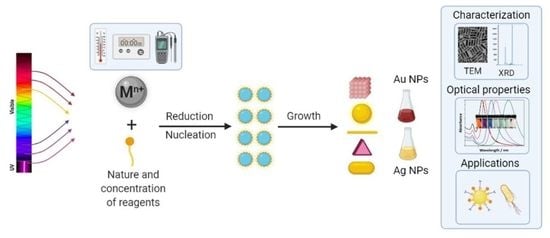
Photochemical Synthesis of Gold and Silver Nanoparticles—A Review
Abstract
:1. Introduction
2. Photochemical Processes
3. Synthesis of Gold and Silver Nanoparticles under UV Light Irradiation
3.1. Gold Nanoparticles (Au NPs)
3.1.1. Influence of pH
3.1.2. Influence of Precursor Concentration
3.1.3. Greener Alternatives
3.2. Silver Nanoparticles (Ag NPs)
3.2.1. Influence of pH
3.2.2. Influence of Reducing Agents
3.3. Bimetallic Silver–Gold Nanoparticles
4. Synthesis of Gold and Silver Nanoparticles under Visible Light Irradiation
4.1. Synthesis of Au NPs
4.2. Synthesis of Ag NPs
4.3. Greener Alternatives
4.4. Impact of Experimental Parameters
5. Applications of Photochemically Produced Ag NPs and Au NPs
6. Conclusions and Future Developments
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016, 6, 4. [Google Scholar] [CrossRef]
- Lin, S.K.; Cheng, W.T. Fabrication and characterization of colloidal silver nanoparticle via photochemical synthesis. Mater. Lett. 2020, 261, 261. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Khan, S.A.; Lee, C.-S. Green Biological Synthesis of Nanoparticles and Their Biomedical Applications. In Applications of Nanotechnology for Green Synthesis; Springer: Cham, Switzerland, 2020; pp. 247–280. [Google Scholar] [CrossRef]
- Khezri, K.; Saeedi, M.; Dizaj, S.M. Application of nanoparticles in percutaneous delivery of active ingredients in cosmetic preparations. Biomed. Pharmacother. 2018, 106, 1499–1505. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Senthilnathan, R.; Megarajan, S.; Anbazhagan, V. Sunlight mediated synthesis of silver nanoparticles using redox phytoprotein and their application in catalysis and colorimetric mercury sensing. J. Photochem. Photobiol. B Biol. 2015, 151, 39–45. [Google Scholar] [CrossRef]
- Bárta, J.; Procházková, L.; Vaněček, V.; Kuzár, M.; Nikl, M.; Čuba, V. Photochemical synthesis of nano- and micro-crystalline particles in aqueous solutions. Appl. Surf. Sci. 2019, 479, 506–511. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Species selection for the design of gold nanobioreactor by photosynthetic organisms. J. Nanopart. Res. 2012, 14, 1–17. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Wijesekera, K.; Filipe, C.D.; Brennan, J.D. Stoichiometrically controlled production of bimetallic Gold-Silver alloy colloids using micro-alga cultures. J. Colloid Interface Sci. 2014, 416, 67–72. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Varca, G.H.C.; Batista, J.G.D.S.; Lugão, A.B. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Armijo García, D.; Mendoza, L.; Vizuete, K.; Debut, A.; Arias, M.; Gavilanes, A.; Terencio, T.; Ávila, E.; Jeffryes, C.; Dahoumane, S. Sugar-Mediated Green Synthesis of Silver Selenide Semiconductor Nanocrystals under Ultrasound Irradiation. Molecules 2020, 25, 5193. [Google Scholar] [CrossRef]
- Kumar, S.; Bafana, A.P.; Pawar, P.; Rahman, A.; Dahoumane, S.A.; Jeffryes, C.S. High conversion synthesis of <10 nm starch-stabilized silver nanoparticles using microwave technology. Sci. Rep. 2018, 8, 5106. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.V.; Bafana, A.P.; Pawar, P.; Faltane, M.; Rahman, A.; Dahoumane, S.A.; Kucknoor, A.; Jeffryes, C.S. Optimized production of antibacterial copper oxide nanoparticles in a microwave-assisted synthesis reaction using response surface methodology. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 170–178. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Lin, J.; Dahoumane, S.A.; Jeffryes, C. A Mechanistic View of the Light-Induced Synthesis of Silver Nanoparticles Using Extracellular Polymeric Substances of Chlamydomonas reinhardtii. Molecules 2019, 24, 3506. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.; Rajasekar, P.; Vijayaprasath, G.; Ravi, G.; Manikandan, R.; Prabhu, N.M. A green route to synthesis silver nanoparticles using Sargassum polycystum and its antioxidant and cytotoxic effects: An in vitro analysis. Mater. Lett. 2017, 189, 196–200. [Google Scholar] [CrossRef]
- Bagur, H.; Medidi, R.S.; Somu, P.; Choudhury, P.W.J.; Karua, C.S.; Guttula, P.K.; Melappa, G.; Poojari, C.C. Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Mater. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Korbekandi, H.; Mohseni, S.; Jouneghani, R.M.; Pourhossein, M.; Iravani, S. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif. Cells Nanomed. Biotechnol. 2014, 44, 235–239. [Google Scholar] [CrossRef]
- Mourato, A.; Gadanho, M.; Lino, A.R.; Tenreiro, R. Biosynthesis of Crystalline Silver and Gold Nanoparticles by Extremophilic Yeasts. Bioinorg. Chem. Appl. 2011, 2011, 546074. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.B.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Wujcik, E.K.; Jeffryes, C. Noble metal, oxide and chalcogenide-based nanomaterials from scalable phototrophic culture systems. Enzym. Microb. Technol. 2016, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Dahoumane, S.A.; Mechouet, M.; Alvarez, F.J.; Agathos, S.N.; Jeffryes, C. Microalgae: An outstanding tool in nanotechnology. Bionatura 2016, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology—A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Yugay, Y.; Usoltseva, R.; Silant’Ev, V.; Egorova, A.; Karabtsov, A.; Kumeiko, V.; Ermakova, S.; Bulgakov, V.; Shkryl, Y. Synthesis of bioactive silver nanoparticles using alginate, fucoidan and laminaran from brown algae as a reducing and stabilizing agent. Carbohydr. Polym. 2020, 245, 116547. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Brayner, R. Design of magnetic akaganeite-cyanobacteria hybrid biofilms. Thin Solid Films 2010, 518, 5432–5436. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, J.; Jaramillo, F.E.; Bazylinski, D.A.; Jeffryes, C.; Dahoumane, S.A. In Vivo Biosynthesis of Inorganic Nanomaterials Using Eukaryotes—A Review. Molecules 2020, 25, 3246. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Biosynthetic Conversion of Ag+ to highly Stable Ag0 Nanoparticles by Wild Type and Cell Wall Deficient Strains of Chlamydomonas reinhardtii. Molecules 2018, 24, 98. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Zambonino, M.; Quizhpe, E.; Jaramillo, F.; Rahman, A.; Vispo, N.S.; Jeffryes, C.; Dahoumane, S. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, I.G.; Bachmatiuk, A.; Bezugly, V.; Kunstmann, J.; Gemming, T.; Liu, Z.; Cuniberti, G.; Rümmeli, M.H. Electron-beam induced synthesis of nanostructures: A review. Nanoscale 2016, 8, 11340–11362. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Osone, S.; Kim, T.; Higashi, H.; Seto, T. Synthesis of Nanoparticles by Laser Ablation: A Review. KONA Powder Part. J. 2017, 34, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef] [Green Version]
- Daruich De Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloy. Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. Conventional and Microwave Hydrothermal Synthesis and Application of Functional Materials: A Review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef] [Green Version]
- Elsupikhe, R.F.; Ahmad, M.B.; Shameli, K.; Ibrahim, N.A.; Zainuddin, N. Photochemical Reduction as a Green Method for the Synthesis and Size Control of Silver Nanoparticles in κ-Carrageenan. IEEE Trans. Nanotechnol. 2016, 15, 209–213. [Google Scholar] [CrossRef]
- Gabriel, J.S.; Gonzaga, V.A.; Poli, A.L.; Schmitt, C.C. Photochemical synthesis of silver nanoparticles on chitosans/montmorillonite nanocomposite films and antibacterial activity. Carbohydr. Polym. 2017, 171, 202–210. [Google Scholar] [CrossRef]
- Le, N.H.; Hajjar-Garreau, S.; Bonne, M.; Megías-Sayago, C.; Louis, B.; Lebeau, B.; Balan, L. Photo-induced generation of size controlled Au nanoparticles on pure siliceous ordered mesoporous silica for catalytic applications. Microporous Mesoporous Mater. 2020, 295, 109952. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Kumar, V.; Gundampati, R.K.; Singh, D.K.; Bano, D.; Jagannadham, M.V.; Hasan, S.H. Photoinduced green synthesis of silver nanoparticles with highly effective antibacterial and hydrogen peroxide sensing properties. J. Photochem. Photobiol. B Biol. 2016, 162, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Annadhasan, M.; Kasthuri, J.; Rajendiran, N. Green synthesis of gold nanoparticles under sunlight irradiation and their colorimetric detection of Ni2+ and Co2+ ions. RSC Adv. 2015, 5, 11458–11468. [Google Scholar] [CrossRef]
- Mukha, I.; Khodko, A.; Vityuk, N.; Severynovska, O.; Pivovarenko, V.; Kachalova, N.; Smirnova, N.; Eremenko, A. Light-driven formation of gold/tryptophan nanoparticles. Appl. Nanosci. 2019, 10, 2827–2833. [Google Scholar] [CrossRef]
- Dong, S.-A.; Zhou, S.-P. Photochemical synthesis of colloidal gold nanoparticles. Mater. Sci. Eng. B 2007, 140, 153–159. [Google Scholar] [CrossRef]
- Lazzaroni, S.; Ravelli, D.; Protti, S.; Fagnoni, M.; Albini, A. Photochemical synthesis: Using light to build C–C bonds under mild conditions. Comptes Rendus Chim. 2017, 20, 261–271. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujistuka, M.; Majima, T. Light as a construction tool of metal nanoparticles: Synthesis and mechanism. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 33–56. [Google Scholar] [CrossRef]
- Oelgemöller, M. Solar Photochemical Synthesis: From the Beginnings of Organic Photochemistry to the Solar Manufacturing of Commodity Chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef]
- Eremenko, A.M.; Smirnoval, N.P.; Mukhal, I.P.; Yashan, H.R. Silver and gold nanoparticles in silica matrices: Synthesis, properties, and application. Theor. Exp. Chem. 2010, 46, 65–88. [Google Scholar] [CrossRef]
- de Oliveira, P.F.M.; Torresi, R.M.; Emmerling, F.; Camargo, P.H.C. Challenges and opportunities in the bottom-up mechanochemical synthesis of noble metal nanoparticles. J. Mater. Chem. A 2020, 8, 16114–16141. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of nanocomposite thin films containing silver and gold nanoparticles with 2-thioxanthone thioacetic acid-dioxide and their role in photocatalytic degradation of methylene blue. Surf Interfaces 2021, 22, 100793. [Google Scholar] [CrossRef]
- Reddy, G.B.; Madhusudhan, A.; Ramakrishna, D.; Ayodhya, D.; Venkatesham, M.; Veerabhadram, G. Green chemistry approach for the synthesis of gold nanoparticles with gum kondagogu: Characterization, catalytic and antibacterial activity. J. Nanostruct. Chem. 2015, 5, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Anh, M.N.T.; Nguyen, D.T.D.; Thanh, N.V.K.; Phong, N.T.P.; Nguyen, D.H.; Nguyen-Le, M.-T. Photochemical Synthesis of Silver Nanodecahedrons under Blue LED Irradiation and Their SERS Activity. Processes 2020, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.A.; Paterno, L.G.; Moreira, S.G.C.; Sales, M.J.A. Original photochemical synthesis of Ag nanoparticles mediated by potato starch. SN Appl. Sci. 2019, 1, 554. [Google Scholar] [CrossRef] [Green Version]
- Majdalawieh, A.; Kanan, M.C.; El-Kadri, O.; Kanan, S.M. Recent advances in gold and silver nanoparticles: Synthesis and applications. J. Nanosci. Nanotechnol. 2014, 14, 4757–4780. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Element Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Qi, H.; Kinkead, B.; Hegmann, T. Unprecedented Dual Alignment Mode and Freedericksz Transition in Planar Nematic Liquid Crystal Cells Doped with Gold Nanoclusters. Adv. Funct. Mater. 2008, 18, 212–221. [Google Scholar] [CrossRef]
- Choudhary, A.; Singh, G.; Biradar, A.M. Advances in gold nanoparticle–liquid crystal composites. Nanoscale 2014, 6, 7743–7756. [Google Scholar] [CrossRef]
- Watanabe, K. Photochemistry on Nanoparticles. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Cambridge, MA, USA, 2018; pp. 563–572. [Google Scholar]
- Srinoi, P.; Chen, Y.-T.; Vittur, V.; Marquez, M.D.; Lee, T.R. Bimetallic Nanoparticles: Enhanced Magnetic and Optical Properties for Emerging Biological Applications. Appl. Sci. 2018, 8, 1106. [Google Scholar] [CrossRef] [Green Version]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.J.; Kiely, C. Synthesis and reactions of functionalised gold nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 16, 1655–1656. [Google Scholar] [CrossRef]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Pinchuk, A.O. Noble Metal Nanomaterials: Synthetic Routes, Fundamental Properties, and Promising Applications. In Solid State Physics; Elsevier: Oxford, UK, 2015; pp. 131–211. [Google Scholar]
- Abu Bakar, N.H.H.; Ismail, J.; Abu Bakar, M. Synthesis and characterization of silver nanoparticles in natural rubber. Mater. Chem. Phys. 2007, 104, 276–283. [Google Scholar] [CrossRef]
- Jagiello, K.; Chomicz, B.; Avramopoulos, A.; Gajewicz, A.; Mikolajczyk, A.; Bonifassi, P.; Papadopoulos, M.G.; Leszczynski, J.; Puzyn, T. Size-dependent electronic properties of nanomaterials: How this novel class of nanodescriptors supposed to be calculated? Struct. Chem. 2016, 28, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C 2016, 65, 199–204. [Google Scholar] [CrossRef]
- Njoki, P.; Lim, I.-I.S.; Mott, D.; Park, H.-Y.; Khan, B.; Mishra, S.; Sujakumar, R.; Luo, A.J.; Zhong, C.-J. Size Correlation of Optical and Spectroscopic Properties for Gold Nanoparticles. J. Phys. Chem. C 2007, 111, 14664–14669. [Google Scholar] [CrossRef]
- Lee, K.-S.; El-Sayed, M.A. Gold and Silver Nanoparticles in Sensing and Imaging: Sensitivity of Plasmon Response to Size, Shape, and Metal Composition. J. Phys. Chem. B 2006, 110, 19220–19225. [Google Scholar] [CrossRef]
- Piñón-Segundo, E.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D. Nanoparticles as Dental Drug-Delivery Systems. In Nanobiomaterials in Clinical Dentistry; Elsevier: Oxford, UK, 2013; pp. 475–495. [Google Scholar]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2011, 4, 1871–1880. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Aval, S.F.; Kouhi, M.; Akbarzadeh, A.; Nasrabadi, H.T.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2014, 42, 173–180. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial Silver Nanoparticles for Wound Healing Application: Progress and Future Trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.; Khlebtsov, N. Immunological properties of gold nanoparticles. Chem. Sci. 2017, 8, 1719–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Physics X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Kueh, A.B.H.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Amirjani, A.; Firouzi, F.; Haghshenas, D.F. Predicting the Size of Silver Nanoparticles from Their Optical Properties. Plasmonics 2020, 15, 1077–1082. [Google Scholar] [CrossRef]
- Samai, S.; Qian, Z.; Ling, J.; Guye, K.N.; Ginger, D.S. Optical Properties of Reconfigurable Polymer/Silver Nanoprism Hybrids: Tunable Color and Infrared Scattering Contrast. ACS Appl. Mater. Interfaces 2018, 10, 8976–8984. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Lee, Y.R.; Sethuraman, M.G. Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 161, 122–129. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, T.; Rahman, M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Scaiano, J.; Billone, P.; Gonzalez, C.M.; Marett, L.; Marin, M.L.; McGilvray, K.L.; Yuan, N. Photochemical routes to silver and gold nanoparticles. Pure Appl. Chem. 2009, 81, 635–647. [Google Scholar] [CrossRef]
- El-Sheikh, M.A. A Novel Photosynthesis of Carboxymethyl Starch-Stabilized Silver Nanoparticles. Sci. World J. 2014, 2014, 514563. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K.; Saion, E. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Prakash, A.; Radhakrishnan, E.K. Sunlight mediated rapid synthesis of small size range silver nanoparticles using Zingiber officinale rhizome extract and its antibacterial activity analysis. Inorg. Nano-Metal Chem. 2018, 48, 139–145. [Google Scholar] [CrossRef]
- Mallick, K.; Wang, Z.L.; Pal, T. Seed-mediated successive growth of gold particles accomplished by UV irradiation: A photochemical approach for size-controlled synthesis. J. Photochem. Photobiol. A Chem. 2001, 140, 75–80. [Google Scholar] [CrossRef]
- Darroudi, M.; Ahmad, M.B.; Zak, A.K.; Zamiri, R.; Hakimi, M. Fabrication and Characterization of Gelatin Stabilized Silver Nanoparticles under UV-Light. Int. J. Mol. Sci. 2011, 12, 6346–6356. [Google Scholar] [CrossRef] [Green Version]
- Kempa, T.; Farrer, R.A.; Giersig, M.; Fourkas, J.T. Photochemical Synthesis and Multiphoton Luminescence of Monodisperse Silver Nanocrystals. Plasmonics 2006, 1, 45–51. [Google Scholar] [CrossRef]
- Huang, W.-C.; Chen, Y.-C. Photochemical synthesis of polygonal gold nanoparticles. J. Nanopart. Res. 2007, 10, 697–702. [Google Scholar] [CrossRef]
- Sengani, M.; Grumezescu, A.M.; Rajeswari, V.D. Recent trends and methodologies in gold nanoparticle synthesis—A prospective review on drug delivery aspect. OpenNano 2017, 2, 37–46. [Google Scholar] [CrossRef]
- Slepička, P.; Kasálková, N.S.; Siegel, J.; Kolská, Z.; Švorčík, V. Methods of Gold and Silver Nanoparticles Preparation. Materials 2019, 13, 1. [Google Scholar] [CrossRef] [Green Version]
- Valandro, S.; Poli, A.; Neumann, M.; Schmitt, C. Photochemical Synthesis of Ag and Au Nanoparticles Using a Thioxanthone Substituted Chitosan as Simultaneous Photoinitiator and Stabilizer. J. Braz. Chem. Soc. 2019, 30, 2642–2648. [Google Scholar] [CrossRef]
- Courrol, L.; Silva, F.R.D.O.; Gomes, L. A simple method to synthesize silver nanoparticles by photo-reduction. Colloid Surf A Physicochem. Eng. Asp. 2007, 305, 54–57. [Google Scholar] [CrossRef]
- Łukowiec, D.; Radoń, A. Self-organization of silver nanoparticles during synthesis of Ag–Au nanoalloy by UV irradiation method. J. Mater. Sci. 2020, 55, 2796–2801. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wei, G.; Guo, C.; Sun, L.; Sun, Y.; Song, Y.; Yang, T.; Li, Z. Photochemical synthesis and self-assembly of gold nanoparticles. Colloid Surf A Physicochem. Eng. Asp. 2008, 312, 148–153. [Google Scholar] [CrossRef]
- Dubas, S.T.; Pimpan, V. Green synthesis of silver nanoparticles for ammonia sensing. Talanta 2008, 76, 29–33. [Google Scholar] [CrossRef]
- Kora, A.J.; Manjusha, R.; Arunachalam, J. Superior bactericidal activity of SDS capped silver nanoparticles: Synthesis and characterization. Mater. Sci. Eng. C 2009, 29, 2104–2109. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, J.; Zhang, J.; Lian, Y.; Shi, Z.; Cheng, Z.; Gu, M. pH-controlled growth of triangular silver nanoprisms on a large scale. Nanoscale Adv. 2019, 1, 4904–4908. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-H.; Huang, K.-M.; Tseng, W.-L.; Chiu, T.-C.; Lin, Y.-W.; Chang, H.-T. Manipulation of the Growth of Gold and Silver Nanomaterials on Glass by Seeding Approach. Langmuir 2007, 23, 1435–1442. [Google Scholar] [CrossRef]
- Eftekhari-Kenzerki, Z.; Fardid, R.; Behzad-Behbahani, A. Impact of Silver Nanoparticles on the Ultraviolet Radiation Direct and Bystander Effects on TK6 Cell Line. J. Med. Phys. 2019, 44, 118–125. [Google Scholar] [PubMed]
- Chandra, M.; Das, P.K. Green Routes to Noble Metal Nanoparticle Synthesis. Int. J. Green Nanotechnol. Phys. Chem. 2009, 1, P10–P25. [Google Scholar] [CrossRef]
- Huang, L.; Zhai, M.L.; Long, D.W.; Peng, J.; Xu, L.; Wu, G.Z.; Li, J.Q.; Wei, G.S. UV-induced synthesis, characterization and formation mechanism of silver nanoparticles in alkalic carboxymethylated chitosan solution. J. Nanopart. Res. 2008, 10, 1193–1202. [Google Scholar] [CrossRef]
- Abdelrasoul, G.N.; Cingolani, R.; Diaspro, A.; Athanassiou, A.; Pignatelli, F. Photochemical synthesis: Effect of UV irradiation on gold nanorods morphology. J. Photochem. Photobiol. A Chem. 2014, 275, 7–11. [Google Scholar] [CrossRef]
- Kundu, S.; Panigrahi, S.; Praharaj, S.; Basu, S.; Ghosh, S.K.; Pal, A.; Pal, T. Anisotropic growth of gold clusters to gold nanocubes under UV irradiation. Nanotechnology 2007, 18, 075712. [Google Scholar] [CrossRef]
- Rodríguez, G.R.C.; Gauthier, G.H.; Ladeira, L.O.; Cala, J.A.S.; Cataño, D.L. Effect of pH and chloroauric acid concentration on the geometry of gold nanoparticles obtained by photochemical synthesis. J. Phys. Conf. Ser. 2017, 935, 012027. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Ge, L.; Xiong, B.; He, Y. Investigation of pH Effect on Gold Nanorod Synthesis. J. Chin. Chem. Soc. 2011, 58, 822–827. [Google Scholar] [CrossRef]
- Unal, I.S.; Demirbaş, A.; Onal, I.; Ildiz, N.; Ocsoy, I. One step preparation of stable gold nanoparticle using red cabbage extracts under UV light and its catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111800. [Google Scholar] [CrossRef]
- Sanabria-Cala, J.A.; Conde-Rodríguez, G.R.; Gauthier, G.H.; Ladeira, L.O.; Laverde-Cataño, D.A.; Peña-Ballesteros, D.Y.; Merchan-Arenas, D. Gold Nanoparticles Formation Mechanism by Photochemical Synthesis. Chem. Eng. Trans. 2018, 64, 403–408. [Google Scholar]
- Shiraishi, Y.; Tanaka, H.; Sakamoto, H.; Ichikawa, S.; Hirai, T. Photoreductive synthesis of monodispersed Au nanoparticles with citric acid as reductant and surface stabilizing reagent. RSC Adv. 2017, 7, 6187–6192. [Google Scholar] [CrossRef] [Green Version]
- Zewde, B.; Ambaye, A.; Stubbs, J., III; Raghavan, D. A Review of Stabilized Silver Nanoparticles—Synthesis, Biological Properties, Characterization, and Potential Areas of Applications. JSM Nanotechnol. Nanomed. 2016, 4, 1043. [Google Scholar]
- Teixeira, P.R.; Santos, M.S.; Silva, A.L.G.; Báo, S.; Azevedo, R.B.; Sales, M.J.A.; Paterno, L.G. Photochemically-assisted synthesis of non-toxic and biocompatible gold nanoparticles. Colloid Surf B Biointerfaces 2016, 148, 317–323. [Google Scholar] [CrossRef]
- Huang, Y.; Kim, D.-H. Light-controlled synthesis of gold nanoparticles using a rigid, photoresponsive surfactant. Nanoscale 2012, 4, 6312–6317. [Google Scholar] [CrossRef]
- Pal, A. Photochemical synthesis of gold nanoparticles via controlled nucleation using a bioactive molecule. Mater. Lett. 2004, 58, 529–534. [Google Scholar] [CrossRef]
- Liao, L.; Lv, G.; Cai, D.; Wu, L. The sequential intercalation of three types of surfactants into sodium montmorillonite. Appl. Clay Sci. 2016, 119, 82–86. [Google Scholar] [CrossRef]
- Shang, Y.; Min, C.; Hu, J.; Wang, T.; Liu, H.; Hu, Y. Synthesis of gold nanoparticles by reduction of HAuCl4 under UV irradiation. Solid State Sci. 2013, 15, 17–23. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-light mediated green synthesis of silver and gold nanoparticles using Cornelian cherry fruit extract and their comparative effects in experimental inflammation. J. Photochem. Photobiol. B Biol. 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Yulizar, Y.; Utari, T.; Ariyanta, H.A.; Maulina, D. Green Method for Synthesis of Gold Nanoparticles Using Polyscias scutellaria Leaf Extract under UV Light and Their Catalytic Activity to Reduce Methylene Blue. J. Nanomater. 2017, 2017, 3079636. [Google Scholar] [CrossRef] [Green Version]
- Babu, P.J.; Sharma, P.; Saranya, S.; Bora, U. Synthesis of gold nanoparticles using ethonolic leaf extract of Bacopa monnieri and UV irradiation. Mater. Lett. 2013, 93, 431–434. [Google Scholar] [CrossRef]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “Green” Synthesis and Stabilization of Metal Nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef]
- Mittelman, A.M.; Fortner, J.; Pennell, K.D. Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ. Sci. Nano 2015, 2, 683–691. [Google Scholar] [CrossRef]
- Spadaro, D.; Barletta, E.; Barreca, F.; Currò, G.; Neri, F. Synthesis of PMA stabilized silver nanoparticles by chemical reduction process under a two-step UV irradiation. Appl. Surf. Sci. 2010, 256, 3812–3816. [Google Scholar] [CrossRef]
- Berti, L.; Alessandrini, A.; Facci, P. DNA-Templated Photoinduced Silver Deposition. J. Am. Chem. Soc. 2005, 127, 11216–11217. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, L.; Nowak, M.; Trojanowska, A.; Tylkowski, B.; Jastrzab, R. The Effect of pH on the Size of Silver Nanoparticles Obtained in the Reduction Reaction with Citric and Malic Acids. Materials 2020, 13, 5444. [Google Scholar] [CrossRef]
- Babusca, D.; Popescu, L.; Sacarescu, L.; Dorohoi, D.O.; Creanga, D.; Oprica, L.A. Two phase photochemical synthesis of silver nanoparticles and their impact on the chlorophylls. Mol. Cryst. Liq. Cryst. 2020, 698, 56–64. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K.; Limsuwan, P. Photo-irradiation induced green synthesis of highly stable silver nanoparticles using durian rind biomass: Effects of light intensity, exposure time and pH on silver nanoparticles formation. J. Phys. Commun. 2020, 4, 095015. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Y.-L.; Stallworth, A.M.; Ye, C.; Lenhart, J.J. Effects of pH, Electrolyte, Humic Acid, and Light Exposure on the Long-Term Fate of Silver Nanoparticles. Environ. Sci. Technol. 2016, 50, 12214–12224. [Google Scholar] [CrossRef]
- Oprica, L.; Andries, M.; Sacarescu, L.; Popescu, L.; Pricop, D.; Creanga, D.; Balasoiu, M. Citrate-silver nanoparticles and their impact on some environmental beneficial fungi. Saudi J. Biol. Sci. 2020, 27, 3365–3375. [Google Scholar] [CrossRef]
- Alqadi, M.K.; Noqtah, O.A.A.; Alzoubi, F.Y.; Al-Zou’By, J.; Aljarrah, K. pH effect on the aggregation of silver nanoparticles synthesized by chemical reduction. Mater. Sci. 2014, 32, 107–111. [Google Scholar] [CrossRef]
- Rheima, A.M.; Mohammed, M.; Jaber, S.H.; Hameed, S.A. Synthesis of Silver Nanoparticles Using the UV-Irradiation Technique in an Antibacterial Application. J. Southwest Jiaotong Univ. 2019, 54, 5. [Google Scholar] [CrossRef]
- Radoń, A.; Łukowiec, D. Silver nanoparticles synthesized by UV-irradiation method using chloramine T as modifier: Structure, formation mechanism and catalytic activity. CrystEngComm 2018, 20, 7130–7136. [Google Scholar] [CrossRef]
- Huang, H.; Yang, Y. Preparation of silver nanoparticles in inorganic clay suspensions. Compos. Sci. Technol. 2008, 68, 2948–2953. [Google Scholar] [CrossRef]
- Balci, F.M.; Sarisozen, S.; Polat, N.; Guvenc, C.M.; Karadeniz, U.; Tertemiz, A.; Balci, S. Laser assisted synthesis of anisotropic metal nanocrystals and strong light-matter coupling in decahedral bimetallic nanocrystals. Nanoscale Adv. 2021, 3, 1674–1681. [Google Scholar] [CrossRef]
- Bassetto, V.C.; Silva, W.O.; Pereira, C.; Girault, H.H. Flash light synthesis of noble metal nanoparticles for electrochemical applications: Silver, gold, and their alloys. J. Solid State Electrochem. 2020, 24, 1781–1788. [Google Scholar] [CrossRef]
- Boufi, S.; Vilar, M.R.; Ferraria, A.M.; Rego, A.M.B.D. In situ photochemical generation of silver and gold nanoparticles on chitosan. Colloid Surf A Physicochem. Eng. Asp. 2013, 439, 151–158. [Google Scholar] [CrossRef]
- González, C.M.; Martin, B.; Betancourt, T. Photochemical synthesis of bimetallic and anisotropic Au-containing nanoparticles using a one-step protocol. J. Mater. Chem. A 2014, 2, 17574–17585. [Google Scholar] [CrossRef]
- Chen, H.; Jia, J.; Dong, S. Photochemical formation of silver and gold nanostructures at the air–water interface and their electrocatalytic properties. Nanotechnology 2007, 18, 24. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Zhang, H.; Wang, D.; Chen, M. Laser-induced photochemical synthesis of branched Ag@Au bimetallic nanodendrites as a prominent substrate for surface-enhanced Raman scattering spectroscopy. Opt. Express 2017, 25, 7408. [Google Scholar] [CrossRef]
- Kazancioglu, E.O.; Aydin, M.; Arsu, N. Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: Superior photocatalytic activity for degradation of methylene blue. Mater. Chem. Phys. 2021, 269, 124734. [Google Scholar] [CrossRef]
- Korir, D.K.; Gwalani, B.; Joseph, A.; Kamras, B.; Arvapally, R.K.; Omary, M.A.; Marpu, S.B. Facile Photochemical Syntheses of Conjoined Nanotwin Gold-Silver Particles within a Biologically-Benign Chitosan Polymer. Nanomaterials 2019, 9, 596. [Google Scholar] [CrossRef] [Green Version]
- Pal, A.; Esumi, K. Photochemical synthesis of biopolymer coated Aucore-Agshell type bimetallic nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 2110–2115. [Google Scholar] [CrossRef]
- McGilvray, K.L.; Fasciani, C.; Bueno-Alejo, C.; Schwartz-Narbonne, R.; Scaiano, J. Photochemical Strategies for the Seed-Mediated Growth of Gold and Gold–Silver Nanoparticles. Langmuir 2012, 28, 16148–16155. [Google Scholar] [CrossRef]
- Mallik, K.; Mandal, M.; Pradhan, A.N.; Pal, T. Seed Mediated Formation of Bimetallic Nanoparticles by UV Irradiation: A Photochemical Approach for the Preparation of “Core−Shell” Type Structures. Nano Lett. 2001, 1, 319–322. [Google Scholar] [CrossRef]
- Mandal, S.; Selvakannan, P.; Pasricha, R.; Sastry, M. Keggin Ions as UV-Switchable Reducing Agents in the Synthesis of Au Core−Ag Shell Nanoparticles. J. Am. Chem. Soc. 2003, 125, 8440–8441. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Tahami, S.; Naji, M. Synthesis and characterization of core-shell bimetallic nanoparticles for synergistic antimicrobial effect studies in combination with doxycycline on burn specific pathogens. J. Photochem. Photobiol. B Biol. 2017, 169, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Pal, A.; Pande, S.; Sarkar, S.; Panigrahi, S.; Pal, T. Alginate Gel-Mediated Photochemical Growth of Mono- and Bimetallic Gold and Silver Nanoclusters and Their Application to Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 7553–7560. [Google Scholar] [CrossRef]
- Shapoval, L.V.; Gorbunova, V.V.; Boitsova, T. Synthesis of hollow bimetal particles based on silver and gold. Russ. J. Gen. Chem. 2012, 82, 1361–1367. [Google Scholar] [CrossRef]
- Fattori, N.; Maroneze, C.M.; Da Costa, L.P.; Strauss, M.; Mazali, I.O.; Gushikem, Y. Chemical and photochemical formation of gold nanoparticles supported on viologen-functionalized SBA-Colloid Surf A Physicochem. Eng. Asp. 2013, 437, 120–126. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.; Zhu, H.Y. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814–1833. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Luo, Y. Size-controlled preparation of polyelectrolyte-protected gold nanoparticles by natural sunlight radiation. Mater. Lett. 2007, 61, 2164–2166. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Shukla, A.; Maurya, S.; Singh, S.; Uttam, K.N.; Sundaram, S.; Singh, M.; Gopal, R. Direct sunlight enabled photo-biochemical synthesis of silver nanoparticles and their Bactericidal Efficacy: Photon energy as key for size and distribution control. J. Photochem. Photobiol. B Biol. 2018, 188, 42–49. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sastry, T.P.; Kumar, M.S.; Dinesh, M.G.; Kannappan, S.; Suguna, L. Sun light mediated synthesis of gold nanoparticles as carrier for 6-mercaptopurine: Preparation, characterization and toxicity studies in zebrafish embryo model. Mater. Res. Bull. 2012, 47, 2113–2119. [Google Scholar] [CrossRef]
- Bhaduri, G.A.; Little, R.; Khomane, R.B.; Lokhande, S.U.; Kulkarni, B.D.; Mendis, B.G.; Šiller, L. Green synthesis of silver nanoparticles using sunlight. J Photochem Photobiol A Chem 2013, 258, 1–9. [Google Scholar] [CrossRef]
- Pienpinijtham, P.; Han, X.X.; Suzuki, T.; Thammacharoen, C.; Ekgasit, S.; Ozaki, Y. Micrometer-sized gold nanoplates: Starch-mediated photochemical reduction synthesis and possibility of application to tip-enhanced Raman scattering (TERS). Phys. Chem. Chem. Phys. 2012, 14, 9636. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Sun, L.; Li, J.; Zhang, M.; Wang, X. Sunlight-driven synthesis of anisotropic silver nanoparticles. Chem. Eng. J. 2015, 260, 99–106. [Google Scholar] [CrossRef]
- Nguyen, V.T. Sunlight-Driven Synthesis of Silver Nanoparticles Using Pomelo Peel Extract and Antibacterial Testing. Hindawi J. Chem. 2020, 2020, 6407081. [Google Scholar] [CrossRef]
- Susilowati, E.; Maryani, A. Sunlight-assisted synthesis of colloidal silver nanoparticles using chitosan as reducing agent. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; p. 12019. [Google Scholar]
- Singh, J.; Kukkar, P.; Sammi, H.; Rawat, M.; Singh, G.; Kukkar, D. Enhanced catalytic reduction of 4-nitrophenol and congo red dye by silver nanoparticles prepared from Azadirachta indica leaf extract under direct sunlight exposure. Part. Sci. Technol. 2017, 37, 434–443. [Google Scholar] [CrossRef]
- Mankad, M.; Patil, G.; Patel, D.; Patel, P.; Patel, A. Comparative studies of sunlight mediated green synthesis of silver nanoparaticles from Azadirachta indica leaf extract and its antibacterial effect on Xanthomonas oryzae pv. oryzae. Arab. J. Chem. 2020, 13, 2865–2872. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Jeffryes, C.; Mechouet, M.; Agathos, S.N. Biosynthesis of Inorganic Nanoparticles: A Fresh Look at the Control of Shape, Size and Composition. Bioengineering 2017, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Jamila, N.; Khan, N.; Bibi, A.; Haider, A.; Khan, S.N.; Atlas, A.; Nishan, U.; Minhaz, A.; Javed, F.; Bibi, A. Piper longum catkin extract mediated synthesis of Ag, Cu, and Ni nanoparticles and their applications as biological and environmental remediation agents. Arab. J. Chem. 2020, 13, 6425–6436. [Google Scholar] [CrossRef]
- Jayapriya, M.; Dhanasekaran, D.; Arulmozhi, M.; Nandhakumar, E.; Senthilkumar, N.; Sureshkumar, K. Green synthesis of silver nanoparticles using Piper longum catkin extract irradiated by sunlight: Antibacterial and catalytic activity. Res. Chem. Intermed. 2019, 45, 3617–3631. [Google Scholar] [CrossRef]
- Huang, H.; Shan, K.; Liu, J.; Tao, X.; Periyasamy, S.; Durairaj, S.; Jiang, Z.; Jacob, J.A. Synthesis, optimization and characterization of silver nanoparticles using the catkin extract of Piper longum for bactericidal effect against food-borne pathogens via conventional and mathematical approaches. Bioorgan. Chem. 2020, 103, 104230. [Google Scholar] [CrossRef]
- Firdaus, M.; Andriana, S.; Elvinawati; Alwi, W.; Swistoro, E.; Ruyani, A.; Sundaryono, A. Green synthesis of silver nanoparticles using Carica Papaya fruit extract under sunlight irradiation and their colorimetric detection of mercury ions. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2017; p. 12029. [Google Scholar]
- Wei, X.; Luo, M.; Li, W.; Yang, L.; Liang, X.; Xu, L.; Kong, P.; Liu, H. Synthesis of silver nanoparticles by solar irradiation of cell-free Bacillus amyloliquefaciens extracts and AgNO3. Bioresour. Technol. 2012, 103, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Xu, W.; An, J.; Li, D.; Zhao, B. A simple method to synthesize triangular silver nanoparticles by light irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2006, 64, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.N.; Nguyen, T.D.; Cao, M.T.; Pham, V.V. Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloid Surf A Physicochem. Eng. Asp. 2020, 594, 124695. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.A.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Silver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahoumane, S.A.; Djediat, C.; Yéprémian, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Recycling and adaptation of Klebsormidium flaccidum microalgae for the sustained production of gold nanoparticles. Biotechnol. Bioeng. 2012, 109, 284–288. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. A global approach of the mechanism involved in the biosynthesis of gold colloids using micro-algae. J. Nanopart. Res. 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Yéprémian, C.; Djédiat, C.; Couté, A.; Fiévet, F.; Coradin, T.; Brayner, R. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J. Nanopart. Res. 2016, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Shabnam, N.; Pardha-Saradhi, P. Photosynthetic Electron Transport System Promotes Synthesis of Au-Nanoparticles. PLoS ONE 2013, 8, e71123. [Google Scholar] [CrossRef]
- Shabnam, N.; Sharmila, P.; Kim, H.; Pardha-Saradhi, P. Light Mediated Generation of Silver Nanoparticles by Spinach Thylakoids/Chloroplasts. PLoS ONE 2016, 11, e0167937. [Google Scholar] [CrossRef]
- Rawat, V.; Sharma, A.; Bhatt, V.P.; Singh, R.P.; Maurya, I.K. Sunlight mediated green synthesis of silver nanoparticles using Polygonatum graminifolium leaf extract and their antibacterial activity. Mater. Today Proc. 2020, 29, 911–916. [Google Scholar] [CrossRef]
- Félix-Domínguez, F.; Carrillo-Torres, R.C.; Lucero-Acuña, A.; Sanchez-Zeferino, R.; Alvarez-Ramos, M. Sunlight-driven phytochemical synthesis of silver nanoparticles using aqueous extract of Albizia lebbeck (L.) Benth. Mater. Res. Express 2019, 6, 125060. [Google Scholar] [CrossRef]
- Sooraj, M.P.; Nair, A.S.; Vineetha, D. Sunlight-mediated green synthesis of silver nanoparticles using Sida retusa leaf extract and assessment of its antimicrobial and catalytic activities. Chem. Pap. 2021, 75, 351–363. [Google Scholar] [CrossRef]
- Bharali, P.; Das, S.; Bhandari, N.; Das, A.K.; Kalita, M.C. Sunlight induced biosynthesis of silver nanoparticle from the bark extract of Amentotaxus assamica D.K. Ferguson and its antibacterial activity against Escherichia coli and Staphylococcus aureus. IET Nanobiotechnol. 2019, 13, 18–22. [Google Scholar] [CrossRef]
- Connor, D.M.; Broome, A.-M. Gold Nanoparticles for the Delivery of Cancer Therapeutics. Adv. Cancer Res. 2018, 139, 163–184. [Google Scholar] [CrossRef]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25, 2193. [Google Scholar] [CrossRef]
- Dizman, H.M.; Eroglu, G.O.; Kuruca, S.E.; Arsu, N. Photochemically prepared monodisperse gold nanoparticles as doxorubicin carrier and its cytotoxicity on leukemia cancer cells. Appl. Nanosci. 2021, 11, 309–320. [Google Scholar] [CrossRef]
- Licciardi, M.; Volsi, A.L.; Mauro, N.; Scialabba, C.; Cavallaro, G.; Giammona, G. Preparation and Characterization of Inulin Coated Gold Nanoparticles for Selective Delivery of Doxorubicin to Breast Cancer Cells. J. Nanomater. 2016, 2016, 2078315. [Google Scholar] [CrossRef] [Green Version]
- Kumar-Krishnan, S.; Prokhorov, E.; Iturriaga, M.H.; Mota-Morales, J.; Vazquez-Lepe, M.; Kovalenko, Y.; Sanchez, I.C.; Luna-Bárcenas, G. Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 2015, 67, 242–251. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef]
- Pham, T.B.; Hoang, T.H.C.; Pham, V.H.; Nguyenvan, C.; Van Nguyen, T.; Vu, D.C.; Bui, H. Detection of Permethrin pesticide using silver nano-dendrites SERS on optical fibre fabricated by laser-assisted photochemical method. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Zhang, H.; Tian, Y.; Jiao, A.; Chen, F.; Chen, M. Photochemical synthesis of ZnO@Au nanorods as an advanced reusable SERS substrate for ultrasensitive detection of light-resistant organic pollutant in wastewater. Talanta 2019, 194, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lin, M.; Chen, L.; Wang, Y.; Guo, X.; Peng, L.; Guo, X.; Ding, W. Thickness-dependent SERS activities of gold nanosheets controllably synthesized via photochemical reduction in lamellar liquid crystals. Chem. Commun. 2015, 51, 5116–5119. [Google Scholar] [CrossRef]
- Darbha, G.K.; Rai, U.S.; Singh, A.K.; Ray, P.C. Gold-Nanorod-Based Sensing of Sequence Specific HIV-1 Virus DNA by Using Hyper-Rayleigh Scattering Spectroscopy. Chem. Eur. J. 2008, 14, 3896–3903. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Clavijo, C.D.F.; Medina, E.; Sinche, F.; Vispo, N.S.; Dahoumane, S.A.; Alexis, F. Biomedical Science to Tackle the COVID-19 Pandemic: Current Status and Future Perspectives. Molecules 2020, 25, 4620. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015, 33 Pt 1, 666–680. [Google Scholar] [CrossRef]
- Hu, S.; Huang, P.-J.J.; Wang, J.; Liu, J. Dissecting the Effect of Salt for More Sensitive Label-Free Colorimetric Detection of DNA Using Gold Nanoparticles. Anal. Chem. 2020, 92, 13354–13360. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Foti, A.; D’Andrea, C.; Villari, V.; Micali, N.; Donato, M.G.; Fazio, B.; Maragò, O.M.; Gillibert, R.; De La Chapelle, M.L.; Gucciardi, P.G. Optical Aggregation of Gold Nanoparticles for SERS Detection of Proteins and Toxins in Liquid Environment: Towards Ultrasensitive and Selective Detection. Materials 2018, 11, 440. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.-Q.; Duo, H.-H.; Zhang, Y.-G.; Zhang, X.-W.; Fang, W.; Liu, Y.-L.; Shen, A.-G.; Hu, J.-M.; Huang, W.-H. Photochemical Synthesis of Shape-Controlled Nanostructured Gold on Zinc Oxide Nanorods as Photocatalytically Renewable Sensors. Anal. Chem. 2016, 88, 3789–3795. [Google Scholar] [CrossRef]
- Çinko, T.; Koyuncu, U.; Ömür, B.C.; Altındal, A.; Arsu, N. In-situ photochemical synthesis of Au nanoparticles in polymer matrix with one-component thioxanthone disulfide for detection of benzene, toluene and xylene vapours. Prog. Org. Coat. 2019, 132, 125–131. [Google Scholar] [CrossRef]
- Liu, C.; Ding, Y.; Li, Q.; Lin, Y. Photochemical synthesis of glutathione-stabilized silver nanoclusters for fluorometric determination of hydrogen peroxide. Microchim. Acta 2017, 184, 2497–2503. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.K.; Mohan, S.; Bano, D.; Gundampati, R.K.; Hasan, S.H. Green synthesis of silver nanoparticle for the selective and sensitive colorimetric detection of mercury (II) ion. J. Photochem. Photobiol. B Biol. 2017, 168, 67–77. [Google Scholar] [CrossRef]







| Metal | Precursor | Reducing Agent | Stabilizer/ Surfactant | Irradiation Source, Wavelength and Power | Exposure Time | pH | Size (nm) | Shape | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Au NPs | HAuCl4 | - | - | 6 W, λ = 365 nm | 1 h | - | - | Nanorods | [149] |
| Au NPs | HAuCl4 | Sodium dodecyl benzene sulfonate (SDBS) | Id. * | 300 W high-pressure mercury lamp | 12 h | - | 3–4 | Spherical NPs | [117] |
| Au NPs | HAuCl4 | Sodium dodecyl sulfate (SDS) | Id. | 300 W high-pressure mercury lamp | 12 h | - | 4–5 | Spherical NPs | [117] |
| Au NPs | HAuCl4 | Extract of cornelian cherry | Id. | 6 W UV lamp (365 nm) | 2 h | - | 19 | Spherical NPs | [118] |
| Ag NPs | AgNO3 | Extract of cornelian cherry | Id. | 6 W UV lamp (365 nm) | 2.5 h | - | 16 | Spherical NPs | [118] |
| Au NPs | HAuCl4 | Leaf extract of Polycias scutellaria | Id. | UV lamp | 2 h | - | 5–20 | Spherical NPs | [119] |
| Au NPs | HAuCl4 | Extract of red cabbage | Id. | 6 W power UV (365 nm) | 20 min | 7 | 25 | Spherical NPs | [109] |
| Au NPs | HAuCl4 | Ethanolic leaf extract of Bacopa monnieri | Id. | UV lamp (254 nm) | 15 min | - | 11 | Spherical NPs | [120] |
| Ag NPs | AgNO3 | Poly(methacrylic acid) (PMA) | Id. | 8 W UV lamp (365 nm) | 1 h | 4 | 8 | Spherical NPs | [98] |
| Ag NPs | AgNO3 | Chitosan | Id. | UV-LED (365 nm) | 15 min | - | 30 | Spherical NPs | [94] |
| Ag NPs | AgNO3 | PMA | Id. | 6 W UV lamp and 25 W UV lamp | 1 h | 9 | 10 | Spherical NPs | [123] |
| Ag NPs | AgNO3 | - | Laponite aqueous suspension | UV light 0.362 mW cm−2 | 3 h | - | 60 110 | Mainly nanoprisms and pentagons | [133] |
| Ag NPs | AgNO3 | Glucose | Id. | UV light (λ = 365 nm; 125 W) | 30 min | - | 20 | Hexagonal and spherical NPs | [131] |
| Ag NPs | AgNO3 | Thiopyran-3-4-dicarboximide (TXICh) | Id. | UV LED (λ = 365 nm; 92 mW cm−2) | 4 h | - | 2–24 | Self-assembled spherical NPs | [94] |
| TXLCh/Triethanolamine (TEOH) | 15 min | - | 2.5 | Stable spherical NPs | |||||
| Ag NPs | AgNO3 | - | Gelatin | UV reactor (UV-A, 6 W) | 24 h | - | 19 | - | [89] |
| Ag NPs | AgNO3 | Poly(vinyl pyrrolidone) (PVP) | - | UV light (λ = 365 nm) | 10 min | - | 11.7 ± 7.2 | Cubes, rods and spheres | [132] |
| Ag NPs | AgNO3 | - | Poly(acrylic acid) (PAA) | Low-pressure mercury lamp (λ = 253.7 nm) | 1 h | - | 30–50 nm in width | Nanofilaments | [138] |
| Au NPs | HAuCl4 | 10–30 | NPs |
| Metal | Precursor | Reducing Agent | Stabilizer/Surfactant | Irradiation Source | Exposure Time | pH | Size (nm) | Shape | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ag NPs | AgNO3 | - | Sodium dodecyl sulfate (SDS) | Sunlight (50.3 mW cm−2) | 1 h | - | 5–10 | - | [155] |
| Ag NPs | AgNO3 | Zingiber officinale extract | - | Sunlight | 2–20 h | - | 4–15 | Spherical NPs | [87] |
| Ag NPs | AgNO3 | Sodium citrate and sodium borohydride | - | Sodium lamp (70 W; λ = 589 nm) | 1 h | - | 40–110 | Truncated triangular NPs | [168] |
| Ag NPs | AgNO3 | - | Cell-free extract of Bacillus amyloliquefaciens | Sunlight | 100 min | 7.2 | 14.6 | Circular and triangular NPs | [167] |
| Ag NPs | AgNO3 | Piper longum extract | - | Sunlight | - | - | 15–40 | Monodisperse spherical NPs | [164] |
| Ag NPs | AgNO3 | Carica Papaya extract | - | Sunlight | 15 min | 4.5 | 35–50 | - | [166] |
| Ag NPs | AgNO3 | Azadirachta indica leaf extract | - | Sunlight | 5 min | - | 67.94 | - | [161] |
| Ag NPs | AgNO3 | A. indica leaf extract | - | Sunlight | 60 min | 10 | 75.87–185 | - | [160] |
| Ag NPs | AgNO3 | Polygonatum graminifolium leaf extract | Id. * | Sunlight | 30 min | - | 3–15 | Spherical, circular, and triangular NPs | [177] |
| Ag NPs | AgNO3 | Pomelo peel extract | - | Sunlight | 30 min | 3.5 | 20–30 | - | [158] |
| Ag NPs | AgNO3 | Albizia lebbeck extract/ Citrate + A. lebbeck extract | - | Sunlight (~788 lux) | 60 min | - | 10–20 | Spheroidal NPs | [178] |
| Ag NPs | AgNO3 | Sodium citrate | - | Sunlight (~788 lux) | 60 min | - | - | Large triangular and hexagonal nanoprisms; small spheroidal NPs | [178] |
| Ag NPs | AgNO3 | Sida retusa extract | Id. | Sunlight | 30 min | - | 20–40 | Spherical | [179] |
| Ag NPs | AgNO3 | Amentotaxus assamica | - | Sunlight | 32 min | - | 39.41 | Spherical | [180] |
| Ag NPs | AgNO3 | - | Ferredoxin-NADP+ reductase and ferredoxin (FNR/FD) | Sunlight | 150 min | 8 | 10–15 | Spherical | [6] |
| Ag NPs | AgNO3 | Pleurotus citrinopileatus extract | Id. | Sunlight | 180 min | - | 7.08 ± 2.92 | - | [153] |
| Ag NPs | AgNO3 | P. citrinopileatus extract | Id. | Blue sunlight | 180 min | - | 3.18 ± 0.72 | - | [153] |
| Au NPs | HAuCl4 | - | Poly(vinyl pyrrolidone) | Xenon flash lamp | 20 ms | - | 25.3 ± 11.0 | Spherical | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jara, N.; Milán, N.S.; Rahman, A.; Mouheb, L.; Boffito, D.C.; Jeffryes, C.; Dahoumane, S.A. Photochemical Synthesis of Gold and Silver Nanoparticles—A Review. Molecules 2021, 26, 4585. https://doi.org/10.3390/molecules26154585
Jara N, Milán NS, Rahman A, Mouheb L, Boffito DC, Jeffryes C, Dahoumane SA. Photochemical Synthesis of Gold and Silver Nanoparticles—A Review. Molecules. 2021; 26(15):4585. https://doi.org/10.3390/molecules26154585
Chicago/Turabian StyleJara, Nicole, Nataly S. Milán, Ashiqur Rahman, Lynda Mouheb, Daria C. Boffito, Clayton Jeffryes, and Si Amar Dahoumane. 2021. "Photochemical Synthesis of Gold and Silver Nanoparticles—A Review" Molecules 26, no. 15: 4585. https://doi.org/10.3390/molecules26154585
APA StyleJara, N., Milán, N. S., Rahman, A., Mouheb, L., Boffito, D. C., Jeffryes, C., & Dahoumane, S. A. (2021). Photochemical Synthesis of Gold and Silver Nanoparticles—A Review. Molecules, 26(15), 4585. https://doi.org/10.3390/molecules26154585