; Pawliszyn, J. Solid-phase microextraction in metabolomics. TrAC Trends Anal. Chem. 2014, 61, 168–180. [Google Scholar] [CrossRef]
- Dang, N.A.; Janssen, H.G.; Kolk, A.H.J. Rapid diagnosis of TB using GC-MS and chemometrics. Bioanalysis 2013, 5, 3079–3097. [Google Scholar] [CrossRef]
- Beccaria, M.; Franchina, F.A.; Nasir, M.; Mellors, T.; Hill, J.E.; Purcaro, G. Investigation of mycobacteria fatty acid profile using different ionization energies in GC–MS. Anal. Bioanal. Chem. 2018, 410, 7987–7996. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in1H NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate : A Practical and Powerful Approach to Multiple Testing. ournal R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Langley, P. The changing science of machine learning. Mach. Learn. 2011, 82, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Leao, S.C.; Tortoli, E.; Paul Euzé, J.; Garcia, M.J. Proposal that Mycobacterium massiliense and Mycobacterium bolletii be united and reclassified as Mycobacterium abscessus subsp. bolletii comb. nov., designation of Mycobacterium abscessus subsp. abscessus subsp. nov. and emended description of Mycobacteri. Int. J. Syst. Evol. Microbiol. 2011, 61, 2311–2313. [Google Scholar] [CrossRef] [Green Version]
- Tortoli, E.; Kohl, T.A.; Brown-Elliott, B.A.; Trovato, A.; Leão, S.C.; Garcia, M.J.; Vasireddy, S.; Turenne, C.Y.; Griffith, D.E.; Philley, J.V.; et al. Emended description of mycobacterium abscessus mycobacterium abscessus subsp. Abscessus and mycobacterium abscessus subsp. bolletii and designation of mycobacterium abscessus subsp. massiliense comb. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 4471–4479. [Google Scholar] [CrossRef]
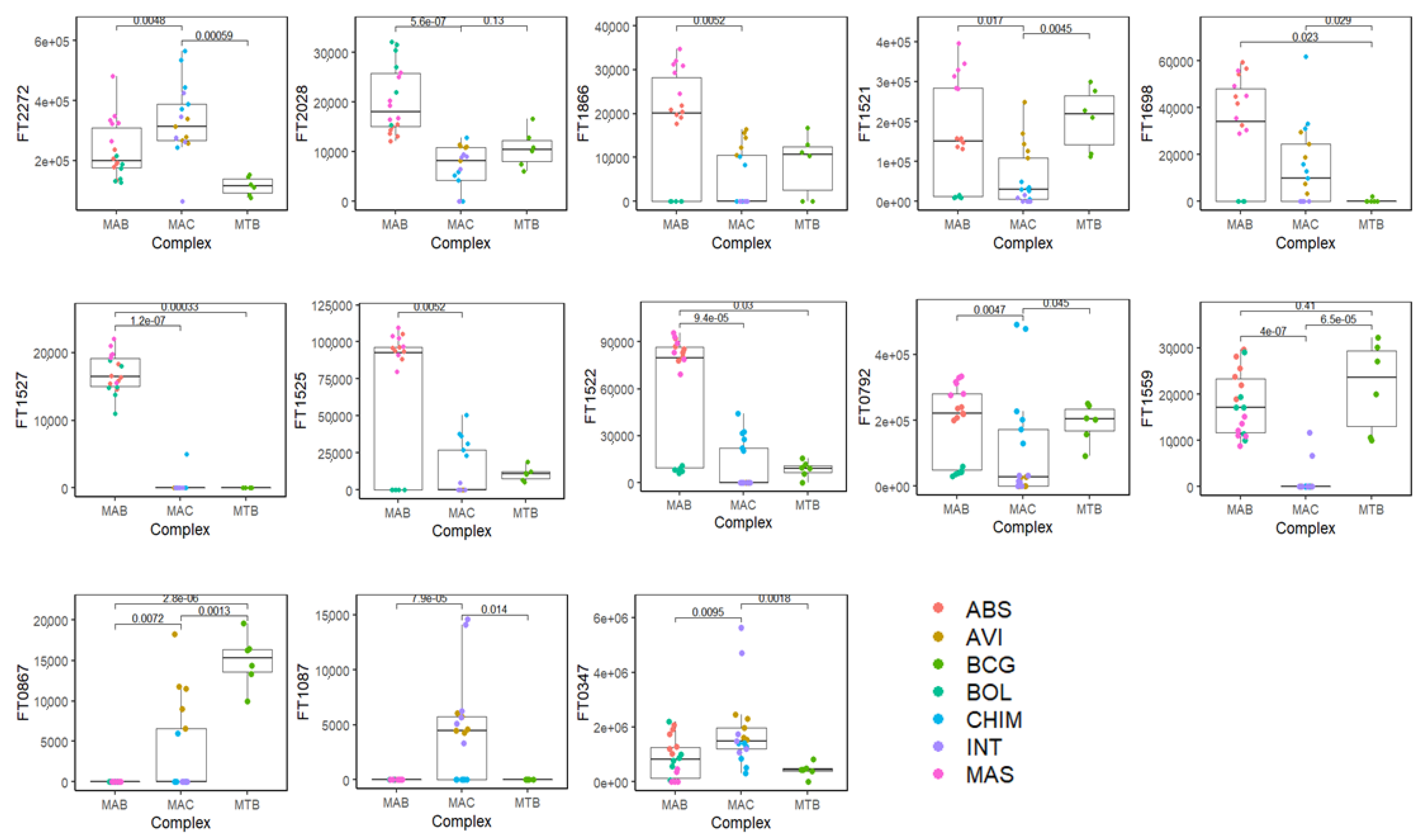
| FT n. | VOC | CAS | MS% | LRI | LRI lib | Rt |
|---|---|---|---|---|---|---|
| FT0347 | 2-Butanol, 2,3-dimethyl- | 594-60-5 | 83 | 649 | 645 | 2.31 |
| FT1087 | Hexanal | 66-25-1 | 94 | 800 | 801 | 5.31 |
| FT0867 | Furan, 2-butyl- | 4466-24-4 | 83 | 888 | 890 | 7.98 |
| FT1559 | Furan, 2-methyl-3-(methylthio)- | 63012-97-5 | 84 | 942 | 946 | 10.23 |
| FT0792 | Phenylacetaldehyde | 122-78-1 | 85 | 1037 | 1045 | 14.50 |
| FT1522 | unknown | 1074 | 16.27 | |||
| FT1525 | (Z)-2-Hexenal diethyl acetal | 87383-46-8 | 81 | 1078 | 1077 | 16.49 |
| FT1527 | Decanal | 112-31-2 | 81 | 1171 | 1187 | 20.86 |
| FT1698 | 2-Nonenoic acid, methyl ester | 111-79-5 | 81 | 1189 | 1191 | 21.79 |
| FT1521 | unknown | 1270 | 25.51 | |||
| FT1866 | unknown | 1326 | 28.03 | |||
| FT2028 | unknown | 1462 | 33.85 | |||
| FT2272 | Ethyl 4-t-butylbenzoate | 5406-57-5 | 80 | 1498 | 1487 | 35.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beccaria, M.; Franchina, F.A.; Nasir, M.; Mellors, T.; Hill, J.E.; Purcaro, G. Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning. Molecules 2021, 26, 4600. https://doi.org/10.3390/molecules26154600
Beccaria M, Franchina FA, Nasir M, Mellors T, Hill JE, Purcaro G. Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning. Molecules. 2021; 26(15):4600. https://doi.org/10.3390/molecules26154600
Chicago/Turabian StyleBeccaria, Marco, Flavio A. Franchina, Mavra Nasir, Theodore Mellors, Jane E. Hill, and Giorgia Purcaro. 2021. "Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning" Molecules 26, no. 15: 4600. https://doi.org/10.3390/molecules26154600
APA StyleBeccaria, M., Franchina, F. A., Nasir, M., Mellors, T., Hill, J. E., & Purcaro, G. (2021). Investigating Bacterial Volatilome for the Classification and Identification of Mycobacterial Species by HS-SPME-GC-MS and Machine Learning. Molecules, 26(15), 4600. https://doi.org/10.3390/molecules26154600







